 World Journal of Cardiovascular Diseases, 2013, 3, 458-463 WJCD http://dx.doi.org/10.4236/wjcd.2013.37072 Published Online October 2013 (http://www.scirp.org/journal/wjcd/) White blood cell count and mortality in acute myocardial infarction Negar Salehi1, Rahimeh Eskandarian2*, Hamid Reza Sanati3, Ata Firouzi3, Farshad Shakerian3, Seyfollah Abdi3, Homan Bakhshandeh4, Mojde Nasiri Ahmad Abadi5, Negin Nouri6, Anoushiravan Vakili-Zarch3 1College of Osteopathic Medicine, Michigan State University, Michigan, USA 2Department of Interventional Cardiology, Semnan University of Medical Sciences, Semnan, Iran 3Department of Interventional Cardiology, Tehran University of Medical Sciences, Tehran, Iran 4Department of Epidemiology, Tehran University of Medical Sciences, Tehran, Iran 5Department of Epidemiology and Biostatistic, Michigan State University, Michigan, USA 6Digestive Disease Research Center, Tehran University of Medical Sciences, Tehran, Iran Email: *rheskandarian@yahoo.com Received 8 August 2013; revised 8 September 2013; accepted 25 September 2013 Copyright © 2013 Negar Salehi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Introduction: Coronary atherosclerosis is increasingly viewed as an inflammatory process. We assessed the relation between WBC count on admission and mor- tality in STEMI patients treated with primary PCI. Material & Method: Totally 205 patients with STEMI less than 24 hours before ad mission who admitted for primary angioplasty enrolled in study. Study end points were defined as myocardial adverse cardiac event (MACE) and mortality at one month and one year follow-up. Result: Totally 205 patients (166 men) with mean age 56 ± 11 were enrolled in study. The mean WBC count was 8983 ± 34 and mean follow-up was 12.24 months. WBC count remained a significant predictor of mortality after multivariable adjustment in one month and 12 months follow-up (p = 0.02, p = 0.04). Conclusion: Our results extend previous find- ings that WBC count is an independent marker of cardiac mortality. Keywords: Myocardial Infarction; Primary Percutaneous Coronary Intervention; WBC Count; Inflammation 1. INTRODUCTION Coronary atherosclerosis is increasingly viewed as an inflammatory process [1-3]. Previous studies confirmed an association between inflammatory markers and athe- rosclerosis plaque formation, progression, and instability of plaque, adverse clinical outcome and development of myocardial infarction [4,5]. Leucocytes are major me- diators of inflammation. They have a key role in host defense to injury. Increasing of white blood cell (WBC) count is a risk factor for future cardiovascular events in individuals without cardiovascular disease [6,7]. It is as- sociated with worse outcome in patients with stable an- gina and acute coronary syndrome [8-15]. The baseline WBC count is an independent predictor of mortality in ST elevation myocardial infarction (STEMI) patients [16, 17]. Moreover, in STEMI patients undergoing primary percutaneous coronary intervention (PCI) WBC count predicts short term mortality [18]. Although in one study this association was not significant. Gurm et al. showed that WBC count is an independent predictor for long term mortality in patients undergoing PCI either in the setting of stable angina or primary PCI [19,20]. The aim of our study was to assess the relation between on ad- mission WBC count and clinical data and short term and long term mortality in STEMI patients treated with pri- mary PCI. 2. MATERIAL & METHOD This prospective study was done from Nov 2008 to Dec 2011. Totally 205 patients with STEMI (ST elevation ≥0.1 mV in more than 2 limb leads or ≥0.2 mV in chest lead or new LBBB at presentation, patients with chest pain during less than 24 hours before presentation who admitted for primary angioplasty in Shahid Rajaie hos- pital in Tehran, Iran enrolled in study. In all patients, primary angioplasty was performed *Corresponding author. OPEN ACCESS  N. Salehi et al. / World Journal of Cardiovascular Diseases 3 (2013) 458-463 459 according standard technique after loading dose of ASA 325 mg and 600 mg of clopidoigrel. Integrilin prescribed based on discretion physician performing procedure. Demographic, clinical and angiographic data including sex, age, coronary risk factor, MI location, blood pres- sure, heart rate, previous presentation before MI and time from onset to admission was determined and recorded. Exclusion criteria included the following: any form of steroid medication or non steroidal anti-inflammatory drugs or antibiotics use in the preceding month, acute infection in the last 2 weeks, surgery within the last 2 weeks, history of liver or renal failure, history of malig- nancy or vacuities and admission more than 24 hour from onset of symptoms. Pre- and post-procedural an- giograms were analyzed by two operators blinded to the study. Study protocol was approved by the local ethics committee. Study end point was major adverse cardiac events (MACE) including mortality, MI, target vessel revascu- larization (TVR) and stent thrombosis. Follow up per- formed based on records of monthly visits in outpatient clinic and telephone interview to evaluate symptoms and admission note. Baseline WBC count values were analyzed as con- tinuous variable. To assess the relation between WBC count and baseline clinical data multivariable linear re- gression was applied. Study end points were defined as MACE and mortality at one month and one year follow-up (minimum 1moth to 33 months for all subjects). Continuous data are pre- sented as mean values with standard deviation and com- pared by use of Student’s t-test. Categorical data are pre- sented as frequencies and analyzed with χ2 tests. The relation between one-year mortality and clinical factors’ including WBC count is examined with stepwise, multi- variable logistic regression. Risk ratios are reported with regression model that adjust for factors that are inde- pendently associated with the outcome variable. Data analysis is performed using SPSS 16.0. 3. RESULT Total of 205 patients (166 men and 39 women) with AMI with mean age 56.9 ± 11.154 (range 28 - 86 years) were enrolled in study. The mean WBC count was 8983 ± 34 and mean follow up was 12.24 month (range 1 to 33). In patient with current smoking, mean WBC was signifi- cantly higher than non smoker patients [10,021 ± 53 versus 8319 ± 55 (p = 0.002)]. Others risk factors such as diabetes mellitus, hypertension, family history of coro- nary disease, hypercholesterolemia, age and sex had no significant relation with WBC (Table 1). Table 1 De- scribes baseline characteristics of patients including cor- onary risk factors and association with mean WBC. We assessed the relation of MI location, history of pre- vious PCI or CABG, MI history and symptoms before the presentation and presence or absence of shock and their relationship with WBC count. There was a signifi- cant statistical relation with history of PCI (Table 2). WBC count in patients with single vessel and multi vessel involvement had no significant difference in our data. LAD lesion diagnosed in 53.7%, LCX lesion in 12.2%, and RCA lesion in 32.7% of patients (p = 0.028). Moreover, 80.5% of patient had thrombotic lesion. Data Table 1. Baseline characteristics of patients. Variables Number Percent WBC count mean ± SD p value Male 166 81 9091 ± 65 Sex Female 39 19 8535 ± 48 0.739 Current 87 42.4 10,021 ± 53 Smoking Non 118 57.6 8319 ± 55 0.002 Yes 43 21 8323 ± 71 Diabetes No 162 79 9169 ± 36 0.179 Yes 72 35.1 8360 ± 78 Hypertension No 133 64.9 9350 ± 45 0.077 Yes 24 11.7 8962 ± 38 Family history of coronary disease No 181 88.3 8986 ± 38 0.976 Yes 82 40 8713 ± 33 Hypercholesterolemia No 123 60 9190 ± 12 0.384 >70 25 13.2 9204 ± 32 Age Up to 70 178 88.8 8953 ± 25 0.765 Copyright © 2013 SciRes. OPEN ACCESS 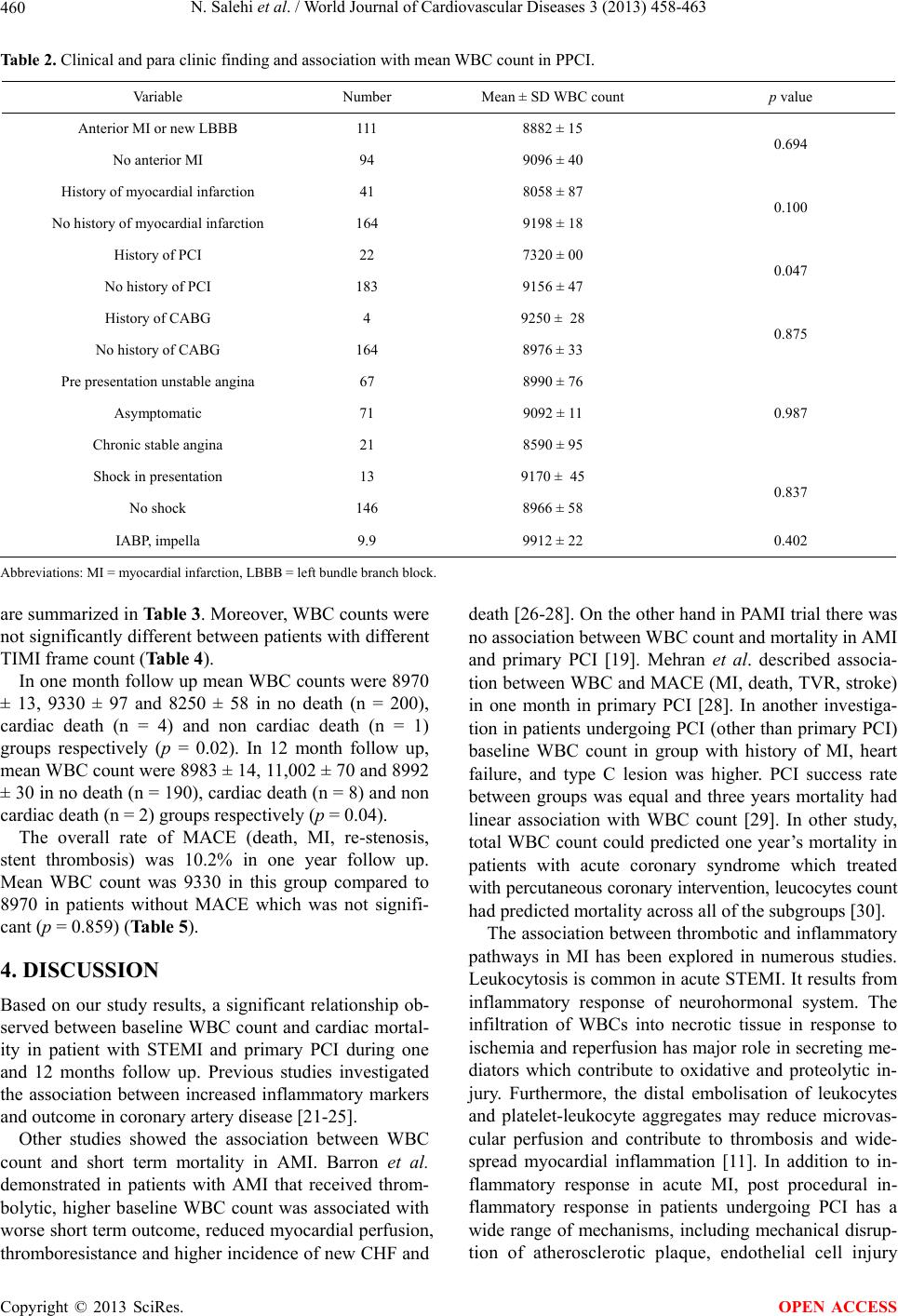 N. Salehi et al. / World Journal of Cardiovascular Diseases 3 (2013) 458-463 460 Table 2. Clinical and para clinic finding and association with mean WBC count in PPCI. Variable Number Mean ± SD WBC count p value Anterior MI or new LBBB 111 8882 ± 15 No anterior MI 94 9096 ± 40 0.694 History of myocardial infarction 41 8058 ± 87 No history of myocardial infarction 164 9198 ± 18 0.100 History of PCI 22 7320 ± 00 No history of PCI 183 9156 ± 47 0.047 History of CABG 4 9250 ± 28 No history of CABG 164 8976 ± 33 0.875 Pre presentation unstable angina 67 8990 ± 76 Asymptomatic 71 9092 ± 11 Chronic stable angina 21 8590 ± 95 0.987 Shock in presentation 13 9170 ± 45 No shock 146 8966 ± 58 0.837 IABP, impella 9.9 9912 ± 22 0.402 Abbreviations: MI = myocardial infarction, LBBB = left bundle branch block. are summarized in Table 3. Moreover, WBC counts were not significantly different between patients with different TIMI frame count (Table 4). In one month follow up mean WBC counts were 8970 ± 13, 9330 ± 97 and 8250 ± 58 in no death (n = 200), cardiac death (n = 4) and non cardiac death (n = 1) groups respectively (p = 0.02). In 12 month follow up, mean WBC count were 8983 ± 14, 11,002 ± 70 and 8992 ± 30 in no death (n = 190), cardiac death (n = 8) and non cardiac death (n = 2) groups respectively (p = 0.04). The overall rate of MACE (death, MI, re-stenosis, stent thrombosis) was 10.2% in one year follow up. Mean WBC count was 9330 in this group compared to 8970 in patients without MACE which was not signifi- cant (p = 0.859) (Table 5). 4. DISCUSSION Based on our study results, a significant relationship ob- served between baseline WBC count and cardiac mortal- ity in patient with STEMI and primary PCI during one and 12 months follow up. Previous studies investigated the association between increased inflammatory markers and outcome in coronary artery disease [21-25]. Other studies showed the association between WBC count and short term mortality in AMI. Barron et al. demonstrated in patients with AMI that received throm- bolytic, higher baseline WBC count was associated with worse short term outcome, reduced myocardial perfusion, thromboresistance and higher incidence of new CHF and death [26-28]. On the other hand in PAMI trial there was no association between WBC count and mortality in AMI and primary PCI [19]. Mehran et al. described associa- tion between WBC and MACE (MI, death, TVR, stroke) in one month in primary PCI [28]. In another investiga- tion in patients undergoing PCI (other than primary PCI) baseline WBC count in group with history of MI, heart failure, and type C lesion was higher. PCI success rate between groups was equal and three years mortality had linear association with WBC count [29]. In other study, total WBC count could predicted one year’s mortality in patients with acute coronary syndrome which treated with percutaneous coronary intervention, leucocytes count had predicted mortality across all of the subgroups [30]. The association between thrombotic and inflammatory pathways in MI has been explored in numerous studies. Leukocytosis is common in acute STEMI. It results from inflammatory response of neurohormonal system. The infiltration of WBCs into necrotic tissue in response to ischemia and reperfusion has major role in secreting me- diators which contribute to oxidative and proteolytic in- jury. Furthermore, the distal embolisation of leukocytes and platelet-leukocyte aggregates may reduce microvas- cular perfusion and contribute to thrombosis and wide- spread myocardial inflammation [11]. In addition to in- flammatory response in acute MI, post procedural in- flammatory response in patients undergoing PCI has a wide range of mechanisms, including mechanical disrup- tion of atherosclerotic plaque, endothelial cell injury Copyright © 2013 SciRes. OPEN ACCESS 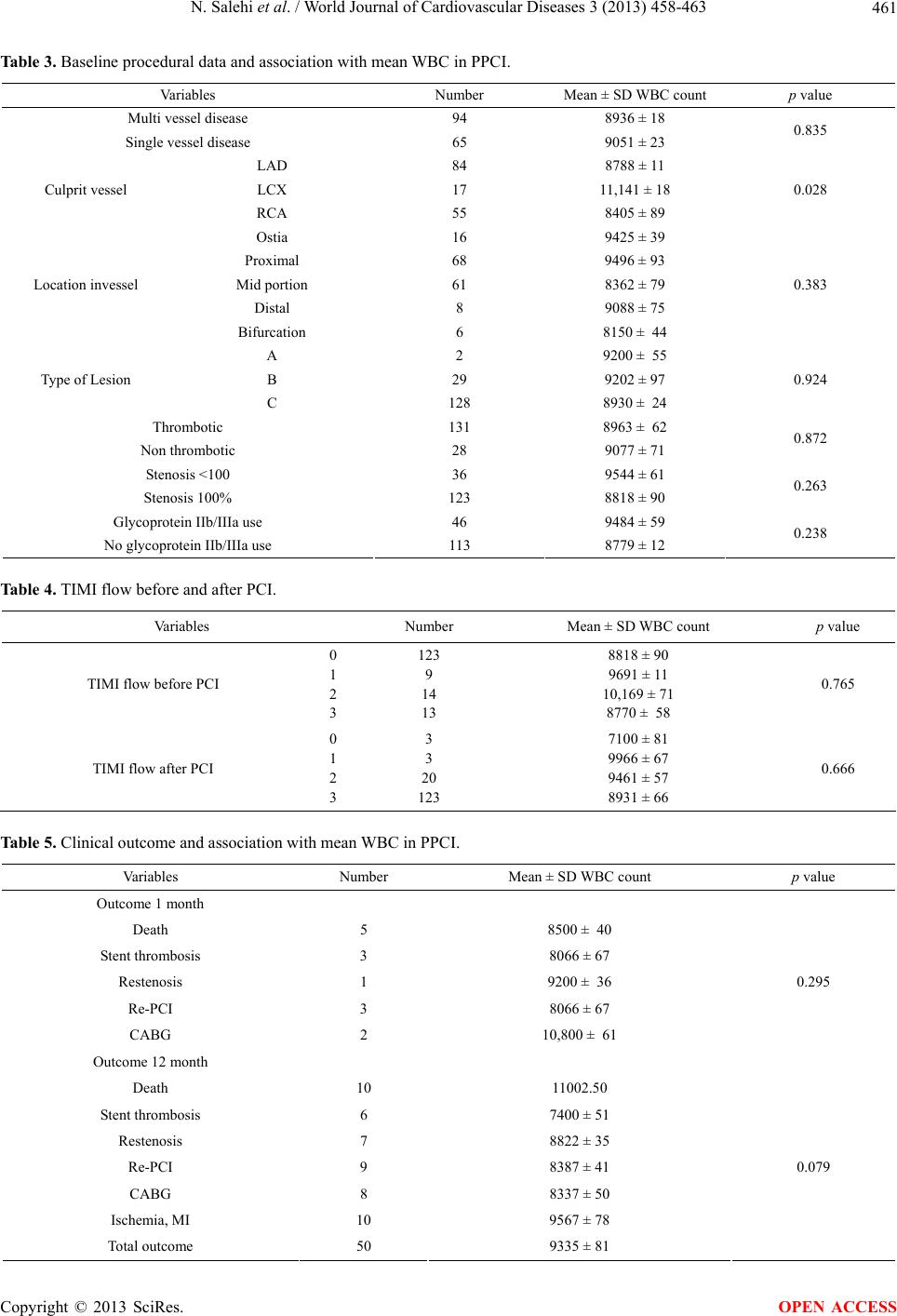 N. Salehi et al. / World Journal of Cardiovascular Diseases 3 (2013) 458-463 461 Table 3. Baseline procedural data and association with mean WBC in PPCI. Variables Number Mean ± SD WBC count p value Multi vessel disease 94 8936 ± 18 Single vessel disease 65 9051 ± 23 0.835 LAD 84 8788 ± 11 LCX 17 11,141 ± 18 Culprit vessel RCA 55 8405 ± 89 0.028 Ostia 16 9425 ± 39 Proximal 68 9496 ± 93 Mid portion 61 8362 ± 79 Distal 8 9088 ± 75 Location invessel Bifurcation 6 8150 ± 44 0.383 A 2 9200 ± 55 B 29 9202 ± 97 Type of Lesion C 128 8930 ± 24 0.924 Thrombotic 131 8963 ± 62 Non thrombotic 28 9077 ± 71 0.872 Stenosis <100 36 9544 ± 61 Stenosis 100% 123 8818 ± 90 0.263 Glycoprotein IIb/IIIa use 46 9484 ± 59 No glycoprotein IIb/IIIa use 113 8779 ± 12 0.238 Table 4. TIMI flow before and after PCI. Variables Number Mean ± SD WBC count p value TIMI flow before PCI 0 1 2 3 123 9 14 13 8818 ± 90 9691 ± 11 10,169 ± 71 8770 ± 58 0.765 TIMI flow after PCI 0 1 2 3 3 3 20 123 7100 ± 81 9966 ± 67 9461 ± 57 8931 ± 66 0.666 Table 5. Clinical outcome and association with mean WBC in PPCI. Variables Number Mean ± SD WBC count p value Outcome 1 month Death 5 8500 ± 40 Stent thrombosis 3 8066 ± 67 Restenosis 1 9200 ± 36 Re-PCI 3 8066 ± 67 CABG 2 10,800 ± 61 0.295 Outcome 12 month Death 10 11002.50 Stent thrombosis 6 7400 ± 51 Restenosis 7 8822 ± 35 Re-PCI 9 8387 ± 41 CABG 8 8337 ± 50 Ischemia, MI 10 9567 ± 78 Total outcome 50 9335 ± 81 0.079 Copyright © 2013 SciRes. OPEN ACCESS  N. Salehi et al. / World Journal of Cardiovascular Diseases 3 (2013) 458-463 462 caused by proteolytic and oxidative stress, arterial wall injury, myocardial necrosis due to distal embolisation, vessel plugging effecting the blood flow through the car- diac microvasculature, hyper coagulable state with de- crease epicardial patency and increased ischemic burden [31,32]. Moreover, reduced patency of coronary arteries and higher thrombus burden was observed in patients with elevated WBC count [33]. It is possible that endothelial dysfunction and mi- crovascular plugging due to elevated level of WBC play an important role in developing of future heart failure in STEMI patients [34]. 5. CONCLUSION Our results extend previous findings that WBC count can be an independent marker of cardiac mortality, in short term and long-term mortality prediction in STEMI treated with revascularization. Increased WBC count is a simple non-specific marker of inflammation; it may serve as an available and inexpensive tool for risk stratification in acute SEMI. REFERENCES [1] Bhatt, D.L. and Topol, E.J. (2002) Need to test the arte- rial inflammation hypothesis. Circulation, 106, 136-140. http://dx.doi.org/10.1161/01.CIR.0000021112.29409.A2 [2] Alexander, R.W. (1994) Inflammation and coronary ar- tery disease. New England Journal of Medicine, 331, 468-469. http://dx.doi.org/10.1056/NEJM199408183310709 [3] Gurm, H.S., Bhatt, D.L., Lincoff, A.M., Tcheng, J.E., Kereiakes, D.J., Kleiman, N.S., Jia, G. and Topol, E.J. (2003) Impact of preprocedural white blood cell count on long term mortality after percutaneous coronary interven- tion: Insights from the EPIC, EPILOG, and EPISTENT trials. Heart, 89, 1200-1204. http://dx.doi.org/10.1136/heart.89.10.1200 [4] Ross, R. (1999) Atherosclerosis—An inflammatory dis- ease. New England Journal of Medicine, 340, 115-126. http://dx.doi.org/10.1056/NEJM199901143400207 [5] Libby, P., Ridker, P.M. and Maseri, A. (2002) Inflamma- tion and atherosclerosis. Circulation, 105, 1135-1143. http://dx.doi.org/10.1161/hc0902.104353 [6] Friedman, G.D., Klatsky, A.L. and Siegelaub, A.B. (1974) The leukocyte count as a predictor of myocardial infarc- tion. New England Journal of Medicine, 290, 1275-1278. http://dx.doi.org/10.1056/NEJM197406062902302 [7] Mänttäri, M., Manninen, V., Koskinen, P., Huttunen, J.K., Oksanen, E., Tenkanen, L., Heinonen, O.P. and Frick, M.H. (1992) Leukocytes as a coronary risk factor in a dyslipidemic male population. American Heart Journal, 123, 873-877. http://dx.doi.org/10.1016/0002-8703(92)90690-W [8] Schlant, R.C., Forman, S., Stamler, J. and Canner, P.L. (1982) The natural history of coronary heart disease: Prognostic factors after recovery from myocardial infarc- tion in 2789 men. The 5-year findings of the coronary drug project. Circulation, 66, 401-414. http://dx.doi.org/10.1161/01.CIR.66.2.401 [9] Lowe, G.D., Machado, S.G., Krol, W.F., Barton, B.A. and Forbes, CD. (1985) White blood cell count and hae- matocrit as predictors of coronary recurrence after myo- cardial infarction. Thromb Haemost, 54, 700-703. [10] Sabatine, M.S., Morrow, D.A., Cannon, C.P., Murphy, S.A., Demopoulos, L.A., DiBattiste, P.M., McCabe, C.H., Braunwald, E. and Gibson, C.M. (2002) Relationship between baseline white blood cell count and degree of coronary artery disease and mortality in patients with acute coronary syndromes: A TACTICS-TIMI 18 (treat angina with aggrastat and determine cost of therapy with an invasive or conservative strategy-thrombolysis in myocardial infarction 18 trial) substudy. Journal of the American College of Cardiology, 40, 1761-1768. http://dx.doi.org/10.1016/S0735-1097(02)02484-1 [11] Mueller, C., Neumann, F.J., Perruchoud, A.P. and Buett- ner, H.J. (2003) White blood cell count and long term mortality after non-ST elevation acute coronary syn- drome treated with very early revascularisation. Heart, 89, 389-392. http://dx.doi.org/10.1136/heart.89.4.389 [12] Cannon, C.P., McCabe, C.H., Wilcox, R.G., Bentley, J.H. and Braunwald, E. (2001) Association of white blood cell count with increased mortality in acute myocardial in- farction and unstable angina pectoris. OPUS-TIMI 16 Investigators. American Journal of Cardiology, 87, 636- 639. http://dx.doi.org/10.1016/S0002-9149(00)01444-2 [13] Hajj-Ali, R., Zareba, W., Ezzeddine, R. and Moss, A.J. (2001) Relation of the leukocyte count to recurrent car- diac events in stable patients after acute myocardial in- farction. American Journal of Cardiology, 88, 1221-1224. http://dx.doi.org/10.1016/S0002-9149(01)02080-X [14] Furman, M.I., Becker, R.C., Yarzebski, J., Savegeau, J., Gore, J.M. and Goldberg, R.J. (1996) Effect of elevated leukocyte count on in-hospital mortality following acute myocardial infarction. American Journal of Cardiology, 78, 945-948. http://dx.doi.org/10.1016/S0002-9149(96)00473-0 [15] Barron, H.V., Harr, S.D., Radford, M.J., Wang, Y. and Krumholz, H.M. (2001) The association between white blood cell count and acute myocardial infarction mortal- ity in patients ≥65 years of age: Findings from the coop- erative cardiovascular project. Journal of the American College of Cardiology, 38, 1654-1661. http://dx.doi.org/10.1016/S0735-1097(01)01613-8 [16] Cannon, C.P., McCabe, C.H., Wilcox, R.G., et al. (2001) Association of white blood cell count with increased mortality in acute myocardial infarction and unstable an- gina pectoris. OPUS-TIMI 16 investigators. American Journal of Cardiology, 87, 636-639. http://dx.doi.org/10.1016/S0002-9149(00)01444-2 [17] Furman, M.I., Becker, R.C., Yarzebski, J., Savegeau, J., Gore, J.M. and Goldberg, R.J. (1996) Effect of elevated leukocyte count on in-hospital mortality following acute myocardial infarction. American Journal of Cardiology, 78, 945-948. http://dx.doi.org/10.1016/S0002-9149(96)00473-0 Copyright © 2013 SciRes. OPEN ACCESS  N. Salehi et al. / World Journal of Cardiovascular Diseases 3 (2013) 458-463 463 [18] Kruk, M., Karcz, M., Przyłuski, J., Bekta, P., Kepka, C., Kalińczuk, Ł., Pregowski, J., Kaczmarska, E., Demkow, M., Chmielak, Z., Witkowski, A. and Ruzyłło, W. (2007) White blood cell count adds prognostic information to the thrombolysis in myocardial infarction in risk index in pa- tients following primary percutaneous intervention (ANIN myocardial infarction registry). International Journal of Cardiology, 116, 376-382. http://dx.doi.org/10.1016/j.ijcard.2006.03.061 [19] Pellizzon, G.G., Dixon, S.R., Stone, G.W., Cox, D.A., Mattos, L., Boura, J.A., Grines, L.L., Addala, S., O’Neill, W.W. and Grines, C.L. (2003) Stent PAMI Investigators. Relation of admission white blood cell count to long-term outcomes after primary coronary angioplasty for acute myocardial infarction (The Stent PAMI Trial). American Journal of Cardiology, 91, 729-731. http://dx.doi.org/10.1016/S0002-9149(02)03416-1 [20] Gurm, H., Gupta, R., Lauer, M., et al. (2001) The base- line leucocyte count is a strong and independent predictor of mortality in patients undergoing percutaneous coro- nary artery interventions: Implications of arterial inflame- mation. Circulation, 104, II775. [21] Danesh, J., Collins, R., Appleby, P., et al. (1998) Asso- ciation of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: Meta-ana- lyses of prospective studies. JAMA, 279, 1477-1482. http://dx.doi.org/10.1001/jama.279.18.1477 [22] Danesh, J., Whincup, P., Walker, M., et al. (2000) Low grade inflammation and coronary heart disease: Prospec- tive study and updated meta-analyses. BMJ, 321, 199- 204. http://dx.doi.org/10.1136/bmj.321.7255.199 [23] Yarnell, J.W., Baker, I.A., Sweetnam, P.M., et al. (1991) Fibrinogen, viscosity, and white blood cell count are ma- jor risk factors for ischemic heart disease. The Caerphilly and Speedwell collaborative heart disease studies. Circu- lation, 83, 836-844. http://dx.doi.org/10.1161/01.CIR.83.3.836 [24] de Labry, L.O., Campion, E.W., Glynn, R.J., et al. (1990) White blood cell count as a predictor of mortality: Results over 18 years from the normative aging study. Journal of Clinical Epidemiology, 43, 153-157. http://dx.doi.org/10.1016/0895-4356(90)90178-R [25] Barron, H.V., Cannon, C.P., Murphy, S.A., et al. (2000) Association between white blood cell count, epicardial blood flow, myocardial perfusion, and clinical outcomes in the setting of acute myocardial infarction: A throm- bolysis in myocardial infarction 10 substudy. Circulation, 102, 2329-2334. http://dx.doi.org/10.1161/01.CIR.102.19.2329 [26] Cannon, C.P., McCabe, C.H., Wilcox, R.G., Bentley, J.H. and Braunwald, E. (2001) Association of white blood cell count with increased mortality in acute myocardial in- farction and unstable angina. American Journal of Cardi- ology, 87, 636-639. http://dx.doi.org/10.1016/S0002-9149(00)01444-2 [27] Furmann, M.I., Becker, R.C., Yarzebski, J., Savegeau, J., Gore, J.M. and Golgberg, R.J. (1996) Effect of elevated leukocyte count on in-hospital mortality following acute myocardial infarction. American Journal of Cardiology, 78, 945-948. http://dx.doi.org/10.1016/S0002-9149(96)00473-0 [28] Mehran, R., Dangas, G., Parise, H., Guagliumi, G., Witzen- bichler, B. and Grinfeld, L. (2008) Prognostic impact of the baseline white blood cell count in patients with acute myocardial infarction undergoing primary PCI: Results from the HORIZONS-AMI trial. Circulation, 118, S973. [29] Jurewitz, D., Pessegueiro, A., Zimmer, R., Bhatia, R., Tobis, J. and Lee, M. (2009) Preprocedural white blood cell count as a predictor of death and major adverse car- diac events in patients undergoing percutaneous coronary intervention with drug-eluting stents. Journal of Invasive Cardiology, 21, 202-206. [30] Ndrepepag, G., Brauns, S., Iijimar, R., Keta, D., Byrner, A., Schulz, S., Mehilli, J., Schomig, A. and Kastrati, A. (2009) Total leucocyte count, but not C-reactive protein, predicts 1-year mortality in patients with acute coronary syndromes treated with percutaneous coronary interven- tion. Clinical Science, 116, 651-658. [31] Ferrieres, J., et al. (2004) Endothelial cell markers and risk of coronary heart disease: The prospective epidemi- ological study of myocardial infarction (PRIME) study. Circulation, 109, 1343-1348. http://dx.doi.org/10.1161/01.CIR.0000120705.55512.EC [32] Avramkis, G., Papadimitraki, E., Papakonstandinou, D., Liakou, K., Zidianakis, M., Dermitzakis, A., et al. (2007) Platelets and white cell subpopulations among patients with myocardial infarction and unstable angina. Platelets, 18, 16-23. http://dx.doi.org/10.1080/09537100600800412 [33] Gibson, C.M., Cannon, C.P., Murphy, S.A., Ryan, K.A., Mesley, R., Marble, S.J., McCabe, C.H., Van De Werf, F. and Braunwald, E. (2000) Relationship of TIMI myocar- dial perfusion grade to mortality after administration of thrombolytic drugs. Circulation, 101, 125-130. http://dx.doi.org/10.1161/01.CIR.101.2.125 [34] Kloner, R.A., Ganote, C.E. and Jennings, R.B. (1974) The “no-reflow” phenomenon after temporary coronary occlusion in the dog. Journal of Clinical Investigation, 54, 1496-1508. http://dx.doi.org/10.1172/JCI107898 Copyright © 2013 SciRes. OPEN ACCESS
|