Paper Menu >>
Journal Menu >>
 Vol.3, No.2, 77-81 (2011) Health doi:10.4236/health.2011.32014 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ Adenylate kinase locus 1 genetic polymorphism and type 2 diabetes Fulvia Gloria-Bottini1*, Elena Antonacci2, Anna Neri1, Andrea Magrini1, Egidio Bottini1 1Division of Biopathology of Human Population and Environmental Pathology, Department of Biopathology and Imaging Diagnos- tics, Unive r si ty of Rome Tor Vergata, Rome, Italy; *Corresponding Author: gloria@med.uniroma2.it 2Centre of Diabetology, Local Sanitary Unit, Penne, Italy. Received 9 August 2010; revised 14 February 2011; accepted 18 August 2011 ABSTRACT AK1 catalyzes the reversible reaction ATP+AMP 2ADP thus contributing to the regulation of relative concentration of these important nu- cleotides. Intracellular ATP is a storage of en- ergy for cellular processes, moreover extracel- lular ATP together with ADP, AMP and adeno- sine are critical signalling molecule for sending messages to nearby cells acting on P1 and P2 receptors. AK1 shows a genetic polymorphism and recently our group has shown that the cor- relation between blood glucose and glycated haemoglobin in T2D is dependent on AK1 phe- notype. In the present paper we have carried further studies on the relationship between AK1 phenotypes and T2D. Possible interactions with ABO blood groups and ACP1 polymorphism have also been investigated. We have re-examined the data on 280 subjects with type 2 diabetes from the White population of Penne (Central Italy). 384 consecutive healthy new- borns from the same population have been also studied. A three way contingency table analysis was carried out according to Sokal and Rohlf and other statistical analyses by SPSS pro- grams. T2D patients with AK12-1 phenotype have higher values of blood glucose level and glycated haemoglobin and an increased ten- dency to dyslipidemia and retinopathy. In addi- tion there is an interaction of AK1 with ABO blood group s and with ACP1 polymorphism. The different activity between AK1 phenotypes could influence the relative concentration of ATP, ADP, AMP and adenosine with important effects on metabolic activity thus explaining the associa- tion of AK1 with clinical manifestation of T2D. Keywords: AK; T2D; Glycemia; ATP; Glycated Haemoglobin 1. INTRODUCTION Adenylate kinase (AK) is an ubiquitous enzyme that catalyzes the nucleotide phosphoryl interconversion ATP + AMP 2ADP. The products of this reaction are in- volved in the regulation of many cellular function and relationship. Intracellular ATP is a storage of energy for cellular processes. The energy from extracellular ATP is used for sending messages to nearby cells. At first, extracellular ATP was seen simply as a neuro- transmitter, subsequently was studied the mechanism of interactions between extracellular ATP signalling with other signalling systems outside the cells named ec- toATPases. This large family of AK enzyme removes from ATP, one by one, its phosphates producing adeno- sine diphosphate (ADP), adenosine monophosphate (AMP) and adenosine that have different effect on sev- eral cells by binding themselfes to P2 family (for ADP, AMP) and to P1 (for adenosine) receptors 1]. The family of AK enzymes includes seven genes, AK1-AK7, with each other different functions,molecular weight and kinetic property. The network of these en- zymes are distributed throughout intracellular compart- ments, interstitial space and body fluids to regulate en- ergetic and metabolic signaling circuits and to fasten an efficient economy of cell energy, signal communication and stress response.Mutations in AK1, AK2 or AK7 genes have been found associated with hemolytic anemia, re- ticular dysgenesis and ciliary dyskinesia 2]. Adenylate kinase locus 1 (AK1) belongs to AK family and plays an important role in the synthesis of nucleo- tides requested for many metabolic functions. It is pre- sent in the cytosol of skeletal muscle, brain, and eryth- rocyte. The enzyme is polymorphic and shows three phenotypes with different activity, in the order AK11 > AK12-1 > AK12 corresponding to the presence of two codominant alleles, AK1*1 and AK1*2 at an autosomal  F. Gloria-Bottini et al. / Health 3 (2011) 77-81 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 78 locus on chromosome 9. Rare alleles AK1*3, AK1*4 and AK1*5 have been also referred. AK1 was identified because of its association with a rare genetic disorder causing nonspherocytic hemolytic anemia where a mutation in the AK1 gene was found to reduce the catalytic activity of the enzyme and the re- placement of Arg-128 with Trp 3]. Our group has recently shown that the correlation between blood glucose and glycated haemoglobin in T2D is dependent on AK1 phenotype. In addition, blood glucose is significantly higher in AK12-1 than in AK11 subjects 4]. This prompted us to study in more details the relationship between AK1 and T2D. We have also considered possible interaction s o f AK 1 with ABO blood groups that is linked to AK1 5] and with ACP1 that is associated with clinical manifestation of T2D 6]. 2. MATERIAL AND METHODS We have re-examined the data on 278 subjects with type 2 diabetes from the population of Penne (Italy) and have studied 384 consecutive healthy newborns from the same population. T2D patients have been considered in a previous study 6] and were a random sample of a popu- lation of about 2000 subjects under care at Centre of Diabetology of the local Hospital. Penne is a rural town located in Eastern side of Central Italy. This homoge- nous population are the descendant of an old Italic po- pulation called “Vestini”. The total number of subjects shown in the tables are not always the same due to some random missing value for t he variables considered. The patients have been controlled in the Centre of Diabetology according to a regular schedule. In occasion of the control blood glucose and glycated haemoglobin levels have been measured (more than 9 hours since the last meal). Blood sample for determination of genetic markers were also obtained. Written informed consent was obtained from patients and from mothers of new- borns to participate to this study that was approved by the Institutional Review Board. 2.1. Laboratory Analysis Serum glucose concentration was measured by the automated Roche/Hitachi cobas C501 system based on enzymatic reaction with exochinase. Glycated haemo- globin was determined using the Menarini Diagnostics HA-8160 automated equipment based on inverse ex- change cationic chromatography. AK1 phenotype was determined by starch gel electro- phoresis of haemolysate 7]. Samples were examined at pH7. The insert were made from Whatman, n˚3 filter paper. After electrophoresis the gels were sliced and then covered with a 0.75% agar solution at 45˚C made in 0.1 M tris buffer pH 8 and containing glucose 10 mM, mag- nesium chloride 20 mM, adenosine diphosphate (ADP) 1 mM, nicotinammide adenine dinucleotide phosphate (NA- DP) 0.4 mM, phenazine methosulphate (PMS) 0.012%, tetrazolium salt (MTT) 0.012%, glucose-6-phosphate de- hydrogenase (G6PD) 0.04 units/ml and hexokinase 0.08 units/ml. The agar was allowed to set and then the gel incubated at 37˚C for two hours. At the sites of AK activity ADP is converted into AMP and ATP. The ATP reacts with glucose in the presence of hexokinase to produce ADP and glucose-6-phosphate (G6P), this is oxidized to 6-phosphogluconate by G6PD with concomitant reduction of NADP. The reduced NADP in the presence of PMS causes the reduction of MTT to give a blue-coloured insoluble formazan, which is thus deposited at the sites of AK activity. In Caucasian populations three distinct types of electrophoretic p attern are recognized referred as AK11, AK12-1 and AK12 cor- responding to the presence of two codominant alleles: AK1*1 and AK1*2 at an autosomal locus. ACP1 phenotype has been determined by starch gel electrophoresis on red blood cell haemolysates according to Spencer et al. 8]. The acid phosphatase pattern is revealed by a solution of phenolphthalein diphosphate: The addendum of ammonium solution reveals the area where phenophtalein has been liberated in the areas of gel where ACP1 activity is present. In European popula- tions, the presence of three common alleles *A, *B and *C determines the occurrence of six phenotypes with enzymatic activity increasing in the order: A < AB < B< AC < BC < C. Each of the homozygous A, B, and C phenotypes are composed of two fractions F and S cor- responding to a fast and slow component of electropho- retic pattern. Heterozygous phenotypes have a pattern corresponding to a mixture of homozygous types. 2.2. Statistical Analysis Statistical analyses have been carried out by SPSS pro- gram 9]. Three way contingency table analysis has been performed according to Sokal and Rohlf 10]. 3. RESULTS Clinical and demographic data in subjects with T2D and in healthy newborns are shown in Table 1. In T2D the frequency of AK12-1 is slightly greater in comparison to healthy newborn s but the difference is not statistically significant (see Table 2). The data suggest that Ak1 may not be an important factor primarily in- volved in the susceptibility to type 2 diabetes. The distributions of blood glucose and glycated Hb are shown in Tab le 3. Both parameters show a greater value in AK12-1 than in AK11 but only for bloo d glucose  F. Gloria-Bottini et al. / Health 3 (2011) 77-81 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 7979 Table 1. Clinical and demographic data of the samples studied. MeanS.E. %ProportionTotal n˚ T2D Age (yrs) 66 0.59 280 Age at onset of disease (yrs) 54 0.65 275 Duration of disease (yrs) 11.90.48 269 BMI 29.50.29 277 Male proportion 47% 279 Dyslipidemia 26.8% 276 Healthy Newborns Male proportion 47.6% 424 Birthweight (g) 3348.322.0 417 Gestational age (wks) 39.70.06 411 Table 2. Distribution of AK1 phenotypes in T2D and in healthy newborns. AK11 AK12-1 Total n˚ T2D subjects 93.5% 6.5% 278 Healthy newborns 94.3% 5.7% 384 Chi square test of independence 2 df p 0.054 1 0.816 Table 3. Blood glucose and glycated haemoglobin levels ac- cording to AK1 phenotype in T2D. Blood glucose Glycated Hb Mean S.E. Mean S.E. AK11 133.9 2.3 7.51 0.11 AK12-1 156.9 11.8 8.18 0.39 T-test for differences between means p = 0.015 p = 0.121 the difference is statistically significant. The proportion of subjects with dyslipidemia and retinopathy is greater in AK12-1 than in AK11 but the difference does not reach the level of statistical signifi- cance (see Table 4). In T2D the proportion of AK2-1 phenotype is very high in B blood group and very low in A group . The dif- ference between A and B blood groups is highly signifi- cant (O.R. = 10.173 C.I. 2.151-39.163). No significant association has been observed between ABO and AK1 phenotypes in healthy newborns (see Table 5). Table 6 shows in T2D subjects the distribution of blood glucose and glycated Hb in relation to the joint ABO-AK1 phenotypes. Both parameters in A and O sub- jects show higher values in those carrying the AK12-1 phenotype than in those carrying the AK11 phenotype while in B subjects there is a slight tenden cy to a reduc- tion of both parameters in AK12-1phenotype as com- pared to Ak1 phenotype. Figure 1 displays the relationship between serum glucose level an d ACP1 enzymatic activity in AK11 and AK12-1 subjects with T2D. In AK11 phenotype the con- centration of serum glucose is positively correlated with ACP1 activity while in AK12-1 phenotype the associa- tion follows an opposite pattern. 4. DISCUSSION The present data show that T2 D patients with AK12-1 phenotype have higher values of blood glucose level and glycated haemoglobin and have an increased tendency to dyslipidemia and retinopathy. In addition there is an in- teraction of AK1 with ABO blood groups and ACP1 polymorphism. AK1 belongs to AK family and, at the sites of AK1 ac- tivity, ADP is converted into AMP and ATP. The reaction is reversible thus contributing to the regulation of rela- tive concentration of these important nucleotides 1]. Extracellular ATP plays a physiological role in the maintenance of glucose homeostasis by regulating insu- lin secretion 11]. ATP is able to stimulate the release of pancreatic insulin modulating glucose transport via GLUT1 acting on P2 purinergic ionotropic (P2X) and P2 metabotropic (P2Y) receptors through a mechanism that involves beta-cell metabolism and a rise of intracellular calcium. P2Y receptors are deficient in fibroblasts from T2D patients 12]. Table 4. Prevalence of dyslipidemia and retinopathy in T2D subjects according to AK1 phenotype. Dyslipidemia Retinopathy % of subjects with dyslipidemia Total n˚ % of subjects with retinopathy Total n˚ AK11 26.0% 258 15.5% 258 AK12-1 38.9% 18 27.8% 18 Chi square test of independence 2 df p 2 df p 1.431 1 0.232 1.938 1 0.152  F. Gloria-Bottini et al. / Health 3 (2011) 77-81 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 80 Table 5. Distribution of the joint ABO-AK1 phenotypes i n T2D and in healthy newborns. ABO phenotypes T2D subjects A B AB O Proportion of AK12-1 phenotype 2.7% 21.9% 0.0% 6.2% Total n˚ 112 32 4 130 Chi square test of independence 2 df p A vs B vs AB vs O 15.497 3 0.001 A vs B vs O 15.012 2 0.0005 A vs B 11.378 3 0.0007 O.R. = 10.173(B vs A/AK12-1 vs AK11) 95% C.I. 2.151-39.163 Healthy newborns Proportion of AK12-1 phenotype 6.8% 9.7% 0.0% 4.5% Total n˚ 162 31 12 178 Chi square test of independence 2 df p A vs B vs AB vs O 2.458 3 0.483 A vs B vs O 1.653 2 0.437 A vs B 0.036 1 0.849 O.R.=1.471(B vs A/AK12-1 vs AK1) 95% C.I. 0.027-6.230 Three way contingency table analysis by a log linear model x = ABO (A vs B); y = AK1 (AK11 vs AK12-1); z = sample (newborns vs T2D) G df p xyz interaction 4.177 1 0.042 ODDS ratio analysis (B vs A)/(T2D vs newborns) AK11 phenotype O.R. = 1.236 95%C.I. 0.656-2.330 AK12-1 phenotype O.R. 8.55 95% C.I. 0.99-92.86 An increase in the AMP/ATP ratio activates adenine monophosphate activated protein kinase (AMPK) that regulates glycolysis, glucose uptake, lipid oxidation, fatty acid synthesis, cholesterol synthesis and gluconeogene- sis. 13,14]. The AMPK channelopathies are present in hyperinsulinemia, neonatal diabetes mellitus and are a risk factor for the aetiology of T2D 15,16]. Ta b l e 6 . Blood glucose and glycated Hb in T2D according to the joint ABO-AK1 phenotype. ABO phenotype Blood glucose A B O AK11 Mean 129.5 141.4 135.3 S.E. 3.6 8.2 3.3 AK2-1 Mean 164.7 139.1 169.5 S.E. 34.3 15.2 20.0 Significance of difference between means (t-Student)(p) N.S. N.S. N.S. Glycated Hb AK11 Mean 7.31 8.15 7.55 S.E. 0.19 0.36 0.15 AK12-1 Mean 8.57 7.35 8.75 S.E. 1.15 0.59 0.54 Significance of difference between means (t-Student) (p)N.S. N.S. 0.042 The differences in enzymatic activity between AK1 phenotypes could influence the relative concentration of ATP, ADP, AMP and adenosine influencing metabolic parameters and contributing to explain the association with clinical manifestation in T2D. Further in vestigation in this area could be rewarding. Our research is in line with the recent interest on genetic variants that through the regulation of insulin secretion by -cells could be involved in the pathogenesis and clinical manifestations of type 2 diabetes. 17]. Genetic variability of ABO blood groups substances that are important components of cell membrane struc- ture may influence AK1 ecto-enzyme activity thus ex- plaining the associations reported in Tables 5 and 6. In general high activity of ACP1 is associated with high blood glucose level. Low AK1 activity modifying the ratio among ATP, ADP and AMP may influence the effect of ACP1 activity on blood glucose resulting in lower glucose level in carriers of high ACP1 activity genotypes. 5. CONCLUSION Low adenylate kinase activity associated to AK12-1 phenotype influencing the relative concentration of ATP, 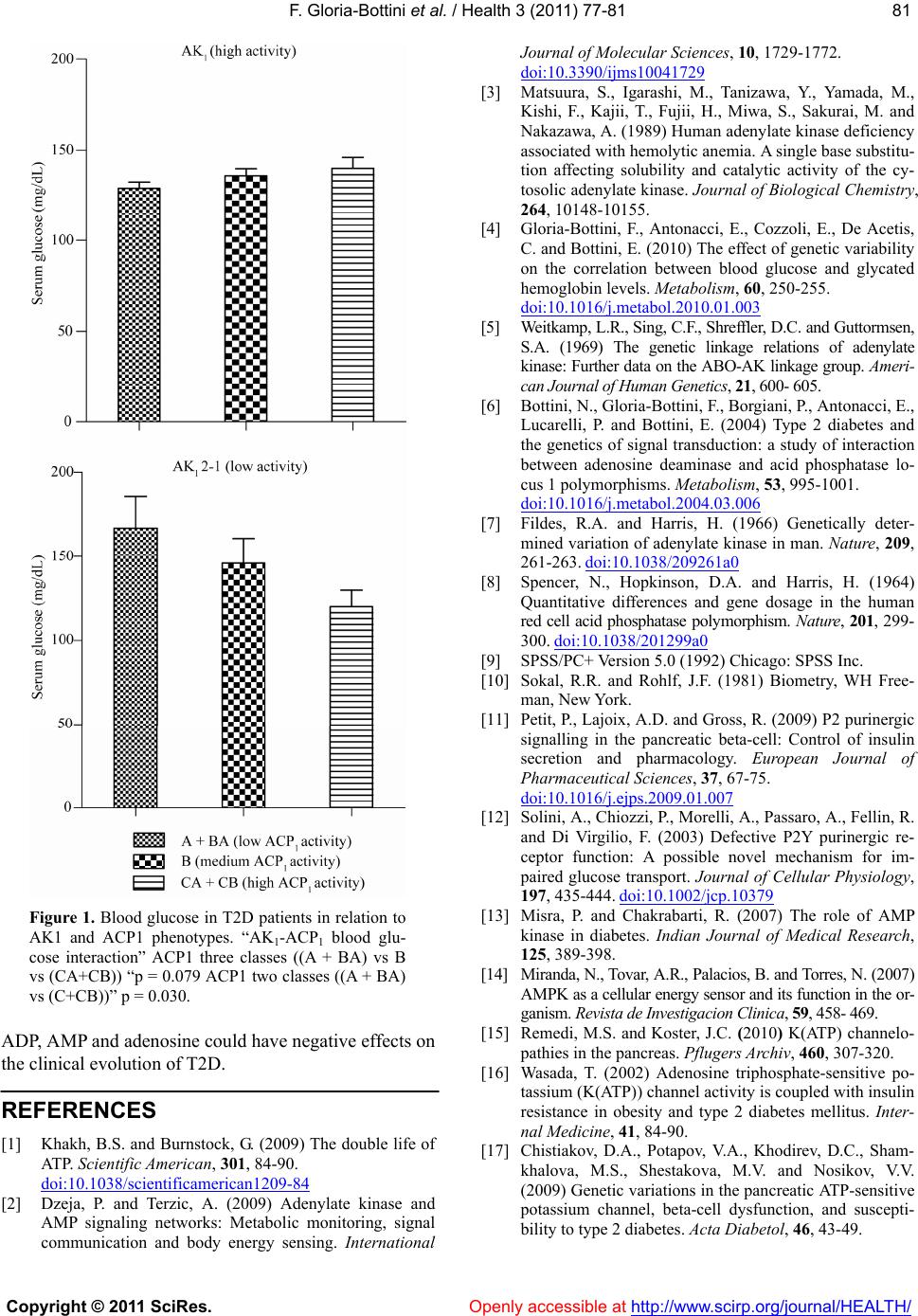 F. Gloria-Bottini et al. / Health 3 (2011) 77-81 Copyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/HEALTH/ 8181 Figure 1. Blood glucose in T2D patients in relation to AK1 and ACP1 phenotypes. “AK1-ACP1 blood glu- cose interaction” ACP1 three classes ((A + BA) vs B vs (CA+CB)) “p = 0.079 ACP1 two classes ((A + BA) vs (C+CB))” p = 0.030. ADP, AMP and adenosine could have negative effects on the clinical evolution of T2D. REFERENCES [1] Khakh, B.S. and Burnstock, G. (2009) The double life of ATP. Scientific American, 301, 84-90. doi:10.1038/scientificamerican1209-84 [2] Dzeja, P. and Terzic, A. (2009) Adenylate kinase and AMP signaling networks: Metabolic monitoring, signal communication and body energy sensing. International Journal of Molecular Sciences, 10, 1729-1772. doi:10.3390/ijms10041729 [3] Matsuura, S., Igarashi, M., Tanizawa, Y., Yamada, M., Kishi, F., Kajii, T., Fujii, H., Miwa, S., Sakurai, M. and Nakazawa, A. (1989) Human adenylate kinase deficiency associated with hemolytic anemia. A single base substitu- tion affecting solubility and catalytic activity of the cy- tosolic adenylate kinase. Journal of Biological Chemistry, 264, 10148-10155. [4] Gloria-Bottini, F., Antonacci, E., Cozzoli, E., De Acetis, C. and Bottini, E. (2010) The effect of genetic variability on the correlation between blood glucose and glycated hemoglobin levels. Metabolism, 60, 250-255. doi:10.1016/j.metabol.2010.01.003 [5] Weitkamp, L.R., Sing, C.F., Shreffler, D.C. and Guttormsen, S.A. (1969) The genetic linkage relations of adenylate kinase : Further data on the ABO-AK link age group. Ameri- can Journal of Human Genetics, 21, 600- 605. [6] Bottini, N., Gloria-Bottini, F., Borgiani, P., Antonacci, E., Lucarelli, P. and Bottini, E. (2004) Type 2 diabetes and the genetics of signal transduction: a study of interaction between adenosine deaminase and acid phosphatase lo- cus 1 polymorphisms. Metabolism, 53, 995-1001. doi:10.1016/j.metabol.2004.03.006 [7] Fildes, R.A. and Harris, H. (1966) Genetically deter- mined variation of adenylate kinase in man. Nature, 209, 261-263. doi:10.1038/209261a0 [8] Spencer, N., Hopkinson, D.A. and Harris, H. (1964) Quantitative differences and gene dosage in the human red cell acid phosphatase polymorphism. Nature, 201, 299- 300. doi:10.1038/201299a0 [9] SPSS/PC+ Version 5.0 (1992) Chicago: SPSS Inc. [10] Sokal, R.R. and Rohlf, J.F. (1981) Biometry, WH Free- man, New York. [11] Petit, P., Lajoix, A.D. and Gross, R. (2009) P2 purinergic signalling in the pancreatic beta-cell: Control of insulin secretion and pharmacology. European Journal of Pharmaceutical Scien ces, 37, 67-75. doi:10.1016/j.ejps.2009.01.007 [12] Solini, A., Chiozzi, P., Morelli, A., Passaro, A., Fellin, R. and Di Virgilio, F. (2003) Defective P2Y purinergic re- ceptor function: A possible novel mechanism for im- paired glucose transport. Journal of Cellular Physiology, 197, 435-444. doi:10.1002/jcp.10379 [13] Misra, P. and Chakrabarti, R. (2007) The role of AMP kinase in diabetes. Indian Journal of Medical Research, 125, 389-398. [14] Miranda, N., Tovar, A.R., Palacios, B. and Torres, N. (2007) AMPK as a cellular energy sensor and its function in the or- ganism. Revista de Investigacion Clinica, 59, 458- 469. [15] Remedi, M.S. and Koster, J.C. (2010) K(ATP) channelo- pathies in the pancreas. Pfl ugers Archiv, 460, 307-320. [16] Wasada, T. (2002) Adenosine triphosphate-sensitive po- tassium (K(ATP)) channel activity is coupled with insulin resistance in obesity and type 2 diabetes mellitus. Inter- nal M edicine, 41, 84-90. [17] Chistiakov, D.A., Potapov, V.A., Khodirev, D.C., Sham- khalova, M.S., Shestakova, M.V. and Nosikov, V.V. (2009) Genetic variations in the pancreatic ATP-sensitive potassium channel, beta-cell dysfunction, and suscepti- bility to type 2 diabetes. Acta Diabetol, 46, 43-49. |

