Paper Menu >>
Journal Menu >>
 Journal of Biomaterials and Nanobiotechnology, 2011, 2, 156-162 doi:10.4236/jbnb.2011.22020 Published Online April 2011 (http://www.scirp.org/journal/jbnb) Copyright © 2011 SciRes. JBNB Synthesis of AgNPs by Bacillus Cereus Bacteria and Their Antimicrobial Potential Anuradha Prakash1, Seema Sharma2*, Naheed Ahmad3, Ashok Ghosh4, Preety Sinha5 1Department of Zoology, A.N. College, Patna, India; 2 Department of Physics, A.N. College, Patna, India; 3 Department of Botany, Patna University, Patna, India; 4 Department of Environment and Water Management, A.N. College, Patna, India; 5 Department of Zoology, A.N. College, Patna, India. Email: seema_sharma26@yahoo.com Received December 26th, 2010; revised January 17th, 2011; accepted January 18th, 2011. ABSTRACT In the present work silver nanoparticles (AgNPs) were synthesized extracellularly by bacteria Bacillus cereus collected from the riverine belt of Gangetic Plain of India. The microbes were isolated, screened and characterized by morpho- logical and biochemical analyses. The silver resistant strain was exposed to different concentrations of silver salt (Ag- NO3). UV-visible spectrum of the supernatant of cell culture showed absorbance peak of AgNPs at ~ 435 nm.The shape and size of AgNPs were ascertained by High Resolution Transmission Electron Micrography (HRTEM), X-ray diffrac- tion (XRD) and Energy Dispersive spectroscopy (EDS). Average size of the synthesized AgNPs was found to be in the range of 10 - 30 nm with spherical shape. AgNPs were tested against antibacterial potential of some common human pathogens. Keywords: AgNPs, XRD, HRTEM, Antimicrobial 1. Introduction Nanotechnology has become the new arena for future technology which is mainly dependent on nanometals and semiconductors. Nanoparticles synthesized by the chemical processes had toxic effect hence there is a growing need to develop environment friendly, cost ef- fective and conveniently reproducible green methods of nanoparticle synthesis. Diversity of microbes is immense and this can be exploited purposefully for synthesis and harvesting of different nanoparticles. A vast range of organic and inorganic materials exist as colloids and na- noparticles in soil, infiltration water and ground water. The cell wall of bacteria is chemically and structurally more complex to the surface of inorganic nanoparticles, and it can adapt itself with the change in the environment. Bioscience can be employed to explore medical sciences in fighting pathogens [1], artificial implants [2], targeted drug delivery [3] etc and understanding the role and ap- plications of microorganisms for the remediation of toxic and radionuclide contaminated sites and antibacte- rial effects. Microorganisms that affect the reactivity and mobility of metals can be used for detoxification and remediation. Many organisms both prokaryotes and eu- karyotes are known to produce many inorganic materials either intracellularly or extracellularly [4]. These specific characteristics of organisms particularly of microorgan- isms can be used in the synthesis of nanoparticles. Metals are present in the environment in trace amount which are used by the organisms for their metabolic activities. Mi- crobes have the capacity to alter their environment by selective interaction with metals. The micro flora of unique ecological niches yields microbial diversity which can be exploited for various ends, including production of nanoparticles. Biosynthesized nanoparticles find utili- zation in the fields of bioremediation, biolabelling, bio- sensors and many more [5]. It is reported that stable sil- ver nanoparticles can be synthesized with controlled size in suitable matrices such as micelles [6], organic small molecules or microbes, [7] linear polymers [8], mesopo- rous materials [9], dendritic polymers [10,11]. The large scale synthesis of silver nanomaterials suffers from is- sues such as polydispersity and stability, especially if the reduction is carried out in aqueous media. Therefore the extracellular biological synthesis of AgNPs can be an attractive and ecologically friendly alternative method for the large quantities because it offers the advantage of easy downstream processing. Moreover, bacteria are easy to handle and can be manipulated genetically without much difficulty. The capability of producing crystalline silver particles through microbes in nanosize, and with  Synthesis of AgNPs by Bacillus Cereus Bacteria and Their Antimicrobial Potential 157 controlled morphology is the basis of using this biologi- cal method in the field of material science. Silver ions promote bone growth and kill surrounding bacteria. They are also reported to be nontoxic to human and most ef- fective against bacteria, viruses and other eukaryotic mi- cro-organisms at very low concentration and without any side effects [12]. As most of the bacteria have developed resistance to antibiotics, there is a need of an alternative antibacterial substance [13]. AgNPs have an important advantage over conventional antibiotics in that it kills all pathogenic microorganisms, and no organism has ever been reported to readily develop resistance to it [14]. The silver nanoparticles synthesized by microbes can be used in target oriented drug delivery, food industry, as an an- tiseptic in waste water treatment, in curing and detecting many diseases, in making electronic devices. In this work, microbes were selected randomly and after several ex- periments one of the strains identified as Bacillus cereus was selected for synthesis of AgNPs and which were found to be fairly dispersed and exhibited effective an- timicrobial effect. 2. Experimental Details 2.1. Culture and Isolation The soil samples were collected from different sites of the wetland area, rich in microfauna of North Bihar of India along the river Ganges. Serial dilutions nutrient agar plates were made and pure culture was isolated after the requisite period of incubation (Figure 1).The identi- fication and characterization of the culture was per- formed on morphological and biochemical basis. One of the most resistant strain Bacillus cereus was selected for further experiment. It was grown in 250 ml MGYP (glu- cose 1%, malt extract 1%, yeast extract 0.3%, peptone 0.5%) medium in 500 ml Erlenmeyer flask. The flasks were incubated at 37.5˚C on a rotary shaker set at 100 rpm for 24 h. 2.2. Synthesis of AgNPs AgNO3 was added to the innoculum and put on shaker for 24 h. One of the set of culture was treated as control for the experiment (without the silver salt). AgNO3 was added in different concentration ranging from 50, 100, 500, 1000, 1500, 20000 ppm respectively. After 24 h, this culture was filtered through Whatman filter paper no. 1 (medium retention, flow rate and porosity which are fre- quently used for clarifying liquids in biological experi- ments) and the cell free supernatant was observed on UV-VIS spectrophotometer with wavelength range of 200-600nm.The absorbance maxima of supernatant was taken at different time intervals after adding AgNO3. The culture was then centrifuged at 5000 rpm for 15 minutes to recover the synthesized nanoparticles in the aliquot and was washed with distilled water 3 - 4 times to avoid any interference of the media in the characterization of the nanoparticles. Then the nanoparticles were allowed to dry and made into fine powder for their characterization through X-ray diffraction, Electron Diffraction Spec- troscopy and Transmission Electron Microscopy. 2.3. Characterization of AgNPs 2.3.1. X- Ray Diffraction Analysis The X-ray Diffraction (XRD) measurements of drop- coated films of AgNPs on glass substrate were recorded in a wide range of Bragg angle 2θ at a scanning rate of 2°min–1 carried out on a Philips PW 1830 instrument that was operated at a voltage of 40 kV and a current of 30 mA with Cu Kα radiation (λ = 1.5405 Å). 2.3.2. TEM and Electron Diffraction Analysis High Resolution Transmission Electron Microscopy (HRTEM) was performed by TECHNAI G20-STWIN (a) (b) Figure 1. (a) Photograph of Bacillus cereus, the bacterial strain (100x magnification); (b) Photograph of Bacillus ce- reus (enlarged section). C opyright © 2010 SciRes. JBNB  Synthesis of AgNPs by Bacillus Cereus Bacteria and Their Antimicrobial Potential 158 (200 KV) machine with a line resolution 2.32 (in ˚A). These images were taken by drop coating AgNPs on a carbon-coated copper grid. Energy Dispersive Absorp- tion Spectroscopy photograph of AgNPs were carried out by the HRTEM equipment as mentioned above. 2.4. Antimicrobial Activity Test for AgNPs After characterization of AgNPs their antibacterial effect was checked by disc diffusion or Kirby-Bauer method [15] on some common pathogens of human, E.coli and Streptococcus. This method is used to determine suscep- tible and resistant values [16]. 3. Results and Discussions 3.1. Synthesis of Silver Nanoparticles The results of this experiment i.e. extracellular synthesis of AgNPs were observed. The colour of the aliquot cha- nged to deep orange after 24 h of agitation with AgNO3 (Figure 2 which further blackened in 72 h. The black colour of the culture confirms the extracellular reduction of silver salt to AgNPs, however the bacterial strain treated with de-ionized water retained its original colour [17]. The absorbance scan taken by UV-VIS spectropho- tometer showed a sharp plasmon peak at ~ 435 nm (Fig- ure not shown here), which confirms silver in nanoscale range. The size, shape and distribution of the nanomate- rial affect the magnitude, peak and width of the spectrum band. As the colour of the culture changed in course of synthesis, its absorbance was recorded after regular in- tervals of time (24 h, 48 h, 72 h, 7days, 15days and 30 days). The suspension was stored for about one month to observe the stability of the synthesized nanocrystallites. The silver nanocrystallites showed the peak at wave- length 430 - 440 nm range. The mechanism for the synthesis of AgNPs by bacteria is not exactly deciphered till date but several possible ways of synthesis is being explained. The possible che- mical reactions in the culture medium may be as follows: C 6H12O6 →CH3-CO-COOH (Glucose) (Pyruvate) NaHCO 3 ↔ Na+ + 3 HCO HCO 3 ↔ OH– + CO2 AgNO 3 +(OH)2 →Ag2O + H2O +N2 Ag(OH) 2→ Ag+↓ + H2O According to this concept, the transmembrane proton gradient effected by respiratory, adenosine trip- hos- phatase, or bacteriorhodopsin activity is used to extrude Na+ from the bacterial cells by means of a Na+ - H+ anti- porter, resulting in the creation of an inwardly directed transmembrane Na+ gradient. The chemical and electrical components of this gradient, either separately or in com- bination, can then be used. Figure 2. Photograph of change in colour of aliquot after addition of AgNO3 during different time period (from left to right): control, 24 hrs, 48 hrs and 72 hrs. to drive the intracellular accumulation of nutrients. The proton gradient can power active transport indirectly, through the formation of sodium ion gradient in micro- organism. In this reaction, Sodium symport (mentioned above) is an important process in cells and is used in sugar and amino acid uptake. ATP binding employ spe- cial substrate binding proteins, which are attached to membrane lipids on the external face of gram positive bacteria (B.cereus). A proton gradient can power active transport indirectly and most of the bacteria which have electrokinetic potential readily attract the cations and this probably acts as an initiator for the biosynthesis of na- noparticles [18]. The mechanism of transformation of oxidase to reduc- tase and vice- versa due to change in pH might have tak- en place at two levels, at cell membrane level, or in the membrane of endoplasmic reticulum (Figure 3). Oxidase gets activated at lower pH whereas reductase gets acti- vated at higher pH in the cell membrane [19,20] which makes the molecular oxygen available for the transfor- mation by the tautomerization of quinones. The molecu- lar oxygen released by both processes is utilized in the conversion of silver to silver oxide. NADH serves as an electron carrier and transfers electron to receptors. The electrons flow from carriers with more negative reduc- tion potential to those with more positive potentials and eventually combine with O2 and H2 to form water. X-ray Diffraction (XRD) pattern (Figure 4) shows in- tense Bragg’s reflections that can be indexed on the basis of the fcc structure of silver [19]. The XRD pattern thus obtained clearly shows [111], [200], [220] planes, and exhibit that the synthesized AgNPs by the Bacillus cer- eus were crystalline in nature. The values agree well with those reported for silver (face centric cubic) by the Joint Committee on Powder Diffraction Standards File No. C opyright © 2011 SciRes. JBNB 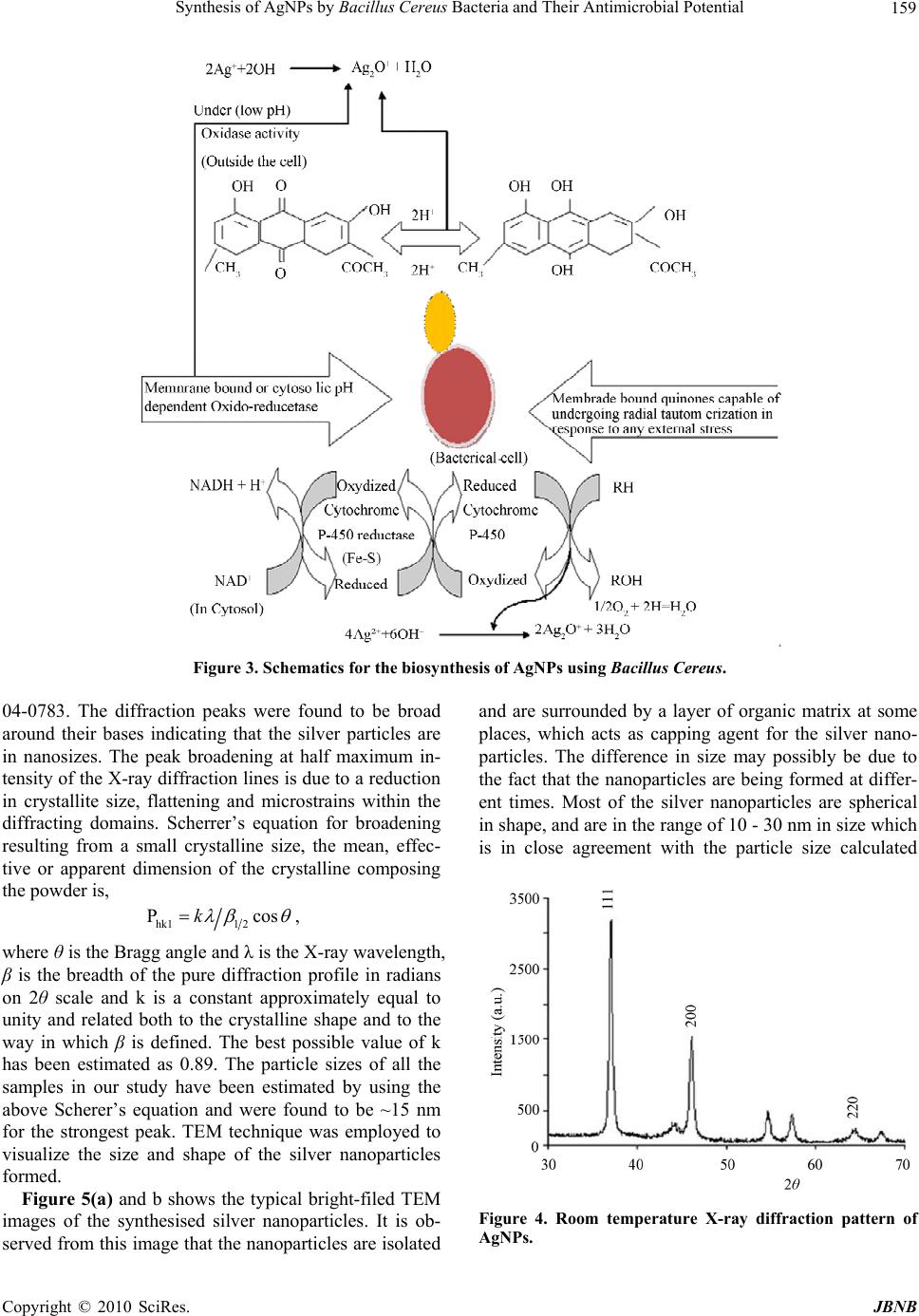 Synthesis of AgNPs by Bacillus Cereus Bacteria and Their Antimicrobial Potential Copyright © 2010 SciRes. JBNB 159 Figure 3. Schematics for the biosynthesis of AgNPs using Bacillus Cereus. 04-0783. The diffraction peaks were found to be broad around their bases indicating that the silver particles are in nanosizes. The peak broadening at half maximum in- tensity of the X-ray diffraction lines is due to a reduction in crystallite size, flattening and microstrains within the diffracting domains. Scherrer’s equation for broadening resulting from a small crystalline size, the mean, effec- tive or apparent dimension of the crystalline composing the powder is, hk11 2 Pckos , where θ is the Bragg angle and λ is the X-ray wavelength, β is the breadth of the pure diffraction profile in radians on 2θ scale and k is a constant approximately equal to unity and related both to the crystalline shape and to the way in which β is defined. The best possible value of k has been estimated as 0.89. The particle sizes of all the samples in our study have been estimated by using the above Scherer’s equation and were found to be ~15 nm for the strongest peak. TEM technique was employed to visualize the size and shape of the silver nanoparticles formed. Figure 5(a) and b shows the typical bright-filed TEM images of the synthesised silver nanoparticles. It is ob- served from this image that the nanoparticles are isolated and are surrounded by a layer of organic matrix at some places, which acts as capping agent for the silver nano- particles. The difference in size may possibly be due to the fact that the nanoparticles are being formed at differ- ent times. Most of the silver nanoparticles are spherical in shape, and are in the range of 10 - 30 nm in size which is in close agreement with the particle size calculated Figure 4. Room temperature X-ray diffraction pattern of AgNPs. 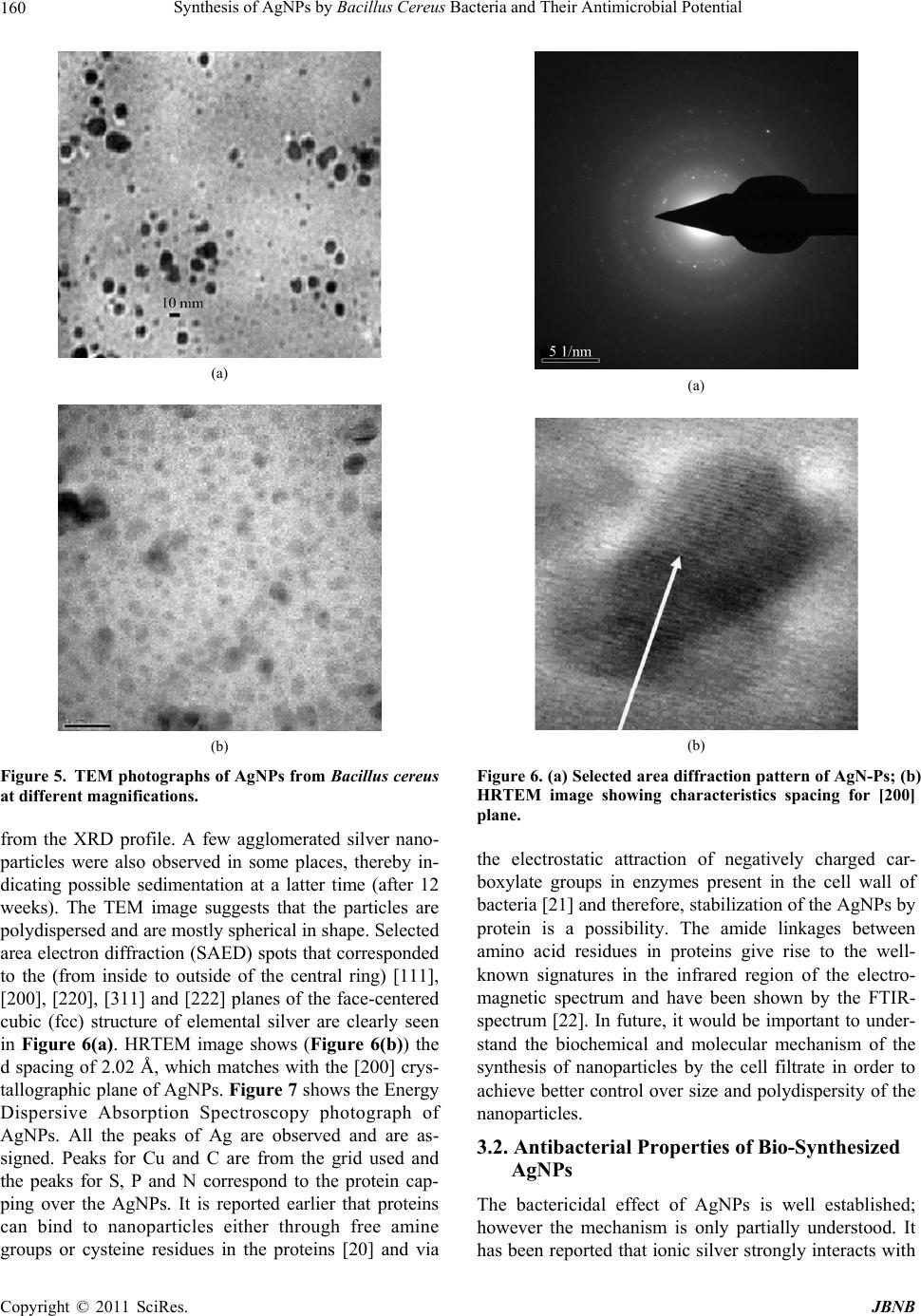 Synthesis of AgNPs by Bacillus Cereus Bacteria and Their Antimicrobial Potential 160 (a) (b) Figure 5. TEM photographs of AgNPs from Bacillus cereus at different magnifications. from the XRD profile. A few agglomerated silver nano- particles were also observed in some places, thereby in- dicating possible sedimentation at a latter time (after 12 weeks). The TEM image suggests that the particles are polydispersed and are mostly spherical in shape. Selected area electron diffraction (SAED) spots that corresponded to the (from inside to outside of the central ring) [111], [200], [220], [311] and [222] planes of the face-centered cubic (fcc) structure of elemental silver are clearly seen in Figure 6(a). HRTEM image shows (Figure 6(b)) the d spacing of 2.02 Å, which matches with the [200] crys- tallographic plane of AgNPs. Figure 7 shows the Energy Dispersive Absorption Spectroscopy photograph of AgNPs. All the peaks of Ag are observed and are as- signed. Peaks for Cu and C are from the grid used and the peaks for S, P and N correspond to the protein cap- ping over the AgNPs. It is reported earlier that proteins can bind to nanoparticles either through free amine groups or cysteine residues in the proteins [20] and via (a) (b) Figure 6. (a) Selected area diffraction pattern of AgN-Ps; (b) HRTEM image showing characteristics spacing for [200] plane. the electrostatic attraction of negatively charged car- boxylate groups in enzymes present in the cell wall of bacteria [21] and therefore, stabilization of the AgNPs by protein is a possibility. The amide linkages between amino acid residues in proteins give rise to the well- known signatures in the infrared region of the electro- magnetic spectrum and have been shown by the FTIR- spectrum [22]. In future, it would be important to under- stand the biochemical and molecular mechanism of the synthesis of nanoparticles by the cell filtrate in order to achieve better control over size and polydispersity of the nanoparticles. 3.2. Antibacterial Properties of Bio-Synthesized AgNPs The bactericidal effect of AgNPs is well established; however the mechanism is only partially understood. It has been reported that ionic silver strongly interacts with C opyright © 2011 SciRes. JBNB 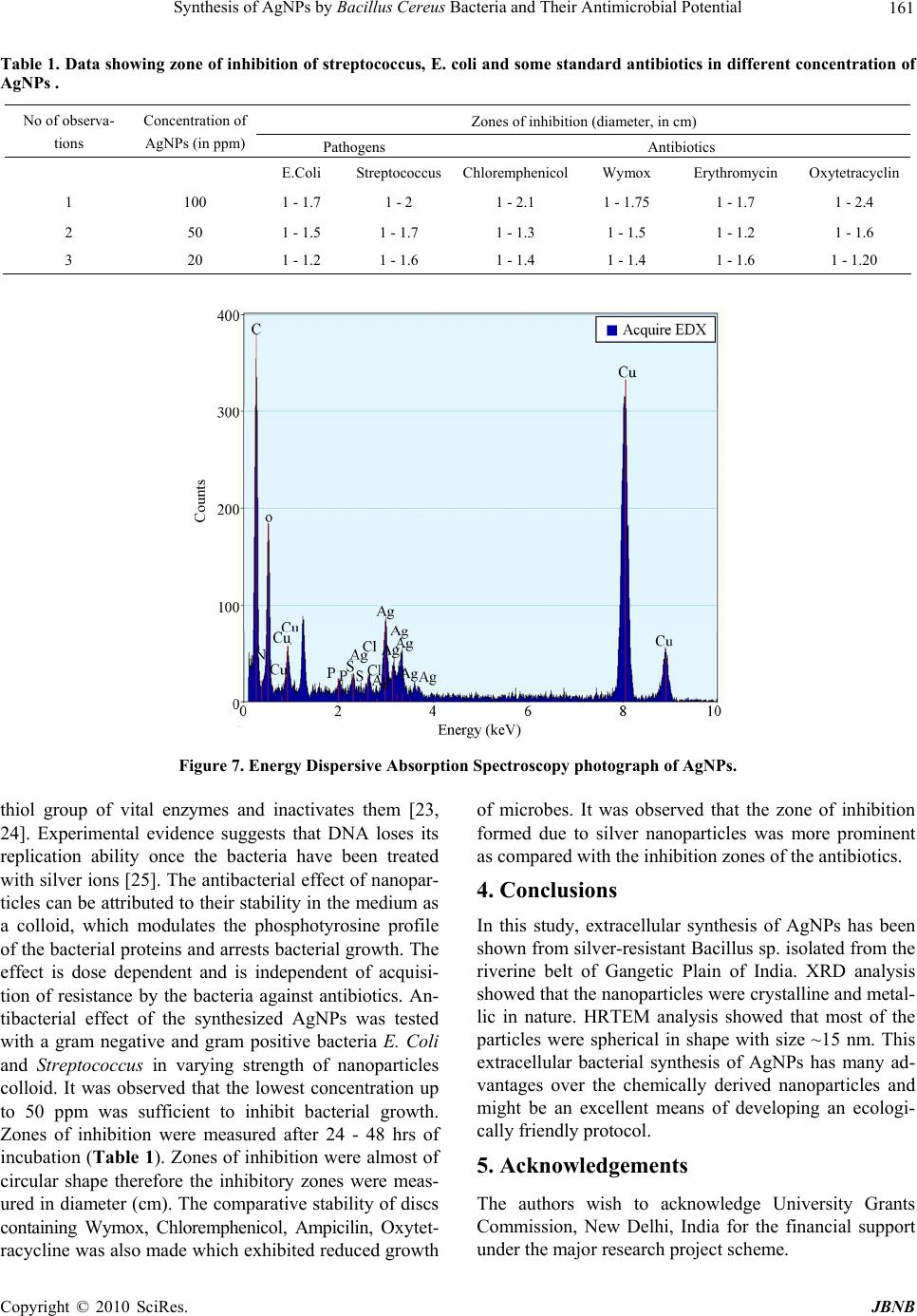 Synthesis of AgNPs by Bacillus Cereus Bacteria and Their Antimicrobial Potential Copyright © 2010 SciRes. JBNB 161 Table 1. Data showing zone of inhibition of streptococcus, E. coli and some standard antibiotics in different concentration of AgNPs . Zones of inhibition (diameter, in cm) No of observa- tions Concentration of AgNPs (in ppm) Pathogens Antibiotics E.Coli StreptococcusChloremphenicolWymox Erythromycin Oxytetracyclin 1 100 1 - 1.7 1 - 2 1 - 2.1 1 - 1.75 1 - 1.7 1 - 2.4 2 50 1 - 1.5 1 - 1.7 1 - 1.3 1 - 1.5 1 - 1.2 1 - 1.6 3 20 1 - 1.2 1 - 1.6 1 - 1.4 1 - 1.4 1 - 1.6 1 - 1.20 Figure 7. Energy Dispersive Absorption Spectroscopy photograph of AgNPs. thiol group of vital enzymes and inactivates them [23, 24]. Experimental evidence suggests that DNA loses its replication ability once the bacteria have been treated with silver ions [25]. The antibacterial effect of nanopar- ticles can be attributed to their stability in the medium as a colloid, which modulates the phosphotyrosine profile of the bacterial proteins and arrests bacterial growth. The effect is dose dependent and is independent of acquisi- tion of resistance by the bacteria against antibiotics. An- tibacterial effect of the synthesized AgNPs was tested with a gram negative and gram positive bacteria E. Coli and Streptococcus in varying strength of nanoparticles colloid. It was observed that the lowest concentration up to 50 ppm was sufficient to inhibit bacterial growth. Zones of inhibition were measured after 24 - 48 hrs of incubation (Table 1). Zones of inhibition were almost of circular shape therefore the inhibitory zones were meas- ured in diameter (cm). The comparative stability of discs containing Wymox, Chloremphenicol, Ampicilin, Oxytet- racycline was also made which exhibited reduced growth of microbes. It was observed that the zone of inhibition formed due to silver nanoparticles was more prominent as compared with the inhibition zones of the antibiotics. 4. Conclusions In this study, extracellular synthesis of AgNPs has been shown from silver-resistant Bacillus sp. isolated from the riverine belt of Gangetic Plain of India. XRD analysis showed that the nanoparticles were crystalline and metal- lic in nature. HRTEM analysis showed that most of the particles were spherical in shape with size ~15 nm. This extracellular bacterial synthesis of AgNPs has many ad- vantages over the chemically derived nanoparticles and might be an excellent means of developing an ecologi- cally friendly protocol. 5. Acknowledgements The authors wish to acknowledge University Grants Commission, New Delhi, India for the financial support under the major research project scheme.  Synthesis of AgNPs by Bacillus Cereus Bacteria and Their Antimicrobial Potential 162 REFERENCES [1] P. K. Stoimenov, R. L. Klinger, G. L. Marchin and K. J. Klabunde, “Metal Oxide Nanoparticles as Bactericidal Agents.” Langmuir, Vol. 18, 2002, pp. 6679-6686. doi:10.1021/la0202374 [2] R. M. Streicher, M. Schmidt, S. Fiorito, “Nanosurfaces and Nanostructures for Artificial Orthopedic Implants,” Nanomedicine, Vol. 2, 2007, pp. 861-874. doi:10.2217/17435889.2.6.861 [3] R. Langer, “Perspective: Drug Delivery-Drug on Target,” Science, Vol. 293, No. 5527, 2001, pp. 58-59. doi:10.1126/science.1063273 [4] A. Ahmad, S. Senapati, R. Kumar and M. Sastry, “Ex- tracellular Biosynthesis of Monodisperse Gold Nanopar- ticles by a Novel Extremophilic Actinomycete, Thermo- monospora sp,” Langmuir, Vol. 19, 2003, pp. 3550-3553. doi:10.1021/la026772l [5] B. Nair and T. Pradeep, “Coalescence of Nanoclusters and the Formation of Submicron Crystallites Assisted by Lactobacillus Strains,” American Chemical Social, Vol. 2, 2002, pp. 293-298. [6] B. L. V. Prasad, S. K. Arumugam, T. Bala and M. Sastry, “Solvent Adaptable Silver Nanoparticles,” Langmuir, Vol. 21, No. 3, 2005, pp. 822-826. doi:10.1021/la047707+ [7] A. Kumar, S. Mandal, P. R. SelvaKannan, R. Pasricha, A. B. Mandale and M. Sastry, “Investigation into the Interac- tion between Surface Bound Alkylamines and Gold Nano- particles,” Langmuir, Vol. 19, No. 15, 2003, pp. 6277-6282. doi:10.1021/la034209c [8] K. A. Bogle, S. D. Dhole and V. N Bhoraskar, “Silver Nanoparticles: Synthesis and Size Control by Electron Ir- radiation,” Nanotech, Vol. 17, 2006, pp. 3204-3208. doi:10.1088/0957-4484/17/13/021 [9] J. C. Lin, W. C Tsai and W.S Lee, “The Improved Elec- trical Contact between a Metal and Porous Silican by Deposition Using a Supercritical Fluid,” Nanotech, Vol. 17, 2006, pp. 2968-2971. doi:10.1088/0957-4484/17/12/024 [10] L. Balogh, D. R Swanson, D. R Tomalia, G. L Hagnauer and A. T. McManus, “Dendrimer-Silver Complexes and Nanocomposites as Antimicrobial Agents,” Nanoletters, Vol. 1, 2001, doi:10.1021/nl005502p [11] X. Shi, T. R. Ganser, K. San, L. P. Balogh and J. R. Baker Jr, “Characterization of Crystalline Dendrimer- Stabilized Gold Nanoparticles,” Nanotechnology, Vol. 17, 2006, pp. 1072-1076. doi:10.1088/0957-4484/17/4/038 [12] C. N. Lok, “Proteomic Analysis of the Mode of Antibac- terial Action of Silver Nanoparticles,” Journal Proteome Research, Vol. 5, 2005, pp. 916-924. doi:10.1021/pr0504079 [13] S. Pal., Y. Tak, and J. M. Song, “Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacte- rium Escherichia Coli,” Applied Environment, Microbi- ology, Vol. 73, 2007, pp. 1712-1720. doi:10.1128/AEM.02218-06 [14] C. T. Dameron, R. N. Reese, R. K. Mehra, A. R. Katari, P. J. Carroll, M. L. Steigerwald, L. E. Brus and D. R., “Winge. Biosynthesis of Cadmium Sulphide Quantum Semicon- ductor Crystallites,” Nature, Vol. 338, 1989, pp. 596-597. doi:10.1038/338596a0 [15] J. M. Boyce, “Reevaluation of the Ability of the Stan- dardized Disk Diffusion Test to Detect Methicillin Resis- tant Strains of Staphylococcus Aureus,” Journal of Clini- cal Microbiology, Vol. 19, 1984, pp. 813-817. [16] W. Lawerence, A. L Barry., R. Otoole and J. Sherris, “Re- liability of the Kirby-Bauer Disc Diffusion Method for Se- lecting Methicilin Resistant Strains of Staphylococcus Aureus,” Applied Micrology, Vol. 24, 1972, pp. 240-247. [17] A. K. Jha, Kamlesh Prasad and K. Prasad, “A Green Low Cost Biosynthesis of Sb2O3 Nanoparticles,” Biochemical English Journal, Vol. 43, No. 3, 2009, pp. 303-306. doi:10.1016/j.bej.2008.10.016 [18] A. Gole, C. Dash, V. Ramachandran, S. R. Sainkar, A. B. Mandale, M. Rao and M. Sastry, “Pepsin-Gold Colloid Conjugates:Preparation, Characterization, and Enzymatic Activity,” Langmuir, Vol. 17, No. 5, 2001, pp. 1674-1679. doi:10.1021/la001164w [19] S. Mandal, S. Phadtare and M. Sastry, “Interfacing Biol- ogy with Nanoparticles,” Current Applied Physics, Vol. 5, No. 2, 2005, pp. 118-127. doi:10.1016/j.cap.2004.06.006 [20] M. Sastry, A. Ahmad, M. IslamKhan and R. Kumar, “Bi- ological Synthesis of Metal Nanoparticles Using Fungi and Actinomycetes,” Current Science, Vol. 85, No. 2, 2003, pp. 162-170. [21] N. Vigneshwaran, N. M. Ashtaputre, P. V. Varadarajan, R. P. Nachane, K. M. Paralikar and R. H. Balasubramanya, “Biological Synthesis of Silver Nanoparticles Using the Fungus Aspergillus Flavus,” Materials Letter, Vol. 61, 2007, pp. 1413-1418. doi:10.1016/j.matlet.2006.07.042 [22] Y. Matsumura, K Yoshikata, S. Kunisaki, and T. Tsuchido, “Mode of Bactericidal Action of Silver Zeolite and Its Comparison with That of Silver Nitrate,” Applied Environ- ment Microbiology, Vol. 69, No. 7, 2003, pp. 4278-4281. [23] A. Gupta, M. Maynes and S. Silver, “Effects of Halides on Plasmid Mediated Silver Resistance in Escherichia Coli,” Applied Environment Micrology, Vol.64, 1998, pp. 5042-5045. [24] Q. L. Feng , J. Wu, G. Q. Chen, F. Z. Cui, T. N. Kim and J. O. Kim, “A Mechanistic Study of the Antibacterial Ef- fect of Silver Ions on Escherichia Coli and Staphylococcus aureus,” Journal of Biomedical Materials Research, Vol. 52, No. 4, 2000, pp. 662-668. doi:10.1002/1097-4636(20001215)52:4<662::AID-JBM10>3. 0.CO;2-3 [25] M. Singh, S. Singh, S. Prasad and I. S. Gambhir, “Nano- Technology in Medicine and Antibacterial Effect of Sil- ver Nanoparticles,” Digest Journal of Nanomaterials and Biostrstructures, Vol. 3, 2008, pp. 115-122. C opyright © 2011 SciRes. JBNB |

