Paper Menu >>
Journal Menu >>
 Engineering, 2012, 5, 172-175 doi:10.4236/eng.2012.410B045 Published Online October 2012 (http://www.SciRP.org/journal/eng) Copyright © 2012 SciRes. ENG The Mechanism of Lipofectamine 2000 Mediated Transmembran e Gene Delivery Shaohui Cui, Shubiao Zhang, Hui ying Chen, Bing Wa ng, Yinan Zhao, Def u Zhi Key Laboratory of Bio-chemistry Engineering - The State Ethnic Affairs Commission-Ministry of Education, Dalian Nationalities University, Dal ian, C hina Email: csh@dlnu.edu.cn Received 2012 ABSTRACT In this paper, the relatived mechanism between lipofectamine 2000 mediated transmembrane gene delivery and endocytic pathway were invest ig ated . Cl ath rin and caveolae-mediated endocytic pathway contributions to transfection efficiency were studied. The inhi- bitors of endocytosis were used to treat HEp-2 cells before lipofectamine 2000/pGFP-N2 transfection. Transfection efficiency was evaluated with green flu ores cence p rotein (GFP) expression assays. Cell viability and cytotoxicity were evaluated with MTT method. The results indicated that inhibitors of clathrin (chlorpromazine or wortmannin) and caveolin (genistein) could reduce the cell trans- fection efficiency o bservabl y. Both clathrin and caveolae-mediated endocytic pathways play important roles in transmembrane gene delivery. Keywords: Gene Delivery; Cationic Liposomes; Transfection Efficienc y; Endocytic Pathwa y; Inhibitor 1. Introduction Non-viral DNA delivery systems have been developed to faci- litate gene entry into mammalian cells[1]. It has shown that non-viral vectors have the advantages over viral ones as they are non-immunogenic, easy to produce and not oncogenic. However, they have the major limitation of inefficien t tran sfec- tion[2]. Indeed, non-viral vectors showing much lower toxicity to cell proliferati on were commerciall y available for li poplexes mediated transfection (lipofection) of cultured cells. However, despite several studies investigating the mechanisms of uptake and intracellular trafficking of non-viral vectors to improve delivery, the current understanding of these processes was still limited. Because cell membrane was the first barrier for lipop- lexes entry into the cell, the mechanism of cationic liposomes mediated transmembrane gene delivery was important as the study could contribute to improving transfection efficiency. There is convincing evidence that endocytosis represents the major pathway of lipoplexes entry into cells that leads to pro- ductive gene expressio n, rather than that genes are tr anslocated into the cytoso l at the level o f the pl asma membrane [3-4]. How- ever, ther e are vario u s endo cytic p ath ways op erated in eukaryo- tic cells, such as clathrin-dependent and clathrin-independent pathways, the latter includes phagocytosis, macropinocytosis and caveolae-mediated internalization[5]. The relative contribu- tion of each pathway in lipoplexes internalization has been poorly defined to date, although involvement of the clath- rin-mediated pathway has been firmly established, while evi- dence was emerging that entry may also occur via macropino- cytosis[4-6]. Endocytotic inhibitors such as chloroquine, chlor- promazine are very helpful for studying drug intracellular re- lease or elucidating a specific endocytosis route[7]. There are still many problems that have not been elucidated on the bio- logical barriers and properties of gene delivery through non- viral vector s, alth ough much effort has been exp ended on them. Understanding the mechanism of transmembrane might play a significant role in overcoming the hurdles in gene delivery. This study was performed with cationic liposome, lipofecta- mine 2000 which showed higher transfection efficiency. The inhibitors of clathrin and caveolin, such as chlorpromazine, wortmannin and genistein were used to treat with cells. Via evaluated inhibitory effects of lipoplexes transfection to explore the underlying transmembrane mech anism in HEp-2 cells. 2. Experimental 2.1. Materials and Equipment Human throat epidermis (HEp-2) cancer cell line was pur- chased from Cell Bank of Chinese Academy of Sciences. RPMI-1640, DMEM, Fetal bovine serum (FBS) were pur- chased from HyClone. Lipofectamine 2000 transfection reagent were purchased from Invitrogen Corporation. Plasmid pGFP-N2 (containing green fluorescent protein gene) was pur- chased from Clontech Company. MTT, Wortmannin, Chlor- promazin e, Genistein were purchased from sig ma–Aldrich. Inverted fluorescence microscope (Olympus IX 71) was purchased from Japan. Microplate reader (Sunrise Tecan) was purchased from Australia. Carbon dioxide incubator (NAPCO 7100) was purchased from France. 2.2. Cell Culture HEp-2 cells were grown in 100 ml culture flask in RPMI-1640 supplemented with 10% fetal bovine and antibiotics (100 U penicillin/ml and 100 mg streptomycin/ml) at 37℃ in 5% CO2 incubator. 2.3. Lipoplexes P reparat ion 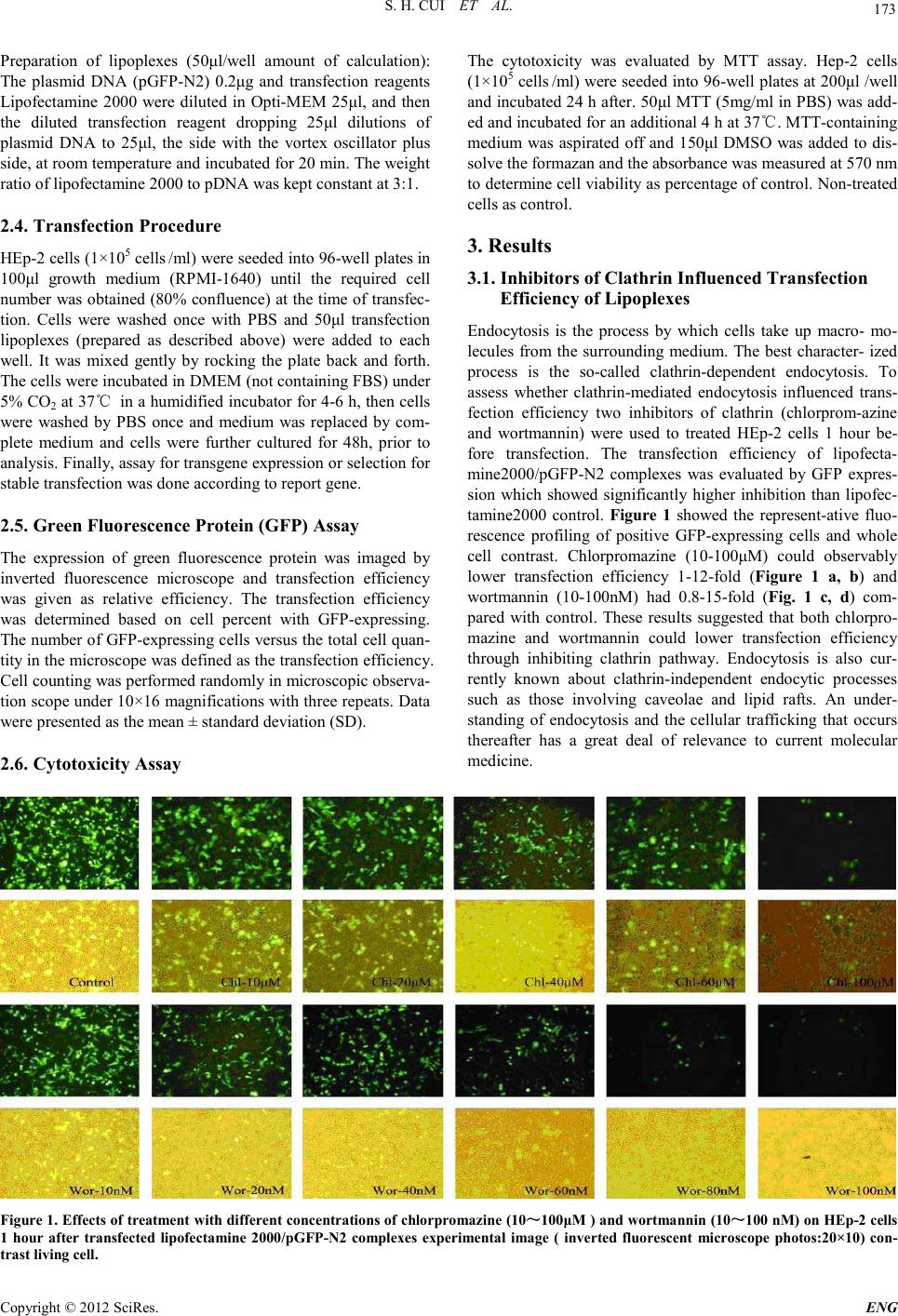 S. H. CUI ET AL. Copyright © 2012 SciRes. E NG 173 Preparation of lipoplexes (50μl/well amount of calculation): The plasmid DNA (pGFP-N2) 0.2μg and transfection reagents Lipofectamine 2000 were diluted in Opti-MEM 25μl, and then the diluted transfection reagent dropping 25μl dilutions of plasmid DNA to 25μl, the side with the vortex oscillator plus side, at room temperature and incubated for 20 min. The weight ratio of lipofectamine 2000 to pDNA was kept constant at 3:1. 2.4. Transfection Procedure HEp-2 cells ( 1×105 cells /ml) were s eeded int o 96-well plates in 100μl growth medium (RPMI-1640) until the required cell number was obtained (80% confluence) at the time of transfec- tion. Cells were washed once with PBS and 50μl transfection lipoplexes (prepared as described above) were added to each well. It was mixed gently by rocking the plate back and forth. The cells were incubated in DMEM (not containing FBS) under 5% CO2 at 37℃ in a humidified incubator for 4-6 h, then cell s were washed by PBS once and medium was replaced by com- plete medium and cells were further cultured for 48h, prior to analysis. Fi nal ly, assay for t ran sgene exp ressio n or selecti on for stable transfection was do ne according to report gene. 2.5. Green Fluorescence Protein (GFP) Assay The expression of green fluorescence protein was imaged by inverted fluorescence microscope and transfection efficiency was given as relative efficiency. The transfection efficiency was determined based on cell percent with GFP-express ing. The number of GFP-expressing cells versus the total cell quan- tity in th e micros cop e was defin ed as th e tran sfectio n efficien cy. Cell counting was performed randomly in microscopic observa- tion scope under 10×16 magnifications with three repeats. Data were presented as the mean ± sta ndard deviatio n ( SD). 2.6. Cytotoxicity Assay The cytotoxicity was evaluated by MTT assay. Hep-2 cells (1×105 cells /ml) were seeded into 96-well plates at 200μl /well and incubated 24 h after. 50μl MTT (5mg/ml in PBS) was add- ed and incubated for an additional 4 h at 37℃. MTT-containing medium was aspirated off and 150μl DMSO was added to dis- solve the formazan and the absorbance was meas ured at 570 nm to determine cell viability as percentage of control. Non-treated cells as contro l. 3. Results 3.1. Inhibitors of Cl athrin In fluenced Tra nsfect i on Efficiency of Lipoplexes Endocytosis is the process by which cells take up macro- mo- lecules from the surrounding medium. The best character- ized process is the so-called clathrin-dependent endocytosis. To assess whether clathrin-mediated endocytosis influenced trans- fection efficiency two inhibitors of clathrin (chlorprom-azine and wortmannin) were used to treated HEp-2 cells 1 hour be- fore transfection. The transfection efficiency of lipofecta- mine2000/pGFP-N2 complexes was evaluated by GFP expres- sion which showed significantly higher inhibition than lipofec- tamine2000 control. Figure 1 showed the represent-ative fluo- rescence profiling of positive GFP-expressing cells and whole cell contrast. Chlorpromazine (10-100μM) could observably lower transfection efficiency 1-12-fold (Figure 1 a, b) and wortmannin (10-100nM) had 0.8-15-fold (Fig. 1 c, d) com- pared with control. These results suggested that both chlorpro- mazine and wortmannin could lower transfection efficiency through inhibiting clathrin pathway. Endocytosis is also cur- rently known about clathrin-independent endocytic processes such as those involving caveolae and lipid rafts. An under- standing of endocytosis and the cellular trafficking that occurs thereafter has a great deal of relevance to current molecular medicine. Fig ure 1 . Effects of treatment with diff erent concentration s of chlorpromazine (10~100μM ) and wortmannin (10~100 nM) o n HE p-2 cells 1 hour after transfected lipofectamine 2000/pGFP-N2 complexes experimental image ( inverted fluorescent microscope photos:20×10) con- trast livin g cell. 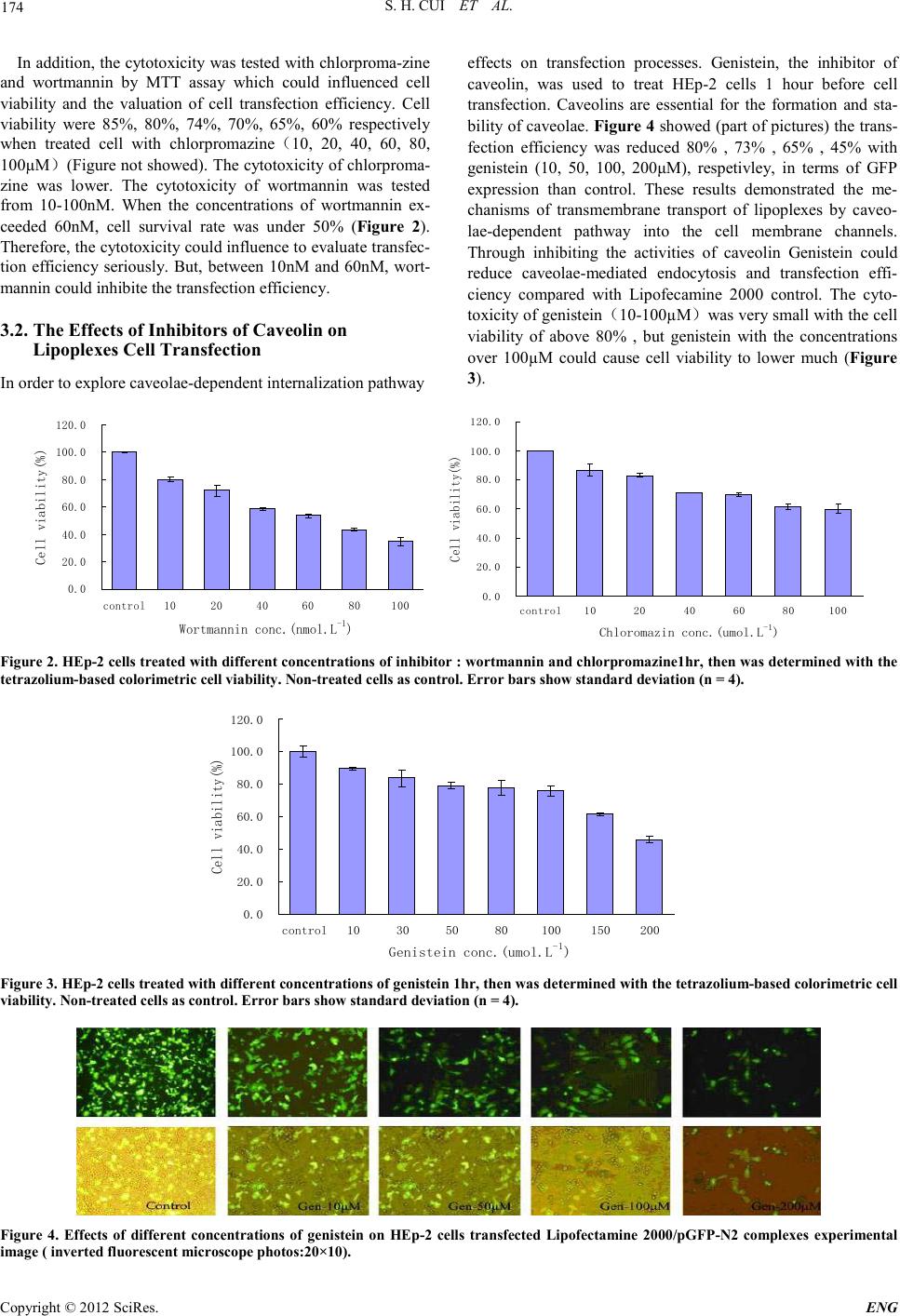 S. H. CUI ET AL. Copyright © 2012 SciRes. ENG 174 In addition, the cytotoxicity was tested with chlorproma-zine and wortmannin by MTT assay which could influenced cell viability and the valuation of cell transfection efficiency. Cell viability were 85%, 80%, 74%, 70%, 65%, 60% respectively when treated cell with chlorpromazine(10, 20, 40, 60, 80, 100µM)(Figure not showed). The cytotoxicity of chlorproma- zine was lower. The cytotoxicity of wortmannin was tested from 10-100nM. When the concentrations of wortmannin ex- ceeded 60nM, cell survival rate was under 50% (Figur e 2). Therefore, the cytot oxicit y could influ ence to eval uate tran sfec- tion efficiency seriously. But, between 10nM and 60nM, wort- mannin could inhibite the transfection efficiency. 3.2. The Effects of Inhibitors of Caveolin on Lipoplexes Cell Transfe c tion In order to explore caveolae-dependent internalization pathway effects on transfection processes. Genistein, the inhibitor of caveolin, was used to treat HEp-2 cells 1 hour before cell transfection. Caveolins are essential for the formation and sta- bili ty of caveolae. Figure 4 showed (part of pictures) the trans- fection efficiency was reduced 80% , 73% , 65% , 45% with genistein (10, 50, 100, 200μM), respetivley, in terms of GFP expression than control. These results demonstrated the me- chanisms of transmembrane transport of lipoplexes by caveo- lae-dependent pathway into the cell membrane channels. Through inhibiting the activities of caveolin Genistein could reduce caveolae-mediated endocytosis and transfection effi- ciency compared with Lipofecamine 2000 control. The cyto- toxicity of genistein(10-100µM)was very small with the cell viability of above 80% , but genistein with the concentrations over 100µM could cause cell viability to lower much (Figure 3). 0.0 20.0 40.0 60.0 80.0 100.0 120.0 control1020 40 60 80100 Wortmannin conc.(nmol.L-1) Cell viability(%) 0.0 20.0 40.0 60.0 80.0 100.0 120.0 control10 20 40 60 80100 Chloromazin conc.(umol.L -1 ) Cell viability(%) Figure 2. HEp-2 cells treated with different concentrations of inhi bitor : wortmannin and chlorpromazine1hr, then was determined with the tetrazolium-based colorimetric cell viability. Non-treated cells as control. Error bars show standard deviation (n = 4). 0.0 20.0 40.0 60.0 80.0 100.0 120.0 control10 30 50 80100150200 Genistein conc.(umol.L -1 ) Cell viability(%) Figure 3. HEp-2 cells treated with different concentrations of genistein 1hr, then was determined with the tetrazolium-based col orimetric cell viability. Non-treated cells as control. Error bars show standard deviation (n = 4). Figure 4. Effects of different concentrations of genistein on HEp-2 cells transfected Lipofectamine 2000/pGFP-N2 complexes experimental image ( inverted fluorescent microscope photos:20×10).  S. H. CUI ET AL. Copyright © 2012 SciRes. E NG 175 4. Discussion Knowledge about the transmembrane uptake mechanism is important for the development of more efficient carriers. Cell membrane was the first barrier for internalization of lipoplexes. Some resul ts indicat ed that endo cytosis was the majo r mechan- ism of entry. Lipoplexes via which endocytic pathways entry into cell is of great importance for the design of further im- proved non-viral gene delivery systems. There are various en- docytic pathways in eukaryotic cells[8-9]. Therefore, the aim of this study was to investigate the endocytic pathways were in- volved in cationic liposome mediated transmembrane gene delivery. A correlation between clathrin-mediated or Caveo- lae-dependent endocytosis and lipoplexes transfection was supported convincingly by several pieces of evidence, including the use of inhibitors of endocytosis, co-localization with path- way-specific markers and defective in clathrin-mediated endo- cytosis. They failed to internalize lipoplexes and concomitantly showed a proportional decrease in transfection efficiency. A combined approach was essential, since the use of inhibitors alone might give rise to unambiguous outcome because o f to xic side effects. Our research focused on plasma membrane endo- cytic pathways to determine the endocytic pathways contri- buted to lipoplexes gene delivery. The results indicated that transfection efficiency was decreased after incubation with inhibitors, both through the clathrin and caveolae-mediated pathways inhibition. However, inhibition of caveolin signifi- cantly reduced the uptake of lipoplexes. It was suggested that the mechanism of cationic liposomes mediated transmembrane gene delivery mainly dependent caveolae-mediated pathway. Taken together, these results demonstrated that uptake of li- poplexes in HEp-2 cells was mediated via both the clath- rin-mediated and caveolae-mediated pathway. It is not definite which is the main. These results are of i mportan ce for success- ful gene transport via clathrin-mediated and caveolae-mediated uptake and rational design of cationic lipid gene delivery sys- tems. 5. Acknowledgements The study was supported by the National Natural Science Foundation of China (21046008 and 21176046) and the Fun- damental Research Funds for the Central Universities (DC120101042). REFERENCES [1] K. A. High. Gene therapy-the moving finger. Nature 435: 577-579, 2005. [2] Y. Zhang, H. M. Li and S. Jing , DC-Chol/DOPE cationic lipo- somes: A comparative study of the influence factors on plasmid pDNA and siRNA gene delivery. International Journal of Phar- maceutics May 10; 390(2):198-207, 2010. [3] J. Rejman, A. Bragonzi and M. Conese, Role of clathrin- and cav eola e med i a t ed en d ocytos i s in g ene t ra n sf er med iat ed by li p o- and polypl exes. M ol Th er 2005; 12:468-74,2005. [4] M. Satyajit and E. P. Richard, Pathways of clathrin-independent endoc ytos is. Nat Rev 8:603-12,2007. [5] K. Sandvig , S. Pust and T. Skotland, Clathrin-independent en- docytosis: mechanisms and function. Curr Opin Cell Bi- ol.Aug;23(4):413-20, 2011. [6] M. T. Howes, S. Mayor and R. G. Parton, Molecules, mechan- isms, and cellular roles of clathrin-independent endocytosis. Curr Opi n Cell Biol 22(4) : 519-27, 2010. [7] B. Emmanuelle, B. Sandrine and G. Lucie, Hepatitis C Virus Entry Depends on Clathrin-Mediated Endocytosis. J Virol. 80(14):6964–6972, July 2006. [8] D. Hoekstra, J. Rejman and L. Wasungu, Gene delivery by ca- tionic lipids: in and out of an endosome.Biochem.Soc Trans. Feb ;3 5( P t 1) :68-71,2007. [9] Y. Obata, G. Ciofani and V. Raffa, Evaluation of cationic lipo- somes composed of an amino acid–based of an endosome. Bio- chemical Society Transactions 35: 68-71,2010. |

