Paper Menu >>
Journal Menu >>
 Journal of Biomaterials and Nanobiotechnology, 2011, 2, 134-143 doi:10.4236/jbnb.2011.22017 Published Online April 2011 (http://www.scirp.org/journal/jbnb) Copyright © 2011 SciRes. JBNB Microcantilever-Based Nanomechanical Studies of the Orphan Nuclear Receptor Pregnane X Receptor-Ligand Interactions Kasey L. Hill, Pampa Dutta, Zhou Long, Michael J. Sepaniak Department of Chemistry, University of Tennessee, Knoxville, USA. Email: msepaniak@utk.edu Received September 10th, 2010; revised January 1st, 2010; accepted January 5th, 2011. ABSTRACT Human pregnane X receptor (PXR) is of vital importance in pharmaceutical and exogenous compound metabolism within the body. This prov ides strong motivation for investiga ting this orphan recep tor’s activation by various pharma- ceuticals, xenobiotics, and endocrine disrupting chemicals (EDCs). A nanomechanical transducer is developed to study xenobiotic and EDC interaction s with the bioreceptor PXR’s ligand binding doma in (LBD). The combina tion of immo- bilized LBD PXR with a nanostructured microcantilever (MC) platform allows for the sensitive, label-free study of li- gand interaction with the receptor. PXR shows real-time, reversible responses when exposed to a specific pharmaceu- tical, EDCs, and xenobiotic ligands. Three EDCs binding interactions are tested, which include phthalic acid, nonyl- phenol, and bisphenol A, with PXR. PXR LBD was exposed to rifampicin, a potent PXR activator, with various injection and recovery times to study their interaction. A two protein array of PXR and estrogen receptor (ER- ) directly compares the nanomechanical responses of these receptors with rifampicin on a single platform. Keywords: Microcantilevers, Nanobiosensing, Pregnane X Receptor, Estrogen Receptor, Endocrine Disrup ting Chemicals, Bioarray 1. Introduction Humans are exposed to harmful chemicals and contami- nants each day. It is essential that these toxins are re- moved or detoxed from the body. These foreign com- pounds or xenobiotics, which include environmental toxins, endogenous hormones, steroids, pharmaceuticals, and dietary supplements, trigger a line of defense me- chanisms within the body. The family of cytochrome P450 enzymes are the main xenobiotic defenders for mammals. These enzymes include four families of Cytochrome P450 monooxygenases: CYP1, CYP2, CYP3, and CYP4 [1]. Cytochrome P4503A4 (CYP3A4), a critical member of our defense system, makes the re- moval of many unwanted xenobiotics possible [2]. CYP3A4 and its isoforms are highly involved in phar- maceutical metabolism, playing a significant role in me- tabolizing approximately 50% of drugs used today [3,4]. When xenobiotic ligands bind to a specific nuclear hor- mone receptor, the interaction transcriptionally activates CYP3A4 [5]. In 1998, a novel orphan human nuclear receptor was identified and termed pregnane X receptor (PXR), steroid and xenobiotic receptor (SXR), pregnane activated receptor (PAR), and NR1I2 [6-9]. Herein, we choose to use the term PXR. PXR is activated by a broad array of structurally di- verse xenobiotics [10]. This wide activation range is pos- sible is due to a unique ligand binding domain (LBD) or pocket, which can expand to fit a variety of sized ligands. PXR’s LBD has two strands that are not present in other nuclear receptors and that allow its expansion [11]. This flexible, hydrophobic LBD allows PXR to be acti- vated by a diverse range of synthetic and naturally occur- ring chemicals making it an interesting candidate to serve as a xenobiotic sensor [12]. Importantly, PXR is activated by numerous pharma- ceuticals with diverse properties, functions, and struc- tures and controls the expression of genes that are vital to pharmaceutical metaolism [13,14]. PXR activation medi- ates transcription of CYP enzymes, which are considered drug metabolizing enzymes [1,12]. Since PXR activation allows for regulation and expression of CYP3A this in- teraction is critical to drug metabolism [2]. Pharmaceuti- cal metabolism and interaction is vital to monitor and  Microcantilever-Based Nanomechanical Studies of the Orphan Nuclear Receptor Pregnane X 135 Receptor-Ligand Interactions prevent drug-drug interactions. Drug-drug interactions can occur when co-administered drugs alter the efficacy of one another. This usually occurs when one drug in- creases or decreases the metabolism of another [3,14]. Contaminants could modify drug concentrations in vivo and skew the prescribed therapeutic dose. High throughput, sensitive screening and detection of xenobiotics are critical to determine harmful toxins and possible drug-drug interactions. A highly sensitive, in- expensive, and rapid sensor utilizing the nuclear receptor PXR has been developed and is demonstrated within. Recently, biosensors based on microcantilevers (MCs) have been used to detect and screen for many harmful environmental contaminants using estrogen and thyroid receptor proteins [16,17]. The high sensitivity and wide- spread availability of inexpensive MCs has generated intense interest in their use as chemical and biological sensors [18-27]. Additionally, MCs can be used with on-chip circuitry and in microcantilever arrays for high throughput and simultaneous differential assays and bio- affinity studies with a very small transducer foo tprint th at potentially could be employed in the field. A MC suitable for biosensing is modified on one side with a nanostructured layer and a receptor phase that has some degree of affinity for the analyte. By exploiting PXR’s affinity for a diverse range of ligands, we are able to screen for xenobiotics quickly and without extensive, time-consuming labeling techniques and cell preparation [10,14,15,28,29]. Specific interactions of the target ana- lytes with the receptor can cause an apparent surface stress and nanomechanical bending that may be conven- iently monitored based on the beam bending technique commonly used in atomic force microscopy. The static bending (tip deflection, zmax) of the MC varies in selec- tivity and sensitivity due to preferential binding of ana- lyte molecules on the functionalized, active MC surface and is governed by Sto ney ’s equation [30] 2 max 2 3(1 ) Et lv z (1) where v and E are, respectively, the Poisson ratio and Young’s modulus for the cantilever, t is the thickness of the MC, l is the cantilever effective length, and is analyte-induced differential surface stress (active side - passive side). We demonstrate that detection and screening for phar- maceuticals and endocrine disrupting chemicals (EDCs) can be accomplished with functionalized MCs. These sensors provide real-time measurements of surface stress changes resulting from ligand interaction with immobi- lized proteins in the low-to-sub-nanomolar range [20]. Sensitivity is critical in biosensors due to the ultra-trace concentrations of many xenobiotics that can impact bio- logical systems. Nanostructured MC biosensors allow detection without labels and in a detection range that is applicable for real biological systems [31]. PXR immobi- lized on a nanostructured MC surface provides sensitive and reversible detection of various pharmaceuticals and environmental contaminants or EDCs. To our knowledge, this is the first time the orphan nuclear receptor PXR has been immobilized on a MC surface. Due to PXR’s u niq ue nature we saw interesting results in our concentration studies with rifampicin as well as surprising responses when utilizing differently tagged PXR-receptors. 2. Materials and Methods 2.1. Reagents Experiments were performed using commercially avail- able silicon arrays of MCs having dimensions 400µm length, 100µm wid th, and approximately 1µm thick (Mi- kro Masch Co., Sunnyvale, CA). Chromium, gold, and silver metals deposited on the MCs were obtained from Kurt J. Lesker, Gatewest, and Alfa Aesar Co., respec- tively, at 99.9% purity. 2-aminoethanethiolhydrochloride (AET), glutaraldehyde (GA), the salts employed for the preparation of buffer solutions, and all other reagents were purchased from Sigma-Aldrich Chemical Co.(St. Louis, MO) or Fisher Scientific at highest available pu- rity and used as received. The test ligands rifampicin, pregnenolone-16-carbonitrile (PCN), 3-methyl-cholanthr- ene (3-MC), phthalic acid, nonylphenol and bisphenol A were also obtained from Sigma-Aldrich. Glutathione- s-transferase (GST)-tagged human PXR, alexa fluor 633 anti-IgG, and estrogen receptor (ER-) were purchased from Invitrogen (Carlsbad, California). 6-histidine (6- HIS)-tagged human PXR was generously provided by Astra Zeneca. Ovalbumin and FITC-anti-IgG was pur- chased from Sigma-Aldrich. Water used to prepare solu- tions was obtained from a Branstead E-pure water filtra- tion system. 2.2. Cantilever Modification The process of preparing and creating nanostructured surfaces on MCs is described in detail elsewhere [32]. The MCs were placed into a physical vapor deposition chamber (Cooke Vacuum Products, Model CVE 30 1, S outh Norwalk, CT) to be coated on on e side with the appropriate metallic films using thermal deposition. To create a nanostructured MC, a thin film (~ 5 n m) of c hr omiu m was app lied to th e surface to act as an adhesion layer followed by a thin film of gold (~1 5 nm). N ex t, a f il m c ons i st in g of g ol d an d si l ve r wa s co-depo-sited. Subsequently, the silver was chemically C opyright © 2010 SciRes. JBNB  Microcantilever-Based Nanomechanical Studies of the Orphan Nuclear Receptor Pregnane X 136 Receptor-Ligand Interactions removed via oxidation from the film (“dealloying”) using an aqueous solution of 5 mg/mL HAuCl4 leaving a gold surface with nanosized, colloid-like features. The thick-ness of the dealloyed gold layer was ~100 or ~150 nm in these studies. Nanostructured MCs were chemically modified for protein immobilization by the process detailed elsewhere [17]. MCs were immersed in 1 mM aqueous solution of AET for on e hour producing a self -assembled monolayer on the cantilever surface. Following thorough rinsing in deionized water, the amino groups were derivatized with the cross linker by immersing the cantilever in a 1% (w/v) solution of GA in 10 mM phosphate buffered saline (PBS), pH 8.0 for three hours [33,34]. Subsequently, immobilization of both the PXR LBD nuclear receptor and the ovalbumin was achieved in random orientation by dipping the functionalized cantilevers into 100 mg/L solutions of protein in 10 mM PBS, pH 7.0 for four hours at 4℃. Although we used an array of MCs, in this study we chemically treated all the cantilevers the same where both PXR and ovalbumin were separately immobilized on the functionalized surfaces of different cantilevers from separate arrays and a single randomly chosen MC within an array response was recorded. For the two pro- tein array, the immobilization of both the PXR and the ER- was achieved in random orientation utilizing a ca- pillary coating method described in detail elsewhere [35]. Each capillary contained 100 mg/L solution of receptor in 10 mM PBS, pH 7.0, which was placed over each lev- er for 1 hour at room temperature. 2.3. Instrumentation The MC deflection measurements were carried out using the optical beam-deflection technique described else- where [16,17]. The apparatus similar to that depicted in Figure 1 included a 5 mW 632 nm diode laser (Coherent Laser Corp., Auburn, CA), a spatial filtering and focus- ing system, and an in-house built position sensitive opti- cal detector. The output of the detector was displayed and recorded using a SRS 850 DSP lock-in amplifier as a multichannel digital recorder (Stanford Research Sys- tems, Sunnyvale, CA). The signal output is recorded as volts (approximately 1 nm zmax per mV output). Data was collected at 1 Hz and then a moving averaging algorithm covering 180 data points was used to generate the figures presented herein. This smoothing did not alter the shape of the true response curves [19]. The cantilever system was mounted inside a ~5µL vo- lume flow cell built in house previously described else- where [16]. A Watec CCD camera (Edmund Indust- rial Optics, Barrington, NJ) was used to image the MC chip in the flow cell and facilitate in aligning the focused laser Figure 1. Schematic of laser array setup with a single PSD. Chip array within flow cell is shown. The lower left shows bending of a single MC due to adsorption-induced differen- tial stress. Note that deformation of the MC is characterized by the radius of curvature, R, and tip deflection, z, ac- cording to Equation (1). beam to reflect off the cantilever tip. Analyte solutions were delivered to the flow cell via a system of vessels connected to three-way valves allowing for switching between diffe r e nt s ol uti o ns. The gravity-dri ven fl ow was generally adjusted to 100 L/minute by adjusting vessel height. Many of the pharmaceuticals and EDCs are sparingly soluble in water. Thus, 10 mM stock solutions of all EDCs and some pharmaceuticals were prepared in pure methanol and then diluted with 10 mM PBS, pH 7.0 to make the desired concentration of each analyte [ Ca u t i o n : because of their potential harmful effects, care must be taken in the handling and disposing of EDC and pharmaceutical solutions]. PCN pharmaceutical stock solution was prepar ed in acetone then dil uted with PBS. PBS was also used as a background solution. MCs mounted in the flow cell were initially allowed to equilibrate in PBS until the signal was stable. For our purposes, tensile and compressive responses involve contraction and expansion of the active MC surface, respectively. MC deflection measurements of single protein func- tionalized chips using a single diode laser or multiple protein arrays can be monitored using an array of vertical cavity surface emitting lasers (VCSELs) which are fo- cused onto the tip of each MC (Figure 1). The reflected beam is captured and monitored by a single position sen- sitive detector as depicted in the figure. Although in the work reported herein employed a single laser setup, we often use a VCSEL setup [36-38]. 3. Results and Discussion Our studies focus on developing MC systems utilizing the nuclear receptor PXR as the immobilized bio receptor C opyright © 2011 SciRes. JBNB 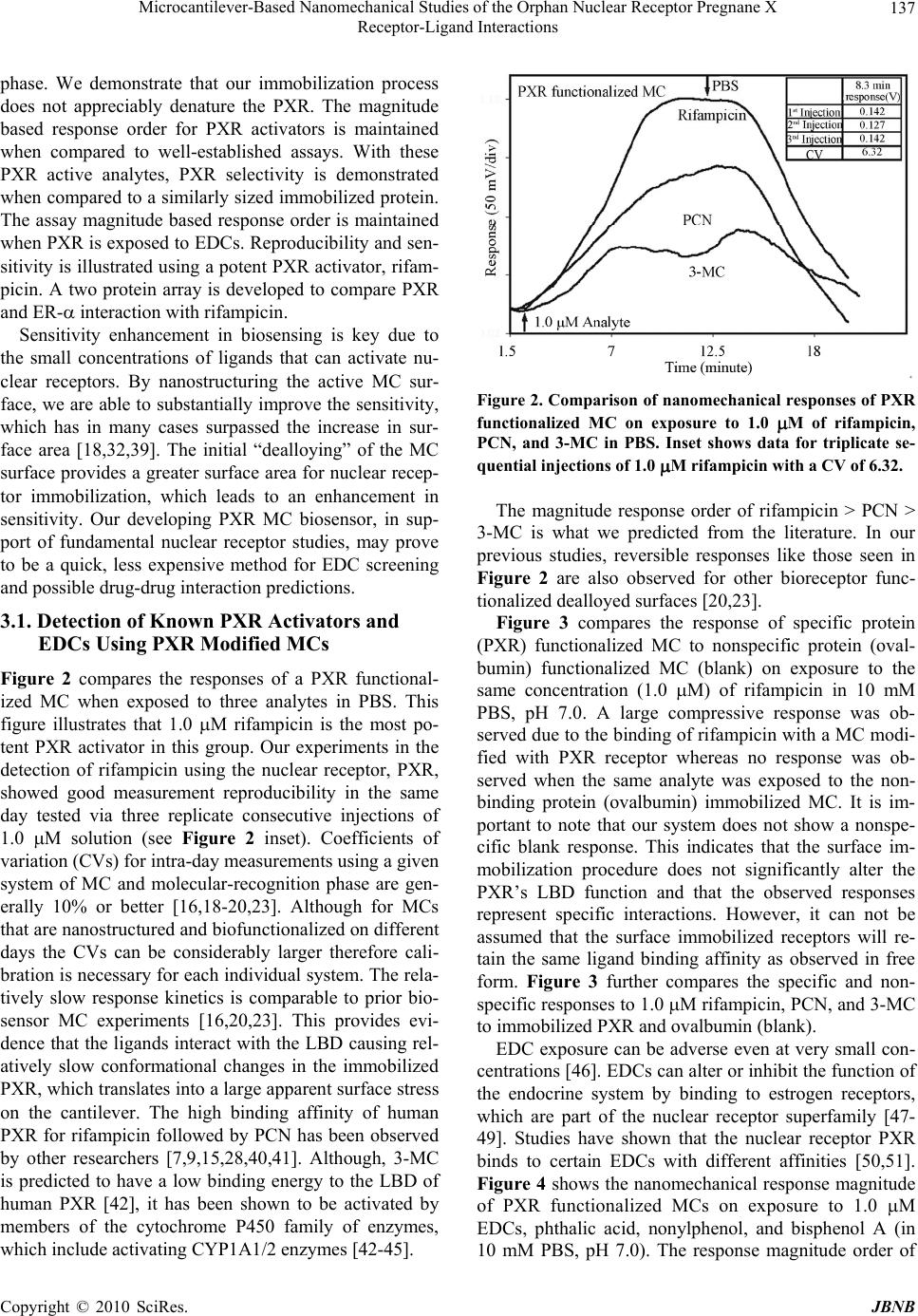 Microcantilever-Based Nanomechanical Studies of the Orphan Nuclear Receptor Pregnane X 137 Receptor-Ligand Interactions phase. We demonstrate that our immobilization process does not appreciably denature the PXR. The magnitude based response order for PXR activators is maintained when compared to well-established assays. With these PXR active analytes, PXR selectivity is demonstrated when compared to a similarly sized immobilized protein. The assay magnitude based response order is maintained when PXR is exposed to EDCs. Reproducibility and sen- sitivity is illustrated using a potent PXR activ ator, rifam- picin. A two protein array is developed to compare PXR and ER- interaction with rifampicin. Sensitivity enhancement in biosensing is key due to the small concentrations of ligands that can activate nu- clear receptors. By nanostructuring the active MC sur- face, we are able to substantially improve the sensitivity, which has in many cases surpassed the increase in sur- face area [18,32,39]. The initial “dealloying” of the MC surface provides a greater surface area for nuclear recep- tor immobilization, which leads to an enhancement in sensitivity. Our developing PXR MC biosensor, in sup- port of fundamental nuclear receptor studies, may prove to be a quick, less expensive method for EDC screening and possible drug-drug interaction predictions. 3.1. Detection of Known PXR Activators and EDCs Using PXR Modified MCs Figure 2 compares the responses of a PXR functional- ized MC when exposed to three analytes in PBS. This figure illustrates that 1.0 M rifampicin is the most po- tent PXR activator in this group. Our experiments in the detection of rifampicin using the nuclear receptor, PXR, showed good measurement reproducibility in the same day tested via three replicate consecutive injections of 1.0 M solution (see Figure 2 inset). Coefficients of variation (CVs) for intra-day measurements using a given system of MC and molecular-recognition phase are gen- erally 10% or better [16,18-20,23]. Although for MCs that are nanostructured and biofunctionalized on different days the CVs can be considerably larger therefore cali- bration is necessary for each individual system. The rela- tively slow response kinetics is comparable to prior bio- sensor MC experiments [16,20,23]. This provides evi- dence that the ligands interact with the LBD causing rel- atively slow conformational changes in the immobilized PXR, which translates into a large apparent surface stress on the cantilever. The high binding affinity of human PXR for rifampicin followed by PCN has been observed by other researchers [7,9,15,28,40,41]. Although, 3-MC is predicted to have a low binding energy to the LBD of human PXR [42], it has been shown to be activated by members of the cytochrome P450 family of enzymes, which include activating CYP1A1/2 enzymes [42-45]. Figure 2. Comparison of nanomechanical responses of PXR functionalized MC on exposure to 1.0 M of rifampicin, PCN, and 3-MC in PBS. Inset shows data for triplicate se- quential injections of 1.0 M rifampicin wi th a CV of 6.32. The magnitude response order of rifampicin > PCN > 3-MC is what we predicted from the literature. In our previous studies, reversible responses like those seen in Figure 2 are also observed for other bioreceptor func- tionalized dealloyed surfaces [20,23]. Figure 3 compares the response of specific protein (PXR) functionalized MC to nonspecific protein (oval- bumin) functionalized MC (blank) on exposure to the same concentration (1.0 M) of rifampicin in 10 mM PBS, pH 7.0. A large compressive response was ob- served due to the binding of rifampicin with a MC modi- fied with PXR receptor whereas no response was ob- served when the same analyte was exposed to the non- binding protein (ovalbumin) immobilized MC. It is im- portant to note that our system does not show a nonspe- cific blank response. This indicates that the surface im- mobilization procedure does not significantly alter the PXR’s LBD function and that the observed responses represent specific interactions. However, it can not be assumed that the surface immobilized receptors will re- tain the same ligand binding affinity as observed in free form. Figure 3 further compares the specific and non- specific responses to 1.0 M rifampicin, PCN, and 3-MC to immobilized PXR and ovalb umin (blank). EDC exposure can be adverse even at very small con- centrations [46]. EDCs can alter or inhibit the function of the endocrine system by binding to estrogen receptors, which are part of the nuclear receptor superfamily [47- 49]. Studies have shown that the nuclear receptor PXR binds to certain EDCs with different affinities [50,51]. Figure 4 shows the nanomechanical response magnitude of PXR functionalized MCs on exposure to 1.0 M EDCs, phthalic acid, nonylphenol, and bisphenol A (in 10 mM PBS, pH 7.0). The response magnitude order of C opyright © 2010 SciRes. JBNB  Microcantilever-Based Nanomechanical Studies of the Orphan Nuclear Receptor Pregnane X 138 Receptor-Ligand Interactions Figure 3. Table compares the nanomechanical responses of PXR and ovalbumin functionalized MCs exposed to 1.0 M rifampicin, PCN, and 3-MC in PBS. Representative traces of ovalbumin and PXR functionalized MCs on exposure to 1.0 M rifampicin are shown. PXR with these three EDCs is similar to prior work with phthalic acid > nonylphenol and with relatively no re- sponse or a very low response to bisphenol A [50]. After MC exposure to EDCs, we injected 1.0 M rifampicin, a potent PXR activator, for comparison. As predicted, the Figure 4. Comparison of response magnitudes in 8.5 min- utes of PXR functionalized MC on exposure to 1.0 M ana- lytes in PBS, which include three EDCs. The inset shows representative responses of PXR functionalized MC ex- posed to 1.0 M nonylphenol and rifampicin. rifampicin caused a large compressive response when compared to the EDCs (see inset). 3.2. Calibration and Sequential Exposure Studies In Figure 5 the kinetic response of the PXR functional- ized cantilever at 7 minutes of expo sure is plotted ag ainst different concentrations of rifampicin in the range of 0.1 nM to 1 M where the response increases with increas- ing concentration. The response magnitude does not in- crease as significantly as predicted with increasing con- centration at relatively high concentrations. This may be due in part to the structural flexibility of the LBD. The expansion of the LBD size to accommodate for rifam- picin’s large size could have an effect on the interaction’s translation to MC surface stress. Rifampicin is one of the largest known ligands for PXR at 823 Da [14]. As illus- trated in the figure, the response increased predictably, but reached a saturation plateau by 100 nM. The inset in Figure 5 shows a linear dynamic range for two orders of magnitude (the first data point corresponds to the lowest concentration in Figure 5 of 0. 1 nM ) i n c on cent rat i o n. The renaturing of the protein after ligand exposure may influence receptor calibration experiments, thus se- quential ligand exposure was studied. Immobilized LBD PXR was exposed to sequential injections of 1.0 M ri- fampicin in 10 mM PBS, pH 7.0 with varying exposure and recovery time. The injection times and recovery times were approximately the same. As injection time increased the response maximum increased as well, which is likely a result of increased exposure time. This longer interaction time may provide larger kinetic re- sponses, which is the nanomechanical measured parame- ter. All injection volumes and times were considerably less than saturation and were reversible, although base- line was not always reached with shorter recovery times. Exposure and recovery times of LBD PXR were also Figure 5. Responses to rifampicin at 6.7 minutes for PXR are plotted over a concentration range from 0.1 nM to 1.0 M, respectively. C opyright © 2011 SciRes. JBNB 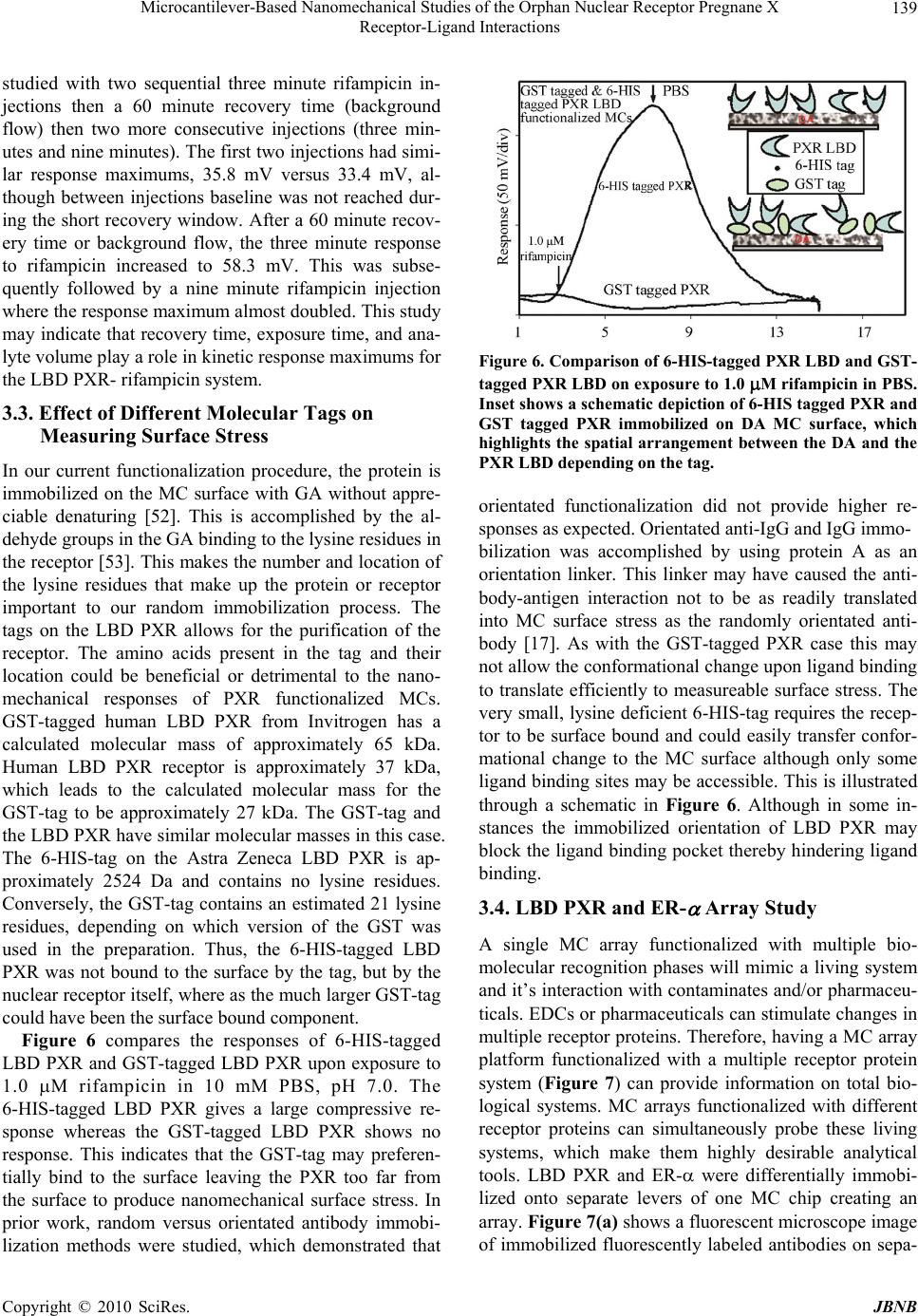 Microcantilever-Based Nanomechanical Studies of the Orphan Nuclear Receptor Pregnane X 139 Receptor-Ligand Interactions studied with two sequential three minute rifampicin in- jections then a 60 minute recovery time (background flow) then two more consecutive injections (three min- utes and nine minutes). The first two injections had simi- lar response maximums, 35.8 mV versus 33.4 mV, al- though between injections baseline was not reached dur- ing the short recovery window. After a 60 minute recov- ery time or background flow, the three minute response to rifampicin increased to 58.3 mV. This was subse- quently followed by a nine minute rifampicin injection where the response maximum almost doubled. This study may indicate that recovery time, exposure time, and ana- lyte volume play a role in kinetic response maximums for the LBD PXR- rifampicin system. 3.3. Effect of Different Molecular Tags on Measuring Surface Stress In our current functionalization procedure, the protein is immobilized on the MC surface with GA without appre- ciable denaturing [52]. This is accomplished by the al- dehyde groups in the GA binding to the lysine residues in the receptor [53]. This makes the number and location of the lysine residues that make up the protein or receptor important to our random immobilization process. The tags on the LBD PXR allows for the purification of the receptor. The amino acids present in the tag and their location could be beneficial or detrimental to the nano- mechanical responses of PXR functionalized MCs. GST-tagged human LBD PXR from Invitrogen has a calculated molecular mass of approximately 65 kDa. Human LBD PXR receptor is approximately 37 kDa, which leads to the calculated molecular mass for the GST-tag to be approximately 27 kDa. The GST-tag and the LBD PXR have similar molecular masses in this case. The 6-HIS-tag on the Astra Zeneca LBD PXR is ap- proximately 2524 Da and contains no lysine residues. Conversely, the GST-tag contains an estimated 21 lysine residues, depending on which version of the GST was used in the preparation. Thus, the 6-HIS-tagged LBD PXR was not bound to the surface by the tag, but by the nuclear receptor itself, where as the much larger GST-tag could have been the surface bound component. Figure 6 compares the responses of 6-HIS-tagged LBD PXR and GST-tagged LBD PXR upon exposure to 1.0 M rifampicin in 10 mM PBS, pH 7.0. The 6-HIS-tagged LBD PXR gives a large compressive re- sponse whereas the GST-tagged LBD PXR shows no response. This indicates that the GST-tag may preferen- tially bind to the surface leaving the PXR too far from the surface to produce nanomechanical surface stress. In prior work, random versus orientated antibody immobi- lization methods were studied, which demonstrated that Figure 6. Comparison of 6-HIS-tagged PXR LBD and GST- tagged PXR LBD on exposure to 1.0 M rifampicin in PBS. Inset shows a schematic depiction of 6-HIS tagged PXR and GST tagged PXR immobilized on DA MC surface, which highlights the spatial arrangement between the DA and the PXR LBD depending on the tag. orientated functionalization did not provide higher re- sponses as expected. Orientated anti-IgG and IgG immo- bilization was accomplished by using protein A as an orientation linker. This linker may have caused the anti- body-antigen interaction not to be as readily translated into MC surface stress as the randomly orientated anti- body [17]. As with the GST-tagged PXR case this may not allow the conformational change upon ligand binding to translate efficiently to measureable surface stress. The very small, lysine deficient 6-HIS-tag requires the recep- tor to be surface bound and could easily transfer confor- mational change to the MC surface although only some ligand binding sites may be accessible. This is illustrated through a schematic in Figure 6. Although in some in- stances the immobilized orientation of LBD PXR may block the ligand binding pocket thereby hindering ligand binding. 3.4. LBD PXR and ER- Array Study A single MC array functionalized with multiple bio- molecular recognition phases will mimic a living system and it’s interaction with contaminates and/or pharmaceu- ticals. EDCs or pharmaceuticals can stimulate changes in multiple receptor proteins. Therefore, having a MC array platform functionalized with a multiple receptor protein system (Figure 7) can provide information on total bio- logical systems. MC arrays functionalized with different receptor proteins can simultaneously probe these living systems, which make them highly desirable analytical tools. LBD PXR and ER- were differentially immobi- lized onto separate levers of one MC chip creating an array. Figure 7(a) shows a fluorescent microscope image of immobilized fluorescently labeled antibodies on sepa- C opyright © 2010 SciRes. JBNB 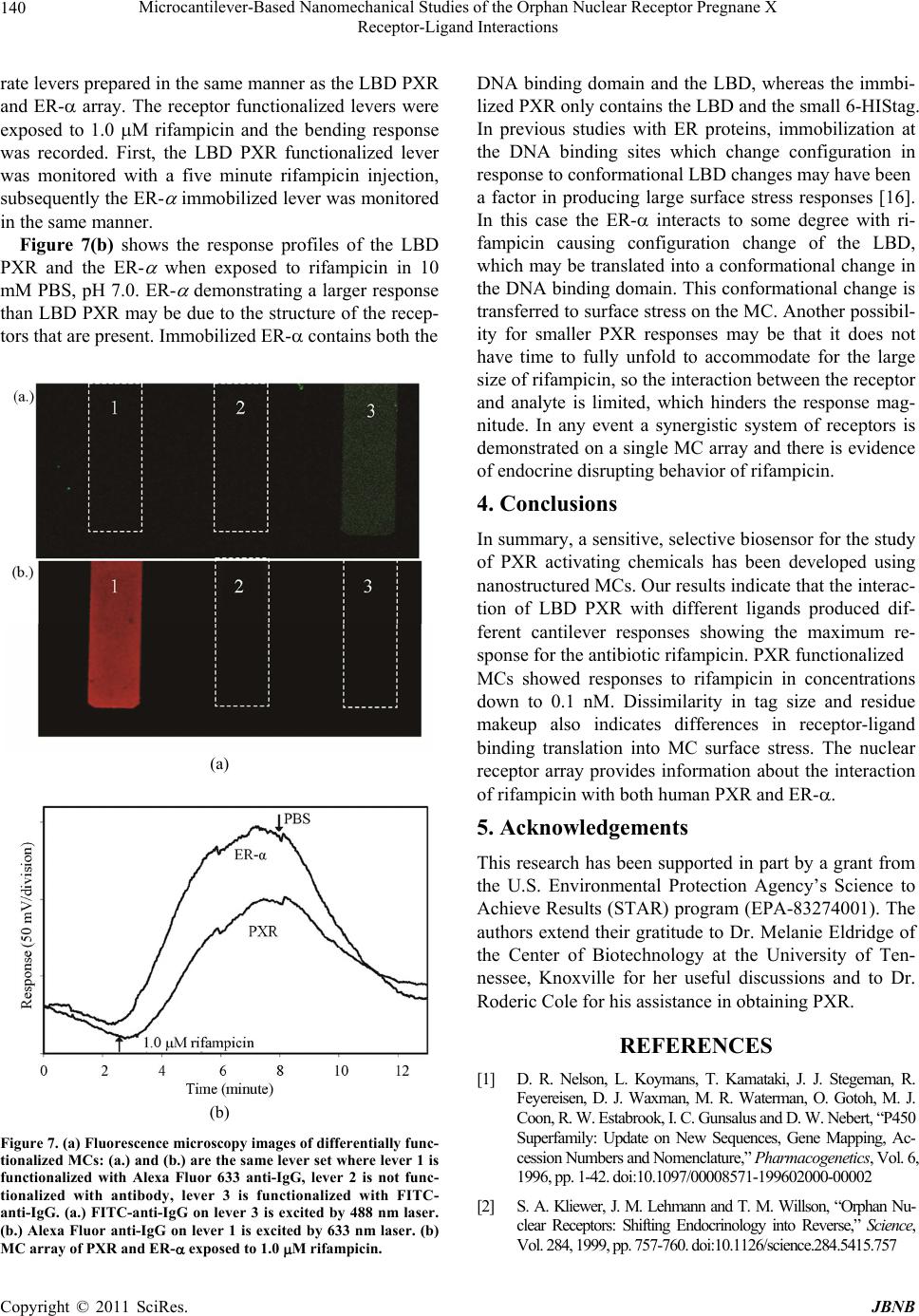 Microcantilever-Based Nanomechanical Studies of the Orphan Nuclear Receptor Pregnane X 140 Receptor-Ligand Interactions rate levers prepared in the same manner as the LBD PXR and ER- array. The receptor functionalized levers were exposed to 1.0 M rifampicin and the bending response was recorded. First, the LBD PXR functionalized lever was monitored with a five minute rifampicin injection, subsequently the ER- immobilized lever was monitored in the same manner. Figure 7(b) shows the response profiles of the LBD PXR and the ER- when exposed to rifampicin in 10 mM PBS, pH 7.0. ER- demonstrating a larger response than LBD PXR may be due to the structure of the recep- tors that are present. Immobilized ER- contains both the (a) (b) Figure 7. (a) Fluorescence microscopy images of differentially func- tionalized MCs: (a.) and (b.) are the same lever set where lever 1 is functionalized with Alexa Fluor 633 anti-IgG, lever 2 is not func- tionalized with antibody, lever 3 is functionalized with FITC- anti-IgG. (a.) FITC-anti-IgG on lever 3 is excited by 488 nm laser. (b.) Alexa Fluor anti-IgG on lever 1 is excited by 633 nm laser. (b) MC array of PXR and ER- exposed to 1.0 M rifampicin. DNA binding domain and the LBD, whereas the immbi- lized PXR only contains the LBD and the small 6-HIStag. In previous studies with ER proteins, immobilization at the DNA binding sites which change configuration in response to conformational LBD changes may have been a factor in producing large surface stress responses [16]. In this case the ER- interacts to some degree with ri- fampicin causing configuration change of the LBD, which may be translated into a conformational chang e in the DNA binding domain. This conformational change is transferred to surface stress on the MC. Another possibil- ity for smaller PXR responses may be that it does not have time to fully unfold to accommodate for the large size of rifampicin, so the interaction between the receptor and analyte is limited, which hinders the response mag- nitude. In any event a synergistic system of receptors is demonstrated on a single MC array and there is evidence of endocrine disrupting behavior of rifampicin. 4. Conclusions In summary, a sensitive, selective b iosensor for the study of PXR activating chemicals has been developed using nanostructur ed MCs. Our r esu lts indicate that the in terac- tion of LBD PXR with different ligands produced dif- ferent cantilever responses showing the maximum re- sponse for the antibiotic rifampicin. PXR functionalized MCs showed responses to rifampicin in concentrations down to 0.1 nM. Dissimilarity in tag size and residue makeup also indicates differences in receptor-ligand binding translation into MC surface stress. The nuclear receptor array provides information about the interaction of rifampicin with both human PXR and ER-. 5. Acknowledgements This research has been supported in part by a grant from the U.S. Environmental Protection Agency’s Science to Achieve Results (STAR) program (EPA-83274001). The authors extend their gratitude to Dr. Melanie Eldridge of the Center of Biotechnology at the University of Ten- nessee, Knoxville for her useful discussions and to Dr. Roderic Cole for his assistance in obtaining PXR. REFERENCES [1] D. R. Nelson, L. Koymans, T. Kamataki, J. J. Stegeman, R. Feyereisen, D. J. Waxman, M. R. Waterman, O. Gotoh, M. J. Coon, R. W. Estabrook, I. C. Gunsalus and D. W. Nebert, “P450 Superfamily: Update on New Sequences, Gene Mapping, Ac- cession Numbers and Nomenclature,” Pharmacogenetics, Vol. 6, 1996, pp. 1-42. doi:10.1097/00008571-199602000-00002 [2] S. A. Kliewer, J. M. Lehmann an d T. M. Wil lson, “Orphan Nu- clear Receptors: Shifting Endocrinology into Reverse,” Science, Vol. 284, 1999, pp. 757-760. doi:10.1126/scien ce.284.5415.757 C opyright © 2011 SciRes. JBNB  Microcantilever-Based Nanomechanical Studies of the Orphan Nuclear Receptor Pregnane X 141 Receptor-Ligand Interactions [3] E. L. Michalets, “Update: Clinically Significant Cyto-Chrome P-450 Drug Interactions,” Pharmacotherapy, Vol. 18, 1998, pp. 84-112. [4] P. R. Ortiz de Montellano, “Cytochrome P450: Structure, Mechanism and Biochemistry,” Plenum Publishers, New York, 2005, pp. 377-425. [5] J. Zhang, P. Kuehl, E. D. Green, J. W. Touchman, P. B. Wat- kins, A. Daly, S. D. Hall, P. Maurel, M. Relling, C. Brimer, K. Yasuda, S. A. Wrighton, M. Hancock , R . B . K i m, S . Str o m , K . Thummel, C. G. Russell, J. R. Hudson, Jr., E. G. Schuetz and M. S. Boguski, “The Human Pregnane X Receptor: Genomic Structure and Identification and Functional Characterization of Natural Allelic Variants,” Pharmacogenetics, Vol. 11, 2001, pp. 555-572. doi:10.1097/00008571-200110000-00003 [6] S. A. Kliewer, J. T. Moore, L. Wade, J. L. Staudinger, M. A. Watson, S. A. Jones, D. D. McKee, B. B. Oliver, T. M. Will- son, R. H. Zetterst röm, T. Perlmann and J. M. Lehmann, “An Orphan Nuclear Receptor Activated by Pregnanes Defines a Novel Steroid Signaling Pathway,” Cell, Vol. 92, 1998, pp. 73-82. doi:10.1016/S0092-8674(00)80900-9 [7] B. Blumberg, W. Sabbagh, Jr., H. Juguilon, J. Bolado, Jr., C. M. van Meter, E. S. Ong and R. M. Evans, “SXR, a Novel Steroid and Xenobiotic-Sensing Nuclear Receptor ,” Genes and Development, Vol. 12, 1998, pp . 3195-3205. doi:10.1101/gad.12.20.3195 [8] G. Bertilsson, J. Heidrich, K. Svensson, M. Åsman, L. Jende- berg, M. Sydow-Bäckman, R. Ohlsson, H. Postlind, P. Blom- quist and A. Berkenstam, “Identification of a Human Nuclear Receptor Defines a New Signaling Pathway for Cyp3a Induc- tion,” Proceedings of the National Academy of Sciences, Vol. 95, 1998, pp. 12208-12213. doi:10.1073/pnas.95.21.1 2208 [9] R . E. Watkins, G. B. Wisel y, L. B. Moore, J. L. Colli ns, M. H. Lambert, S. P. Williams, T. M. Willson, S. A. Kliewer and M. R. Redinbo, “The Human Nuclear Xenobiotic Receptor PXR: Structural Determinants of Directed Promiscuity,” Science, Vol. 292, 2001, pp. 2329-2333 . doi:10.1126/science.1060762 [10] L. B. Moore, J. M. Maglic h, D. D. McKee, B. Wisely, T. M. Willson, S. A. Kliewer, M. H. Lambert and J. T. Moore, “Pregnane X Receptor (PXR), Constitutive Androstane Re- ceptor (CAR) and Benzoate X Receptor (BXR) Define Three Pharmacologically Distinct Classes of Nuclear Receptors,” Molecular Endocrinology, Vol. 16, 2002, pp. 977-986 . doi:10.1210/me.16.5.977 [11] C. Zhou, S. Verma and B. Blumberg, “The Steroid and Xeno- biotic Receptor (Sxr), Beyond Xenobiotic Metabolism,” Nu- clear Receptor Signaling, Vol. 7, 2 009, pp. 1-21. [12] S. A. Kliewer, B. Goodwin and T. M. Willson, “The Nuclear Pregnane X Receptor: A Key Regulator of Xenobiotic Me- tabolism,” Endocrine Reviews, Vol. 23, 2002, pp. 687-702. doi:10.1210/er.2001-0038 [13] J. E. Chrencik, J. Orans, L. B. Moore, Y. Xue, L. Peng, J. L. Collins, G. B. Wisely, M. H . Lambert, S. A. Kliewer and M. R. Redinbo, “Structural Disorder in the Complex of Human Pregnane X Receptor and the Macrolide Antibiotic Rifam- picin,” Molecular Endocrinology, Vol. 19, 2005, pp. 1125- 1134. doi:10.1210 /me.2004-0346 [14] J. T. Moore and S. A. Kliewer, ‘Use of the Nuclear Recaptor Pxr to Predict Drug Interactions,” Toxicology, Vol. 153, 2000, pp. 1-10. doi:10.1016/S0300-483X(00)00300-0 [15] P. Dutta, K. Hill, P. G. Datskos and M. J. Sepaniak, “Devel- opment of a Nanomechanical Biosensor for Analysis of Endo- crine Disrupting Chemicals,” Lab Chip, Vol. 7, 2007, pp. 1184-1191. doi:1 0.1039/b704723a [16] K. Hill, P. Dutta, A. Zareba, M. L. Eldridge and M. J. Sepaniak, “Morphological and Chemical Optimization of Microcantile- ver Surfaces for Thyroid System Sensing and Beyond,” Ana- lytica Chimica Ac ta, Vol. 625, 2008, pp . 55-62. doi:10.1016/j.aca.2008.07.005 [17] C. A. Tipple, N. V. Lavrik, M. Culha, J. Headrick, P. Datskos and M. J. Sepaniak, “Nanostructured Microcantilevers with Functionalized Cyclodextrin Receptor Phases: Self Assembled Monolayers and Vapor Deposited Films,” Analytical Chemis- try, Vol. 74, 2002, pp. 3118-3126. doi:10.1021 /ac020074o [18] P. Dutta, P. J. Chapman, P. G. Datskos and M. J. Sepaniak, “Characterization of Ligand-Functionalized Microcantilevers for Metal Ion Sensing,” Analytical Chemistry, Vol. 77, 2005, pp. 6601-6608. doi:10.1021/ac051082i [19] P. Dutta, J. Sanseverino, P. G. Datskos and M. J. Sepaniak, “Detection of Cytokine Using a Microcantilever Biosensor,” NanoBiotechnology, 1, 2005, pp . 237-244. doi:10.1385/NBT:1:3:237 [20] R. Berger, E. Delamarche, H. P. Lang, C. Gerber, J. K. Gim- zewski, E. Meyer and H. J. Guntherodt, “Surface Stress in the Self-Assembly of Alkanethiols on Gold,” Scie nc e, Vol. 276, 1997, pp. 2021-2024. doi:10.1126/science.276.5321 .2021 [21] T. Thundat, E. A. Wachter, S. L. Sharp and R. J. Warmack, “Detection of Mercury Vapor Using Resonating Microcanti- levers,” Applied Physics Letters, Vol. 66 , 1995, pp. 1695-1697. doi:10.1063/1.113896 [22] P. Dutta, C. A. Tipple, N. V. Lavrik, P. G. Datskos, O. Hof- stetter and M. J. Sepaniak, “Enantioselective Sensors Based on Antibody Mediated Nanomechanics,” Analytical Chemistry, Vol. 75, 2003, pp. 2342-2348. doi:1 0.1021/ac034031z [23] G. Wu, R. H. Datar, K. M. Hansen, T. Thundat, R. J. Cote and A. Majumdar, “Bioassay of Prostate-Specific Antigen (Psa) Using Microcantilevers,” National Biotechnology, Vol. 19, 2001, pp. 856-860. doi:10.1038/nbt0901-856 [24] C. Grogan, R. Raiteri, G. M. O’Connor, T. J. Glynn, V. Cun- ningham, M. Kane, M. Charlton and D. Leech, “Characteriza- tion of an Antibody Coated Microcantilever as a Potential Immuno-Based Biosensor,” Biosensor Bioelectronics, Vol. 17, 2002, pp. 201-207. doi:10.1016/S0956-5663(01)00276-7 [25] J. Fritz, M. K. Baller, H. P. L ang, H. Rothuizen, P. Vettiger, E. Meyer, H. Guntherodt, C. Gerber and J. K. Gimzewski, “Translating Biomolecular Recognition into Nanomechanics,” Science, Vol. 288, 2000, pp. 316-31 8. doi:10.1126/science.288.5464.316 [26] G. Shekhawat, S. H. Tark and V. P. Dravid, “MOS- FET-Embedded Microcantilevers for Measuring Deflection in Biomolecular Sensors,” Science, Vol. 311, 2006, pp. 1592- 1595. doi:10.1126/science.1122588 C opyright © 2010 SciRes. JBNB  Microcantilever-Based Nanomechanical Studies of the Orphan Nuclear Receptor Pregnane X 142 Receptor-Ligand Interactions [27] S. A. Jones, L. B. Moore, J. L. Shenk, G. B. Wisely, G. A. Hamilton, .D. D. McKee, N. C. O. Tomkinson, E. L. LeCluyse, M. H. Lambert, T. M. Willson, S. A. Kliewer and J. T. Moore, “The Pregnane X Receptor: A Promis-Cuous Xenobiotic Re- ceptor That Has Diverged during Evolution,” Molecular En- docrinology, Vol. 14, 2000, pp. 27-3 9. doi:10.1210/me.14.1.27 [28] S. J. Shukla, D. Nguyen, R. MacArthur, A. Simeonov, W. J. Frazee, T. M. Hallis, B. D. Marks, U. Singh, H. C. Eliason, J. Printen, C. P. Austin, J. Inglese and D. S. Auld, “Identification of Pregnane X Receptor Ligands Using Time-Resolved Fluo- rescence Resonance Energy Transfer and Quantitative High- Throughput Screening,” Assay and Drug Development Tech- nologies, Vol. 7, 2009, pp. 143-169. doi:10.1089/adt.2009.193 [29] G. G. Stoney, “The Tension of Metallic Films Deposited by Electrolysis,” Proceeding of the Royal Society of London, Se- rial A, Vol. 82, 1909, pp. 172-177 . [30] N. P. Mahalik, “Micromanufacturing and Nanotechnology,” Springer, Berlin, 2006, pp. 299-321. [31] N. V. Lavrik, C. A. Tipple, M. J. Sepaniak and P. G. Datskos, “Enhanced Chemi-M echanical Tran sduction at Nanos tructured Interfaces,” Chemical Physics Letters, Vol. 336, 2001, pp. 371-376. doi:10.1016/S0009-2614(01)00155-5 [32] J. R. Premkumar, O. Lev, R. S. Marks, B. Polyak, R. Rosen and S. Belkin, “Antibody-Based Immobilization of Biolumi- nescent Bacterial Sensor Cells,” Talanta, Vol. 55, 2001, pp. 1029-1038. doi:10.1016/S0039-9140(01)00533-1 [33] S. W. Park, Y. I. Kim, K. H. C hung, S. I. Hong and S. W. Kim, “Covalent Immobilization of Gl-7-Aca Acylase on Silica Gel Through Silanization,” Reactive Functional Polymers, Vol. 51, 2002, pp. 79-92. doi:10.1016/S1381-5148(02)00028-7 [34] P. J . C ha pm an , Z. Long, P. G. Datskos, R. Archib ald and M. J. Sepaniak, “Ligand-Functionalized Microcantilever Arrays for Metal Ion Sensing,” Analytical Chemistry, Vol. 79, 2007, pp. 7062-7068. doi:10.1021/ac070754x [35] L. R. Senesac, P. Dutta, P. G. Datskos and M. J. Sepaniak, “Analyte Identification Using Differentially-Functionalized Microcantilever Arrays and Artificial Neural Nets,” Analytical Chemistry Acta, Vol. 558 , 2006, pp. 94-101. doi:10.1016/j.aca.2005.11.024 [36] P. J. Chapman, Z. Long, P.G. Datskos, R. Archbald and M. J. Sepaniak, “Ligand-Functionalized Microcantilever Arrays for Metal Ion Sensing,” Analytical Chemistry, Vol. 79, 2007, pp. 7062-7068. doi:10.1021/ac070754x [37] Z. Long, J. Story, S. Lewis and M. J. Sepaniak “Landfill Si- loxane Gas Sensing Using Differentiating, Responsive Phase Coated Microcantilev er Arrays” An alytical Chemistry, Vol. 81, 2009, pp. 2575-2580. doi:10.1021/ac802494v [38] M. J. Sepaniak, P. G. Datskos, N. V. Lavrik and C. Tipple, “Microcantilever Transducers: A New Approach in Sensor Technology,” Analytical Chemistry, Vol. 74, 2002, pp. 568A- 575A. [39] J. L. Staudinger, B. Goodwin, S. A. Jones, D. Hawkins-Brown, K. I. MacKenzie, A. LaTour, Y. Liu, C. D. Klaassen, K. K. Brown, J. Reinhard, T. M. Willson, B. H. Koller and S. A. Kliewer, “The Nuclear Receptor PXR is a Lithocholic Acid Sensor That Protects against Liver Toxicity,” Processings of the National Academy Sciences , Vol. 98, 2001, pp. 33 69-3374. doi:10.1073/pnas.051551698 [40] E. Y. H. Yeung, T. Sueyoshi, M. Negishi and T. K. H. Chang, “Identification of Ginkgo Biloba as a Novel Activator of Pregnane X Receptor,” Drug Metabolism and Dispo sition, Vol. 36, 2008, pp. 2270-2276. doi:10.1124/dmd.108.0234 99 [41] S. Kortagere, D. Chekmarev, W. J. Welsh and S. Ekins, “Hy- brid Scoring and Classification Approaches to Predict Human Pregnane X Receptor Activators,” Pharmaceutical Research, Vol. 26, 2009, pp. 1001-1011. doi:10.1007/s11095-008-9809-7 [42] P. Li, “Evaluation of Luciferin-Isopropyl Acetal as a CYP3A4 Substrate for Human Hepatocytes: Effects of Organics Sol- vents, Cytochrome P450 (P450) Inhibitors and P450 Induc- ers,” Drug Metabolism and Disposition, Vol. 37, 2009, pp. 1598-1603. doi:10.1124/dmd.109.027268 [43] M. Abdelrahim, E. Ariazi, K. Kim, S. Khan, R. Barhoumi, R. Burghardt, S. Liu, D. Hill, R. Finnell, B. Wlodarczyk, V. C. Jordan and S. Safe, “3-Methylcholanthrene and Other Aryl Hydrocarbon Receptor Agonists Directly Activate Estrogen Receptor,” Cancer Research, Vol. 66, 2006, pp. 2459-2467. doi:10.1158/0008-5472.CAN-05-3132 [44] J. P. Jackson, K. K. Kabirov, I. M. Kapetanovic and A. Lyu- bimov, “In Vitro Assessment of p450 Induction Potential of Novel Chemopreventive Agents sr13668, 9-cis-uab30 and Pentamethychromanol In Primary Cultures of Human Hepa- tocytes,” Chemical-Biology Interactive, Vol. 179, 2009, pp. 263-272. doi:10.1016/j.cbi.2008.12.005 [45] H. Kuramitz, J. Natsui, K. Sugawara, S. Itoh and S. Tanaka, “Electrochemical Evaluation of the Interaction between Endo- crine Disruptor Chemicals and Estrogen Receptor Using 17-Estradiol Labe led with Daunomycin,” Analytical Chemistry, Vol. 74, 2002, pp. 533-538. doi:10 .1021/ac010426b [46] G. G. J. M. Kuiper, J. G. L emmen , B. Carlson, J. C. Cor ton, S . H. Safe, P. T. Van Der Saag, B. Van Der Burg a nd J. Gusta fs- son, “Interaction of Estrogenic Chemicals and Phytoestrogens with Estrogen Receptor,” Endocrinology, Vol. 139, 1998, pp. 4252-4263. doi:1 0.1210/en.139.10.4252 [47] S. Stoica, B. S. Katzenellenbogen and M. B. Martin, “Activa- tion of Estrogen Receptor—by the Heavy Metal Cadmium,” Molecular Endocrinology, Vol. 14, 2000, pp. 545-553 . doi:10.1210/me.14.4.545 [48] D. J. Mangelsdorf, C. Thummel, M. Besto, P. Herrlich, G. Schütz, K. Umesono, B. Blumberg, P. Kastner, M. Mark, P. Chambon and R. M. Evans, “The Nuclear Receptor Super- family: The Second Decade,” Cel l, Vol. 83, 1995, pp. 835-839. doi:10.1016/0092-8674(95)90199-X [49] H. Masuyama, Y. Hiramatsu, M. Kunitomi, T. Kudo and P. N . MacDonald, “Endocrine Disrupting Chemicals, Phthalic Acid and Nonylphenol, Activate Pregnane X Receptor-Mediated Transcription,” Molecular Endocrinology, Vol. 14, 2000, pp. 421-428. doi:10.1210/me.14.3.421 [50] H. Masuyama, N. Suwaki, Y. Tateishi, H. Nakatsukasa, T. Seqawa and Y. Hiramatsu, “The Pregnane X Receptor Regu- lates Gene Expression in a Ligand and Promoter-Selective C opyright © 2011 SciRes. JBNB  Microcantilever-Based Nanomechanical Studies of the Orphan Nuclear Receptor Pregnane X Receptor-Ligand Interactions Copyright © 2010 SciRes. JBNB 143 Fashion,” Molecular Endocrinology, Vol. 19, 2005, pp. 1170- 1180. doi:10.1210/me.2004-0434 [51] D. Hopwood, C. R. Allen and M. McCabe, “The Reactions between Glutaraldehyde and Various Protein. An Investigation of Their Kinetics,” Histochemical Journal, Vol. 2, 19 70, pp. 137-150. doi:10.1 007/BF01003541 [52] D. R. Walt and V. I. Agayn, “The Chemistry of Enzy me and Protein Immobilization with Glutaraldehyde,” Trends Analyti- cal Chemistry, Vol. 13, 1994, pp . 425-430. doi:10.1016/0165-9936(94)85023-2 |

