Paper Menu >>
Journal Menu >>
 Engineering, 2012, 5, 159-162 doi:10.4236/eng.2012.410B041 Published Online October 2012 (http://www.SciRP.org/journal/eng) Copyright © 2012 SciRes. ENG An Amperometric Sensor for Sunset Yellow FCF Detection Based on Molecularly Imprinted Polypyrrole Jinfeng X u, Yi Zhang, Hao Zhou, Ming wei Wa ng, Peidong Xu, Juankun Zhang* Key Laboratory of Industrial Fermentation Microbiology, Ministry of Education, College of Biotechnology, Tianjin Key Lab of Industrial Microbiology, College of Biotechnology,Tia njin Uni vers ity of Science an d Technology, Tianjin 300457 , People’s Republic of China Email: *zhangjk@tust.edu.cn Received 2012 ABSTRACT An electrochemical method for fast detecting the concentration of sunset yellow FCF in wine samples was developed in this study. The sensor based on imprinted films which fabricated by electropolymerization of pyrrole on a glassy carbon electrode in the pres- ence of sunset yellow FCF as the template. Comparing to the polypyrrole non-imprinted modified (NIP) electrode, the polypyrrole molecularly imprinted polymer (MIP) electrode improved the electrochemical performance of the sensor significantly. The peak current at about 0.26 V was linear with the concentration of sunset yellow FCF from 0.4 to 2 μM and from 2 to 8 μM. It can be used for more than 10 times to maintain a stable response result. The sensor had the good selectivity on sunset yellow FCF, amaranth and tartrazine, which the selection factors were 1.00, 0.80 and 0.85,respectively. Keywords: E lectrochemical Sensor; Polypyrrole ; Sunset Yellow FCF; Molecularly Imprinted Polymers 1. Introduction Sunset yellow FCF is a synthetic azo dye, which has been widely added into foods and drinks for its high stability to light, oxygen and pH and low production cost [1]. However, studies have shown that sunset yellow FCF can cause many adverse health effects such as high genotoxicity and cytostaticity [ 2] . In Chin a, sunset yellow FCF is permitted and the value of accept- able d aily intake (ADI) is between 0 and 2.5 mg·kg-1 according to the hygienic standards for uses of food additive (GB 2760-1996). Until now, several methods have been applied for the detection of sunset yellow FCF, such as spectrophotometry [3] , HLPC [4,5] and LC/MS [6]. However, the detecting process of these methods was too complicated to proceed, can't meet the d aily food scen e fast d etectio n requ ir ements. Recen tl y, numerous electrochemistry methods are applied to sunset yel- low FCF detection via modified working electrode such as multi-walled carbon nanotubes film-modified electrode [1,7] and Platinum wire–coated electrode [8], which have high sen si- tivity but lower selectivity. A sensor based on MIPs as th e me- rits of high sensitivity and label free evoked much attention. Nevertheless, a sensor based on MIPs for sunset yellow FCF detection has not been reported. Herein, an amperometric sen- sor for sunset yellow FCF measuring was developed in this paper. 2. Experimental 2.1. Chemicals and Apparatus Pyrrole (98%), sunset yellow FCF, amaranth, tartrazine were purchased from Sangon Biotech (Shanghai) Co.,Ltd. All rea- gents were an alytical grade an d were used as received, without further purification. The wine was bought on the market. All experiments were performed t hrough an electrochemical analyzer LK2005A (Tianjin, China) connecting to a computer and carrying out with a conventional three-electrode cell with glassy carb on (GC) electro de(ø = 4mm) which was covered b y non-MIP or MIP polypyrrole film as the working electrode, while an Ag/AgCl/satu rated KCl electrod e and a platinu m wire served as the reference electrode and the auxiliary electrode respecti vely. 2.2. Preparation of Electrodes Based on MIP and NIP Films A GC electrode was polished carefully with aqueous alumina slurry (0.5μm) and repeatedly rinsed with distilled water. The electrolyte solution was prepared with PBS(pH7.0) containing 0.02 M sunset yellow FCF, 0.05 M pyrrole and 0.1 M KCl. The electropolymerization was performed through cyclic voltam- metry of +0.4 V to -1.0 V, with a scan rate of 50 mV·s-1 and 10 consecutive scans. Then , the embedded sunset yellow FCF molecules were removed from the PPy film by overoxidized process at +1.3 V in the 0.1 M NaOH solution for 600 s. And, the molecularly imprinted fi lm electrode was activated to ob- tained MIP film electrodes in 0.2 M PBS (pH6.0) containing 0.1 M KCl by cyclic potential scan . As a control, the non-imprinted PPy-GC electrode was prepared and treated in exactly the same way except that the template molecules were omitted from the electropolymerization stage. 2.3. Electroanalytical Measurements There are t wice current measurements before and after incuba- tion of sunset yellow FCF , which were performed using square wave voltammetry(SWV) in 0.2 M PBS(pH7.0) solution con- *Corresponding author.  J. F. XU ET AL. Copyright © 2012 SciRes. ENG 160 taining 4mM Fe(CN)63-/4-, between 0.6V and 0.0V. After the first current measurement, the imprinted or non-imprinted PPy-GC electrode was dipped into a 0.2 M PBS(pH6.0) con- taining prepared concentration of sunset yellow FCF to adsorp for 10 min, then was washed with distilled water carefully to remove the possible adsorptive substance on the electrode sur- face, and then was transferred to the first detection electro- chemical cell using the same measurements (SWV). All mea- surement s were performed at ro om temperatu re. 2.4. Analysis of Real Sa mples 50.00 mL samples of wine were boiled to eliminate CO2 and ethanol, adjusted pH to 6.0 using 2.5 M NaOH after cooling, and then the wine was transferred to a 50mL flask to volume with distilled water. Standard curve regression method was used to determine the content of the sunset yellow FCF. The treated wine samples were then spiked with appropriate amount of suns et yellow FCF for recovery experiments. 2.5. The Structure of Three Analogue To investigate the selectivity of the MIP and NIP electrode in this research, three same concentration of analogue were de- tected to evaluate the detection response. The structure of sun- set yellow FCF, amaranth and tartrazine were shown in the Figure 1. 3. Results and Discussion 3.1. Electropolymerization of MIP a n d NIP Polypyrrole The thickness of polymer film can easily be adjusted by con- trolling the scan rates and the number of cycles during electro- polymerization process [9]. We developed series of experi- ments in which electro des were fab ricated with d ifferent cycle s of 5, 10 and 15 to optimize the number of CV cycles to use to form the ‘sensing’ layer of the electrode. The results shown that the highest current response of the MIP electrodes to sunset yellow FCF was obtained by applying 10 cycles. Figure 2 showed that one cathodic peak was observed at -0.77 V at the first negative scan, and the current decreased si gnificantly with the increase of cyclic scan times and were stabile after 8-cycle scans. The other cathodic peak was observed at -0.07 V. After the first negative scan and was constant. The reductive peak appeared at 0.06 V and peak current was constant also. As shown in the inset of Figure 2, there was no t a cath o di c peak at -0.77 V, which could correspond to the reduction of sunset yellow FCF molecules. The result s indicat ed that the fo rmation of PPy films on the surface of GC electrode hindered the monomer further accessing to the GC electrode surface, which was the same as Xie’s [ 10] study and the sunset yellow FCF and the PPy backbone could unified together stabliy. It was because t hat th e posi tive ch aracte ri sti c of th e Pp y backbo ne an d the negative charge of sunset yellow FCF molecules were strongly absorbed onto the electrode surface through hydrogen bonding and electrostatic interactions. 3.2. Electrochemical Behavior of Sunset Yellow FCF on the Imprinted PPy-GC Electrode The electro chemical behavio r of sunset yello w FCF was inves- tigated by MIP and NIP electrode. The catalytic effect of the MIP elect rode to different concentrations of sunset yellow FCF were investigated in pH 7.0 PBS by SWV. Sunset yellow FCF gave an oxidation peak response at about 0.250V at the NIP electrod e, while an anod ic peak appeared at about 0.275V with the use of MIP electrode and the peak current decreased with the increased concentration of sunset yellow FCF. The en- hanced peak current response and a shift in the oxidation poten- tial of sunset yellow FCF at about 0.025V in the anodic direc- tion were the clear evidences of the catalytic effect of the MIP electrode towards the oxidation of sunset yellow FCF. The calibration curve for the SWV peak current versus sun- set yellow FCF was observed at MIP electrode under optimum experimental conditions. There were two linear regions in the curve which were 0.002mM to 0.008mM with the correlation coefficient of 0.987 and 0.0004 mM to 0.002mM with the cor- relation coefficient of 0.993. 3.3. Optimization of Experimental Parameters 1) Concentration Selection of Pyrrole and Sunset Yellow FCF The concen trations of monomer (pyrrole) an d template (sun- set yello w FCF) du ring po lymerizatio n det ermine the an alytical behavior of the sensor. The monomer concentration should be proportional to the thickness of the deposit and amount of im- printed molecule (t empl ate) in th e p ol ymeric matri x [9]. So it is necessary to select the proper concentration of sunset yellow FCF a nd pyrrole to both make the response of sunset yellow FCF highest and obtain not a signal for pyrrole. The electro- chemical p erformances of several i mprin ted PP y-GC electr odes which were produced in different solutions of sunset yellow FCF and pyrrole, were investigated in 0.2M PBS pH 7.0 con- taining 1.7×10-5M sunset yellow FCF at the MIP electrode. The response of the MIP electrode to sunset yellow FCF sam- ples were shown in Table 1. The highest current response was obtained at 50mM pyrrole and 20mM sunset yellow FCF(serial number1),which were chosen as the optimum concentrations. O O N N S NaO NN HO SO O NaO sunset yellow FCF N=N HO NaO-S- O O SONa O O SO OONa amaranth S OO ONa N=N O NaO OH S OO NaO tartrazine Figure 1. T he structure of sunset yellow FCF, amaranth and tartrazine. 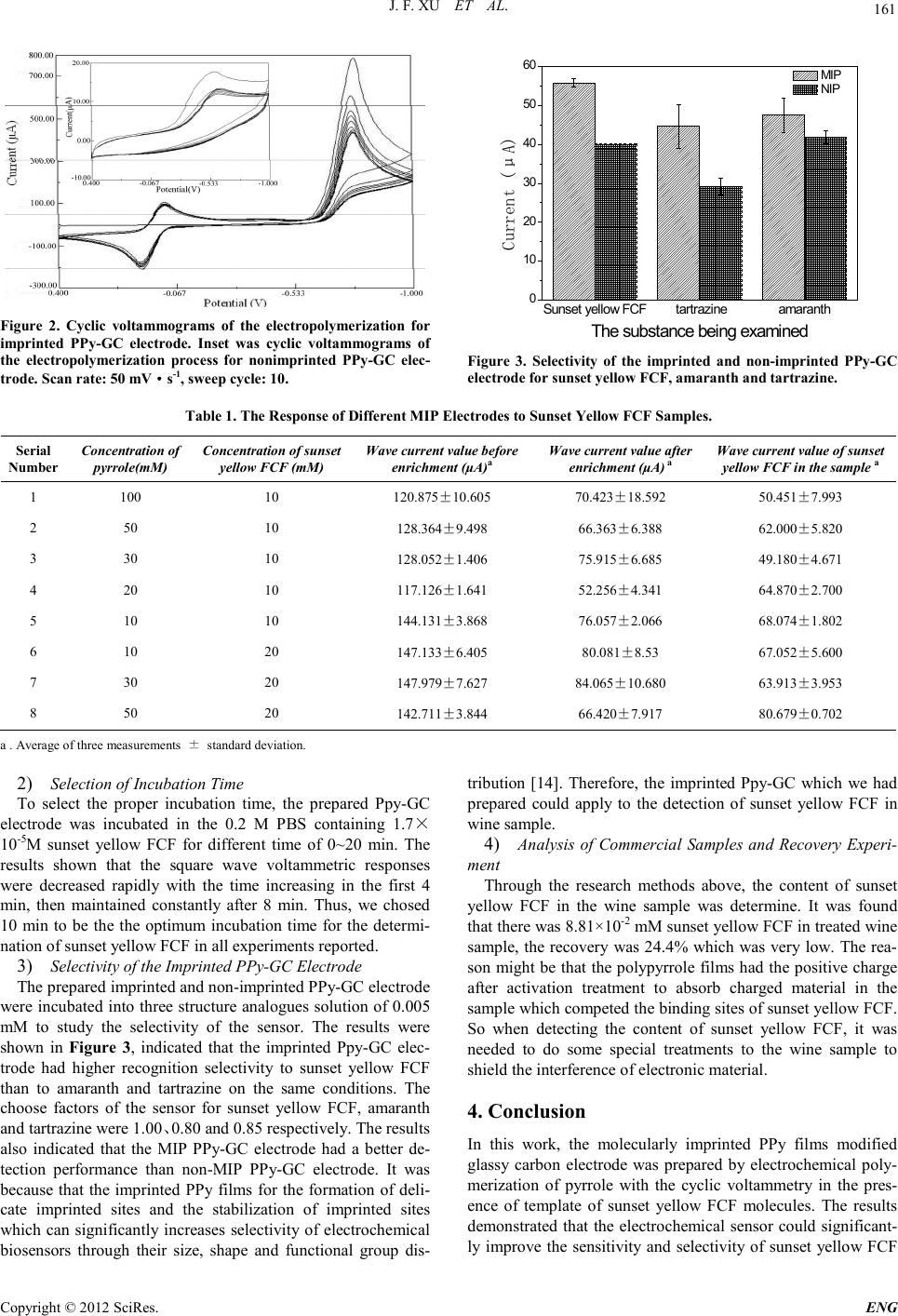 J. F. XU ET AL. Copyright © 2012 SciRes. E NG 161 Figure 2. Cyclic voltammograms of the electropolymerization for imprinted PPy-GC electrode. Inset was cyclic voltammograms of the electropolymerization process for nonimprinted PPy-GC elec- trode. Scan rate: 50 mV·s-1, sweep cycle: 10. Sunset yellow FCF tartrazineamaranth 0 10 20 30 40 50 60 Current (μA) The substanc e being examined MIP NIP Figure 3. Selectivity of the imprinted and non-imprinted PPy-GC electrode for sunset yellow FCF, amaranth and tartrazine. Table 1. The Response of Different MIP Electrodes to Sunset Yel lo w FCF Samp les. Serial Number Concentration of pyrrole(mM) Conc e ntra tion of sunse t yellow FCF (mM) Wav e cur ren t valu e bef o re enrichment (μA)a Wave cur rent va lu e after enrichment (μA) a Wav e c u r re nt va lue of su nse t yellow FCF in the sample a 1 100 10 120.875±10.605 70.4 23±18.592 50.4 51±7.993 2 50 10 128.364±9.498 66.3 63±6.388 62.000±5.820 3 30 10 128.052±1.406 75.9 15±6.685 49.180±4.671 4 20 10 117.126±1.641 52.2 56±4.341 64.870±2.700 5 10 10 144.131±3.868 76.0 57±2.066 68.074±1.802 6 10 20 147.133±6.405 80.081±8.53 67.052±5.600 7 30 20 147.979±7.627 84.0 65±10.680 63.913±3.953 8 50 20 142.711±3.844 66.4 20±7.917 80.679±0.702 a . Average of three measurements ± standard deviation. 2) Selectio n of Incubation Time To select the proper incubation time, the prep ared Ppy-GC electrode was incubated in the 0.2 M PBS containing 1.7× 10-5M sunset yellow FCF for different time of 0~20 min. The results shown that the square wave voltammetric responses were decreased rapidly with the time increasing in the first 4 min, then maintained constantly after 8 min. Thus, we chosed 10 min to be the the optimum incubation time for the determi- nation of sunset yellow FCF in all experiments reported. 3) Selectivity of the Impri nted PPy-GC Electrode The prepared imprinted and non-imprinted PPy-GC electrod e were incubated into three structure analogues solution of 0.005 mM to study the selectivity of the sensor. The results were shown in Figure 3, indicated that the imprinted Ppy-GC elec- trode had higher recognition selectivity to sunset yellow FCF than to amaranth and tartrazine on the same conditions. The choose factors of the sensor for sunset yellow FCF, amaranth and tartrazine were 1.00、0.80 and 0.85 respectively. The resul ts also indicated that the MIP PPy-GC electrode had a better de- tection performance than non-MIP PPy-GC electrode. It was because that the imprinted PP y fi lms for the formation of deli- cate imprinted sites and the stabilization of imprinted sites which can significantly increases selectivity of electrochemical biosensors through their size, shape and functional group dis- tribution [14]. Therefore, the imprinted Ppy-GC which we had prepared could apply to the detection of sunset yellow FCF in wine sample. 4) Analysis of Commercial Samples and Recovery Experi- ment Through the research methods above, the content of sunset yellow FCF in the wine sample was determine. It was found that t here was 8 .81×1 0-2 mM sunset yellow FCF in treated wine sample, t he recovery was 24.4% which was very low. The rea- son might be that the polypyrrole films had th e positive char ge after activation treatment to absorb charged material in the sample which competed the binding sites of sunset yellow FCF. So when detecting the content of sunset yellow FCF, it was needed to do some special treatments to the wine sample to shield the interference of el ectron ic material. 4. Conclusion In this work, the molecularly imprinted PPy films modified glassy carbon electrode was prepared by electrochemical poly- merization of pyrrole with the cyclic voltammetry in the pres- ence of template of sunset yellow FCF molecules. The results demonstrated that the electrochemical sensor could significant- ly improve the sensitivity and selectivity of sunset yellow FCF  J. F. XU ET AL. Copyright © 2012 SciRes. ENG 162 analysis. Therefore, the electrochemical sensor could be poten- tially exploited for detecting the residual analysis of sunset yellow FCF in the wine samples. 5. Acknowledgements The authors thank the Ministry of Science and Technology of the People’s Republic of China (2009GJA10047) to suppo rt the work. REFERENCES [1] P. Wang, X. Z. Hu, Q. Cheng, X. Y. Zhao, X. F. Fu, and K. B. Wu, “Electroch emical detection of amaranth in food based on th e enhanc ement effect o f carbon nanotu be film ,” J. Agric. Food Chem, vol. 58, pp . 12112–12116, 2011. [2] J. Wang, “Investigation on fluorescence spectra of synthetic food dyes, ” [D]. Jiangna n University, 2009. [3] Z. S. Lin, M. Z. Zhang, Y. Z. Jiangi, Z. F. Cai, C. S. Chen, “Study on rapid detection of sunset yellow in beverage by spec- trophotometry, ” Guangzhou Chemical Industry, vol. 39, pp.109-110,2011. [4] Y. L. Zhang, J. Y. Ma, H. Wang, “Simultaneous determination of four synthetic colorants in wine by a HPLC method with variable wavelength detector, ” Journal of Northwest A& F University( Nat. Sci. Ed.), vol. 39,no. 1, Jan 2011. [5] D. Elena, E. C. Petronela, “Study of analytical parameters of the HPLC method for tartrazine and sunset yellow analysis in soft drinks, ” Revista De Chimie,vol. 61, no. 12, pp.1177-1182, Dec 2010 . [6] M. Yamada, A. Kawahara, M. Nakamura, H. Nakazawa, “Anal- ysis of raw materials, intermediates and subsidiary colours in Food Yellow No. 5 (Sunset Yellow FCF) by LC/MS, ” Food ad- ditives and contaminants, vol. 17, no. 8, pp. 665-674, Aug 2000. [7] Y. Z. Song, “Electrochemical reduction of sunset yellow at a multiwalled carbon nanotube (MWCNT)-modi fied glass y carbon electrode and its analytical application, ” Can. J. Chem, vol. 88, no. 7, p p . 6 76-681, 2010. [8] S. Rouhani, “Novel electrochemical sensor for sunset yellow based on a platinum wire–coated electrode, ” Analytical Letters, vol. 42, pp. 141–153, 200 9. [9] L. Ozcan, Y. Sahin, “Determination of paracetamol based on electropolymerized-molecularly imprinted polypyrrole modified penci l graphi te electrod e,” Sensors and Actuators, vol. B 127, pp. 362–369, 2007. [10] C. G. Xie . S. Gao, Q. B. Guo, K. Xu, “Electrochemical sensor for 2,4 -dichloroph enoxy acetic a cid using molecu larly imprin ted polypyrrole membrane as recognition element, ” Microchim Acta, vol. 169, pp. 145–152, 2010. [11] D. Tonelli, B. Ballarin, L. Guadagnini,A. Mi gnan i , E . Sca vet t a , “A novel p otent iometric sens or for l-ascorbic acid based on mo- lecularly imprinted Polypyrrole, ” Electrochimica Acta, vol. 56, pp. 7149– 7154, 2011. [12] Y. Tian, J. R. Wang, M. Liu, K. Shi, F. L. Yang, “Redox s tability of polypyrrole in aqueous electrolyte solutions by a recurrent potential pulse technique, ”Acta Phys. Chim. Sin, vol. 27, no.5, pp. 1116-1121, May 2011. [13] C. G. Xie, H. F. Li, C . Y. Xie, H. K. Zhou , “Prep arat ion of elec- trochemical sensor for salicylic acid based on molecular im- printed film,” Chinese Journal of Analytical Chemistry, vol. 37, no. 7, pp. 1045-1048, July 2009. [14] C. Malitesta, I. Losito, PG. Zambonin, “Molecularly imprinted electrosynthesized polymers: new materials for biomimetic sen- sors, ” Anal Chem. vol. 71, no. 7, pp. 1366–1370, 1999. |

