Paper Menu >>
Journal Menu >>
 Engineering, 2012, 5, 142-145 doi:10.4236/eng.2012.410B037 Published Online October 2012 (http://www.SciRP.org/journal/eng) Copyright © 2012 SciRes. ENG In vitro Rooting of Podophyllum Hexandrum and Transplanting Technique* Qi Guo1,2, Jianping Zhou2, Zhiy e Wang1,2, Hui Yang1,2# 1 Institute of Biology, Gansu Academy of Sciences, L anzhou 730000, China 2Industrial Mi cr obio l o g y Enginee r in g Researc h Cente r in Gansu, Lanzhou 730000, China Email: #yanghui43@163.com Received 2012 ABSTRACT Podophyllum hexandrum Royle is an important medicinal plant that produces podophyllotoxin with anti-cancer properties. In China, it is u sed as a traditional C hinese medicine. Recent years, unplanned exploiting and collection have led to the disappearance of th is species in China and India. Effective methods such as tissue culture should be adopted to conserve it to abtain a large scale. It was an crucial process for the fin al success o f ti s s ue cult ure that seedlings with roots and true l eaves i n flasks were transferred to so il in field. A protocol has been development for in vitro rooting and hardening and en vitro transplant of Podophyllum hexandrum Royle. Roots were efficien tly established on WPM media supplemented with IAA 1.5 mg·L-1 and NAA 0.5 mg·L-1. Before tran sferred to soil con- taining turfysoil and perlite (2:1), rooted plants were exposed to air for 5d for adaptation. Two periods were good for transplanting this plantlets. Keywords: Podophyllum Hexandrum; Podophyllotoxin; in Vitro Rooting; Hardening; Transplanting 1. Introduction Podophyllum hexandrum Royle belonging to the family Berbe- ridaceae is an important medicinal plants of the podophyllo- toxin. It grows in the northern Himalayas and the adjacent range. In China, it is named Sinopodophyllum emodi (Wall.) Ying[1], which distributes in the western area Tibet, Yunnan, Qinghai, Gansu province[2]. Using of dried roots and rhizomes as a drug for deto xification , rheumatism and relieving pain can be found in traditional chinese medicine. In Tibet, it is used to treat patients who suffer from gynecological inflammation[3]. Podophyllotoxin being the main component from the herb is used as a p recursor for the ch emical synth esis of antineoplastic drugs. Etoposide, etopophose and teniposide, these podophyl- lotoxin derivatives, have been success full y utilized in t reatment of a variety of cancers[4]. A novel of series of derivatives of podophyllotoxin showed strong antiHIV-1 activities[5]. In rescent years, the commercial source of podophyllotoxin is wild collected from the alpine Himalayas in India and the west regions of China. Wild harvesting of these plants and li- mited ability to reprodution have depleted the natural popula- tion to such a degree that the plant has become an endangered species[6]. It was at the beginning of the last century that P. hexandrum were cultivated for the first time in India's Hima- layan region[7]. And in China it began in the 1980s. Unfortu- nately cultivation has neither b een carried out scienti fically nor attempted on a large scale. Plant tissue culture seems to be an effective alternative in recovery of some rare and endangered medicinal plants. Al- though some studies related to in vitro propagation techniques for mass shoot multiplication about this plant have been re- ported[8~10], it is desirable to apply methods for enhancing root- ing, hardening and transplant for field trials for large scale res- toration. This protocol seems to be the first time to systemat- ically study on hardening technique of tissue cultured seedlings of Sinopodophyllum emodi (Wall.) Ying after in vitro rooting. These strategies could be adopted to motivate farmers to culti- vate such improtant medicinal plants for its conservation. 2. Materials and Methods Material used: The present study was conducted at Institute of Biology, Gansu Academy of Sciences, Lanzhou in the year 2010. The mature seeds of Sinopodophyllum emodi were col- lected from Xinglong Mountain, in Lanzhou. A sequence of procedures, pretreatment, seed germination, shoot induction and proliferation under in vitro condition were taken. The shoot separated by cutting the multiple shoots were used for devel- opment of roots. The basal medium that consists of woody plant medium(WP M) supplemented with a combination of growth regulators, vitamins and sucrose 3% with a pH adjuste to 5.8 was used to obtain optimal results. In vitro rooting: The shoots excised were transferred to WPM medium with 3% sucrose, 6.5% agar and various com- binations of growth regulators, NAA (0.5mg·L-1), IBA (0.5~ 2.0mg·L-1) and IAA (0.5~2.0mg·L-1) for rooting. The pH of medium was adjusted to 5.8. The medium was dispensed in 100ml glass culture vessels which were sterilized by autoclav- ing at 121℃ for 20min. Cultures were maintained under con- tinuous light conditions (10h) with an illumination of 1500~ *Supported by the National Key Project of Scientific and Technical Sup- porting Programs Funded by Ministry of Science & Technology of China No. 2012BAC01B05, Project of Agricultur al Scientific Achievements Transfer of Gansu No. 1006NCNA116 and Project of Development of Science and Techn ology of Lanzh ou No. 2011-1-72. #Corresponding author. 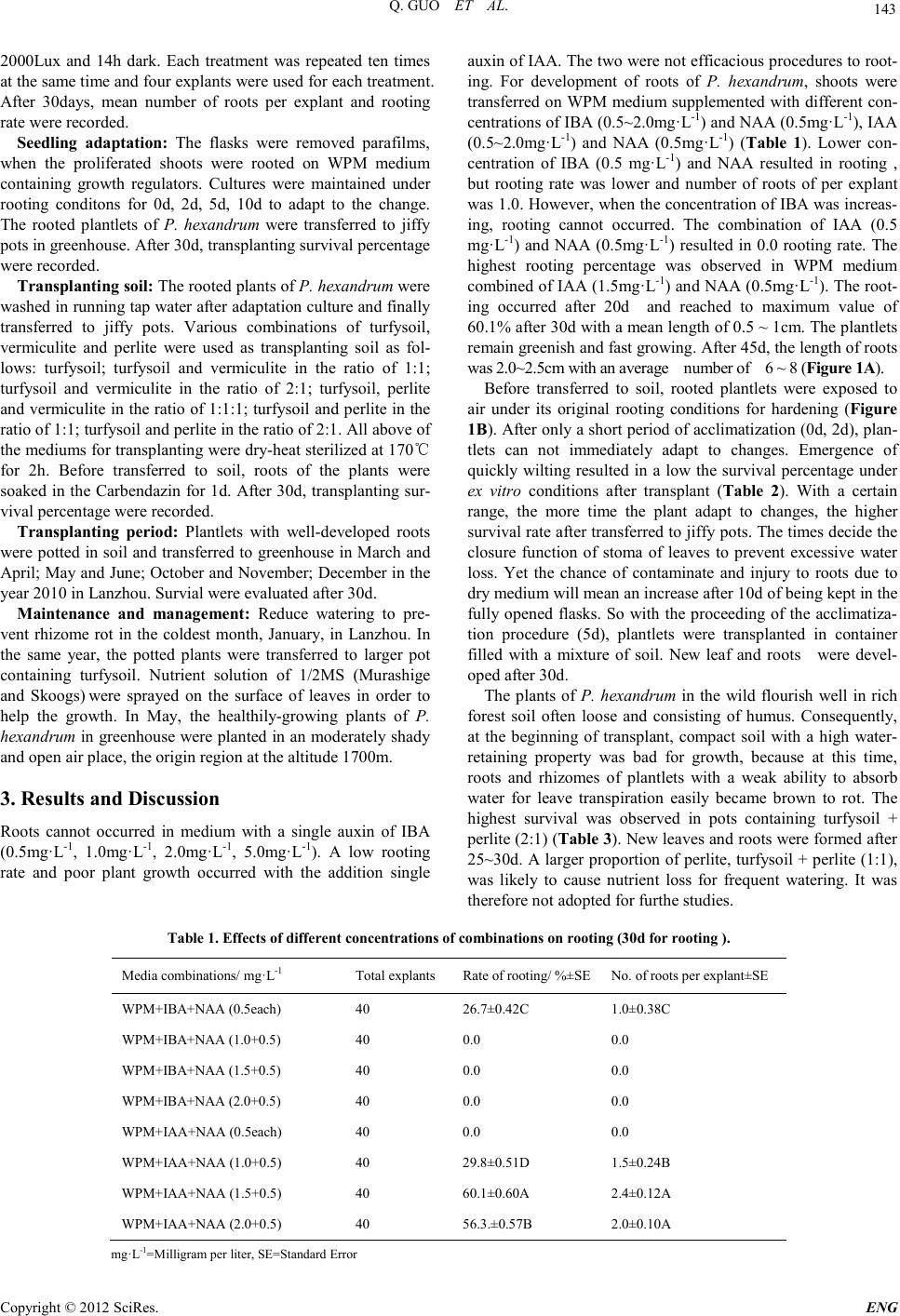 Q. GUO ET AL. Copyright © 2012 SciRes. ENG 143 2000Lux and 14h dark. Each treatment was repeated ten times at the same ti me and four explants were u sed for each treat ment . After 30days, mean number of roots per explant and rooting rate were recorded . Seedling adaptation: The flasks were removed par afilms, when the proliferated shoots were rooted on WPM medium containing growth regulators. Cultures were maintained under rooting conditons for 0d, 2d, 5d, 10d to adapt to the change. The rooted plantlets of P. hexandrum were transferred to jiffy pots in greenhouse. After 30d, transplanting survival percent age were recorded. Tra nspl a nting soi l : The rooted plants of P. hexandrum were washed in running tap water after adap tation cu lture an d finally transferred to jiffy pots. Various combinations of turfysoil, vermiculite and perlite were used as transplanting soil as fol- lows: turfysoil; turfysoil and vermiculite in the ratio of 1:1; turfysoil and vermiculite in the ratio of 2:1; turfysoil, perlite and vermiculite in the ratio of 1:1:1; turfysoil and perlite in the ratio of 1:1; turfysoil and perlite in the ratio of 2:1. All above of the mediums for transplanting were dry-heat sterilized at 17 0℃ for 2h. Before transferred to soil, roots of the plants were soaked in the Carbendazin for 1d. After 30d, transplanting sur- vival percentage were r ecorded. Transplanting period: Plantlets with well-developed roots were potted in soil and transferred to greenhouse in March an d April; M ay and June; Octob er and November; Dec ember in the year 2010 in Lanzhou. Survial were evaluated after 30 d. Maintenance and management: Reduce watering to pre- vent rhizome rot in the coldest month, January, in Lanzhou. In the same year, the potted plants were transferred to larger pot containing turfysoil. Nutrient solution of 1/2MS (Murashige and Skoogs) were sprayed on the surface of leaves in order to help the growth. In May, the healthily-growing plants of P. hexandrum in green house were planted in an mod erately shady and open air place, the origin region at the altitude 1700m. 3. Results and Discussion Roots cannot occurred in medium with a single auxin of IBA (0.5mg·L-1, 1.0mg·L-1, 2.0mg·L-1, 5.0mg·L-1). A low rooting rate and poor plant growth occurred with the addition single auxin of IAA. The two were not efficacious procedures to root- ing. For development of roots of P. hexandrum, shoots were transferred on WPM medium supplemented with different con- centrations of IBA (0.5~2.0mg·L-1) an d NAA (0.5mg·L-1), IAA (0.5~2.0mg·L-1) and NAA (0.5mg·L-1) (Table 1). Lower con- centration of IBA (0.5 mg·L-1) and NAA resulted in rooting , but rooting rate was lower and number of roots of per explant was 1.0. However, when the concentratio n of IBA was increas- ing, rooting cannot occurred. The combination of IAA (0.5 mg·L-1) and NAA (0.5mg·L-1) resulted in 0.0 rooting rate. The highest rooting percentage was observed in WPM medium combined of I AA (1.5mg·L-1) and NAA (0.5mg·L-1). The root- ing occurred after 20d and reached to maximum value of 60.1% after 30d with a mean length of 0.5 ~ 1cm. The plantlets remain greenish and fast growing. After 45d, the length of roots was 2.0~2.5cm wit h an average number of 6 ~ 8 (Figure 1A). Before transferred to soil, rooted plantlets were exposed to air under its original rooting conditions for hardening (Figure 1B). After only a short period of acclimatization (0d, 2d), plan- tlets can not immediately adapt to changes. Emergence of quickly wilting resulted in a low the survival percentage under ex vitro conditions after transplant (Table 2). With a certain range, the more time the plant adapt to changes, the higher survival rate after transferred to jiffy pots. The t imes decid e the closure function of st oma of leaves to prevent excessive water loss. Yet the chance of contaminate and injury to roots due to dry medium will mean an incr eas e after 10d of being kept in the fully opened flasks. So with the proceeding of the acclimatiza- tion procedure (5d), plantlets were transplanted in container filled with a mixture of soil. New leaf and roots were devel- oped after 30d. The plants of P. hexandrum in the wild flourish well in rich forest soil often loose and consisting of humus. Consequently, at the beginning of transplant, compact soil with a high water- retaining property was bad for growth, because at this time, roots and rhizomes of plantlets with a weak ability to absorb water for leave transpiration easily became brown to rot. The highest survival was observed in pots containing turfysoil + perlite (2:1) (Ta ble 3 ). New leaves and roots were formed after 25~30d. A larger proportion of perlite, turfysoil + perlite (1:1), was likely to cause nutrient loss for frequent watering. It was therefore not adopted for furthe studies. Table 1. Effects of different concentrations of combinations on rooting (30d for root ing ). Media combinations/ mg·L-1 Total explants Rate of rooting/ %±S E No. of roots p er explant ±SE WPM+IBA+NAA (0.5each) 40 26.7±0.42C 1.0±0.38C WPM+IBA+NAA (1.0+0.5) 40 0.0 0.0 WPM+IBA+NAA (1.5+0.5) 40 0.0 0.0 WPM+IBA+NAA (2.0+0.5) 40 0.0 0.0 WPM+IAA+NAA (0.5each) 40 0.0 0.0 WPM+IAA+NAA (1.0+0.5) 40 29.8±0.51D 1.5±0.24B WPM+IAA+NAA (1.5+0.5) 40 60.1±0.60A 2.4±0.12A WPM+IAA+NAA (2.0+0.5) 40 56.3.±0.57B 2.0±0.10A mg·L-1=Milligram per liter, SE= St a ndard Error  Q. GUO ET AL. Copyright © 2012 SciRes. ENG 144 Figure 1. In vitro rooting of Podophyllum hexandrum and transplant. A In vitro rooting, B Seedling adaptation, C Transferred plantlets in jiffy pots, D Transferred plantlets in larger pot, E Transferred plants in the origi n region at the alti tude 1700m under a shade, F Roots with the average diameter 0.25 cm. Table 2. Effects of different adaptation periods on hardening of rooted plantlets. Adaptation p eriod/ d Total plants Survival/ %±SE Control (0) 30 20.4±0.56C 2 30 54.7±0.65B 5 30 89.3±0.34A 10 30 90.2±0.41A SE=Standard Error Table 3. Effects of different transplanting soils on hardening of rooted plantlets. Transplanting soil Total plan ts Survival/%±SE turfysoil 30 71.3±0.43C turfysoil+vermiculite (1:1) 30 54.6±0.61D turfysoil+vermiculite (2:1) 30 50.0±0.57D turfysoil+perlite+vermiculite (1:1:1) 30 77.5±0.56B turfysoil+perlite (1:1) 30 95.0±0.23A turfysoil+perlite (2:1) 30 98.2±0.20A SE=Standard Error Micro climate in greenh ouses was so complex that conditions of illumination, temperature, humidity, greenhouse ventilation, etc. affect all activities of plants. Temperature and humidity as a major factor plays an important role in growth of P. hexan- drum. It were not extremely suitable for transplanting this plant in the coldest and hottest months of winter and summer in Lanzhou. Four different periods were chosen in which the pot- ted plants were taken to grow in the greenhouse . The survival are shown in Table 4. Weather was getting cold during October and November in- Lanzhou. The minimum and maximum temperature appeared at night and sunny noon in the greenhouse with an average tem- perature of 23℃, and humidity of 69 ~ 98%. The highest sur- vival was obtained in this period. So it was suitable for the plant of P. hexandrum to grow. Leaves became thicker an d dark green after 20d with new leaves. The period of March and April also was good for transplanting plantlets. Lower temperate and higher humidity are the main reasons for rot of roots and rhi- zomes in December. Wilting and the lowest survial was ob- served during May and June due to the hotter temperate. In th e coldest month, leaves o f the plantlets wither. Simulta- neously, the parts under ground go dormant. Once the weather become warming the next year, shoots unearthed. The results available s ho ws that seedl in gs from tissue cu ltu re t aken to grow under ex vitro conditions have the same characteristics of dor- mancy as the wild one. The potted plants were transferred to larger pot containing turfysoil to grow rapidly (Figure1D). In May when the rainfall was frequent relatively, plants were planted in the origin region at the altitude 1700m under a shade (Figure 1 E). Larger l eave s, deep green , flou ri shed , mor e leaves (2~3) were observed. After two months, the number of roots was 10~12 with the average diameter 0.25cm (Figure 1F). Transplanting was very much successful. 4. Conclusions The endangered plant of P. hexandrum is inherently slow growing. At present the rate of propagation of P. hexandrum in nature is far less than the rate of its exploration. Previous stu- dies were mostly carried out for in vitro productiong of podo- phyllotoxin in cell culture of P. hexandrum. Recent studies were also carried out for in vitro propagation for mass multip- lication of P. hexandrum. Our study focus in enhancing rooting, hardening and transplanting of tissue cultured seedlings. Roots has been developed in WPM medium supplemented with IAA 1.5 mg·L-1 and NAA 0.5 mg·L-1 as optimum for effective re- generation of roots. Before transferred to soil containing turfy- soil and perlite (2:1), rooted plants were exposed to air for 5d. Two periods were good for transplanting plantlets. The ultimate success of this plantlet production undoubtedly depended on the survival and vigorous growth during and after transplanta- tion.  Q. GUO ET AL. Copyright © 2012 SciRes. ENG 145 Tabl e 4. Effects of four transplanting periods on hardening of rooted plants. Transplanting period/m Total explants Minimum temperature/℃ Maximum temperature/ ℃ Minimum humidity/% Maximum Humidity/% Survival/ %±SE Mar. - Apr. 30 10.5 27.3 60.0 98.3 90.2±0.78B May. - Jun. 30 15.5 46 .8 38.5 98.9 38.4±0.55D Oct. - Nov. 30 10.0 36.0 6 9.0 98.2 98.1±0.67A Dec. 30 4.0 19.3 88.6 98.8 56.7±0.60C The data of Mi nimum temperature, Maxi mum temperature, Mi nimum hu midity and Maximum Humidity represent the mean of the period. SE=Sta ndard Error. So far, n ew seeds can h ardly be collected in Gansu Province for the decrease of wild population of this species. In vitro propagation provides an opportunity to abtain seedings of P. Hexandrum. This was the first report of enhancing rooting, hardening and transplanting of seedlings from propagation systematically. It can be valuable for large-scale restoration of this plant. 5. Acknowledgements The authors would like to thank Dr. YANG Hui for support and encouragement and Mr. ZHANG Jian-jun for his help in the greenhouse. REFERENCES [1] YingTsun-shen. "On dysosma woodson and Sinopodophyllum Ying, gen. nov. of the Berberidaceae(in Chinese)," Acta Phyto- taxonomica Sin. Beijing, vol. 17, pp. 15 -26, Feb 1979. [2] Yanghui, Guoqi and ZhaoChang-qi."Research Progress on Cell Engineering and Endophytes of Rare Plant Sinopodophyllum emodi (Wall) Ying(in Chinese)," China Biotechnology. Beijing, vol. 30, pp. 94-99, Nov 2010 . [3] WuZheng-yi. Flora Xizangica (in Chinese). Beijing, Science Press, 1985. [4] S.Farkya, V.S. Bisaria and A.K.Srivastava."Biotechnological aspects of the production of the anticancer drug podophyllotox- in," Appl Microbiol Biotechnol.New York, vol.65, pp. 504-519, Oct 2004. [5] ShiWu-chen, YunHun-wang and YanJin. "Synthesis and anti -HIV-1 activities of novel podophyllotoxin derivatives," Bioorg. Med . Chem Lett. Oxford, vol. 17, pp. 2091-2095, 2007. [6] M.Nadeem, L.M.S.Palni and A.N.Purohit."Propagation and conservation of Podophyllum hexandrum Royle: an important medicinal herb, " Biol. Conservation.Oxford, vol. 92, pp. 121-129, Jan 2000. [7] R.Chatterjee. "Indian Podophyllum," Econ. Bot. New York, vol. 6, pp. 342 -354, Oct-Dec 1952. [8] A.Chakraborty, D.Bhattacharya and S.Ghanta. "An efficient protocol for in vitro regeneration of Podophyllum hexandrum, a critically endangred medicinal plant," Indian Journal of Bio- techn ology. Indian, vol. 9, pp. 217-220, Apr 2010. [9] P.Sultan, A.S.Shawl and P.W.Ramteke. "In vit ro propagat ion for mass multiplication of Podophyllum hexandrum: a high value medicinal herb," Asian Journal of plant Science. New York, vol. 5, pp. 179-184, Apr 20 06. [10] GuoQi, ZhangJun and ZhaoXiao-Feng."In vitro Culture of En- dangered Medicinal Plant Sinopodophylum hexandrum Royle(in Chinese)," Bulletin of Botanical Research. Harbin,vol. 32, pp. 484-487, Aug 2012. |

