Paper Menu >>
Journal Menu >>
 Engineering, 2012, 5, 135-138 doi:10.4236/eng.2012.410B035 Published Online October 2012 (http://www.SciRP.org/journal/eng) Copyright © 2012 SciRes. ENG Formaldehyde Biosensor with Formaldehyde Dehydrogenase Adsorped on Carbon Electrode Modified with Polypyrrole and Carbon Nanotube Mingwei Wa ng, Shuhai Jiang, Yi Che n, X i ang Chen, Li Zhao, J uankun Zhang *, Jinfe ng Xu Key Laboratory of Industrial Microbiology, Ministry of Educati on, College of Biotech nology, Tianjin Key Laboratory of Industry Microbiology, College of Biotechnology, Tianjin Universi ty of Science&Technology, Tia nj in, 30 0 457, C hi na Email: zhangjk@tust.edu.cn Received 2012 ABSTRACT In this study, a carbon electrode electroless polymerization of polypyrrole, which formed a layer of conductive film interface,then absorped a layer of carbon nanotube particles by the way of self-assembled, is studied. The modified electro d e is u sed to elect ro static adherence of formaldehyde dehydrogenase, with Nafion solution capped. Moreover this paper discusses the work o f electrode po si- tion, PH value and scanning speed on the influence of the electrode response, the response potential is -1.2v, the optimal PH value is 7.5, and we also review the l inear r ange o f this electr od e, we disco vered t aht this biosensor have a good linear relationship with the concentration of 1ug/ml-360ug/ml, the Correlation coefficient is 0.9983. In addition,with the stability of this electrode t ested, it can be store d 15 d at 4°C. Keywords: Formaldehyde Dehydrogenase; Eletrostatic Self-assemb ly; Biosensor 1. Introduction Formaldehyde has been identified as carcinogenic and terato- genic substances by the World Health Organization, and it is the recognized source of allergy and a potentially strong muta- gens, ranking second on the list of priority control of toxic chemicals in china[1]. Formaldehyde on human health is main- ly manifest ed in the abno rmal se nse of s mell, irritation, allergy, pulmonary function abnormalities, abnormal liver function and immune function abnormalities[2]. Making use of the catalytic properties of formaldehyde dehydrogenase for detecting formaldehyde, is not only easy to operate and specific, but also suitable for everyday use in the workplace. For example, Vianello’s group[3] have coupled formaldehyde dehydrogenase on field-effect transistor, to detect airborne formaldehyde content by ion FET sensitive transistive, and the detection limit is 0.1ug/ml, much lower than the critical value of the occupational exposure. Mitsubavashi’s group[4] have fixed for mal dehyde d eh ydrogena se on a p lati num electro d e, to detect the production of NADH when enzyme reactions happen, which can reflect the content of formaldehyde. In this study, the l in ear ran ge is 4 0-3 0 0ug/ ml. M. Be n Ali ’s group [13] manufactured a biosensor with formaldehyde dehydrogenase, which was reco mbinated b y Genetic engin eering, and used this biosensor to detect trace amounts of formaldehyde with stripping voltammetry. Kohji. Mitsubayashi [14] developed a convenient biochemical chip for testing wood materials in gaseous formaldehyde. Lilach Bareket [15] developed a electrochemical biosensor based on carbon nanotubes for detecting formaldehyde release from cancer precursors drugs. Yaroslav [ 16] fixed formald ehyde d ehydrogen ase and co factors on the surface of electrode, which avoided adding coenzyme and glutathione when detecting substrates, and the range of this biosensor is 10mM—200mM. In this study, we used the technology of electrostatic self- assembly for sdsorbing enzyme. Firstly, we polymerized polypyrrole on the surface of carbon electrode, Then we made it adsorb carbon nanotubes.At last, we put it in the solution cont aining formalde dehydrogenase, for adsorbing enzyme by electrostatic self-assembly. By this way, we produced a new and excellent biosensor for detecting formaldehyde. 2. Experiment 2.1. Reagents Formaldehyde dehydrogenase was produced according to the papers of[9,10]. Pyrrole is purchased from the company of ke wei of tian jin.Carbon nanotubes was obtained in Shenzhen Nanotech Port Co,Ltd. Potassium ferricyanide is purchased from the company of Chemical Reagent of Yong Da in TianJin. All other reagents like as Na2HPO4.12H2O, NaH2PO4.H2O, NaCl, Anhydrous alcohol were of analytical grade.Pyrrole was freshly distilled before use.Double distilled water was used for the preparation of all buffer solutions. 2.2. Apparatus A LK2005A model potentiostat from lan li ke in tianjin driven by an PHILIPS PC with software was used for electropoly- merization and detection measurements. A three—electrode cell with a saturated Ag /AgCl reference electrode and aplati- num foil counter electrode was used. Electronic analytical bal-  M. W. WANG ET AL. Copyright © 2012 SciRes. ENG 136 ance is the model of FA2004A from the company of shanghai Precision & Scientific instrument. A PH meter is from Shang- hai Magnetic Technology Co.,Ltd. Ultrasonic cleaning instru- ment is from Ningbo Chi Biotechnology Co.,Ltd. 2.3. Characteristics and Functions of Material and Technology Polypyrrole has been in-depth studyed because of its excellent conductivity and simple synthetic process. It has been found that various additives and doped or composite nanoparticles in the polypyrrole ,not only improve the electrical conductivity of polypyrrole,but also enhance its thermal stability and mechanic- cal ductility. In this research,we use the method of electro- chemical polymerization to form a polypyrrole film on the sur- face of carbon electrode[5 ,6]. As a unique nano-materials,Carbon nanotubes has large speci fic sur face area, excellent electric al,chemical properti es and biological affinity. we can modify the carbon nanotubes through replacing, addition and oxidation, whether at the surface, the end or the tube, for introducing functional groups and bio- active co mponen t. This can b e used for enz yme immobi lizatio n materials, or be served as the base electro d e modified materials. In one word, we made a new type of carbon nanotube modified enzyme sensor[7]. Electrostatic self-assembly as a new biomolecular immobili- zation method has been incr easingly used fo r the p reparation of various biological sensors. With the principle of electrostatic self-assembly of supramolecular, it can make positive and ne- gatively charged substances (nanoparticles, dyes, polyion, DNA, protein)attract in g each other, and this method has more advan- tages considering biological activity, performance, stabil- ity, simple preparation,and preparation under mild condi- tions [8]. 2.4. Preparation of Enzyme Electrode[11,12] Polish the carbon electrode with 0.05um Al2O3 suspension until it looks like a mirror, rinse clean,and then ultrasonic clean in distilled water and dilute sulfuric acid solution each with 10 minutes. Get out this processed eletrode,using the Three-elec- trode system to active, under 0.4—﹣1.6v potential, with the speed of 50mv/s, scan by cyclic voltammetry in 0.5mol/L H2SO4 solution with 10 laps. After the activation electrode is removed, rinsed, and dried at room temperature. Then put this electrode into 0.1mol/L pyrrole solution scanning by cyclic voltammetry with 60 laps, at the speed of 100mv/s. Put this electrode modified with polyprrole into carbon nanotube solu- tion for 12 hours, and then transfer it to Formaldehyde dehy- drogenase solution for 24 hours under 4°c, wash by stilled wa- ter, Finally drop nafion solution for capping,dry and put it in PBS solution under 4°c for using. 2.5. The Preparation of the Substrate In this study, all substrates for detecting contant 0.1mol/LPBS, 4.8mol/LNAD+, 1.0mol/L Glutathione,and with different con- centr a t ion of formalde hyde. 2.6. The Mathods of Detection The device of the three-electrode: the reference electrode is Ag/AgCl, the counter electrode is platinum electrode, the working electrode is carbon electrode modified by Formalde- hyde dehydrogenase. Under the condition of experiment, we use cyclic voltammetry and square wave voltammetry to test with the PBS buffer solution at PH=7.0, and the scanning po- tential is ranging from 0.4v to -1.6v at the scanning speed of 50mv/s. The current response of NADH is record by the en- zyme electrode. When the enzyme electrode is not used,stored it in PBS buf fer solut io n u nde r 4°c at the PH value of 7.0. 3. Results and Discussion 3.1. The Preparation of Polypyrrole Film Cyclic voltammetry can make a very uniform film with ele- troless polymerization. The cyclic voltammogram of polypyr- role modified carbon eletrodes is showed in Figure 1, the po- tential is between 0.4v and -1.6v, scanning speed is 100mv/s, the concentration of pyrrole is 0.1mol/L with 60 laps. Accord- ing to this picture, there are two obvious reduction peak in near -0.42 v and -0.78v, and also a oxidation peak in -0.42v, with the gradual increase of the aggregate number of laps. Reduction peaks and oxidation peaks in varying degrees are reduced, in- troducing the conductive properties declined with the polypyr- role increased,and last tend to stability,but the conductivity is also exist. 3.2. The Determination of the Test Potential In order to find out the potential of NADH in reactions, we used square wave voltammetry to test in PBS solution and sub- strate with fo r maldeh yde an d o ther acc esso r y facto rs. In Figure 2, there are two lines ,the line with one peak is the modified electrode in PBS solution,the other is in the substrate. Both of this lines have the same reduction peak in -0.6v, whic h is be- cause of PBS. The second line has two other peaks:-1.2v and -1.5v, in substrates with different concentration formaldehyde. The peak of -1.2v is changed, and the peak of -1.2v is stable, so we can see the peak can show the speed of production of NADH.The peak in -1.5v is not known of its mechanism. Figure 1. Electrochemical Polymerization of polypyrrole. 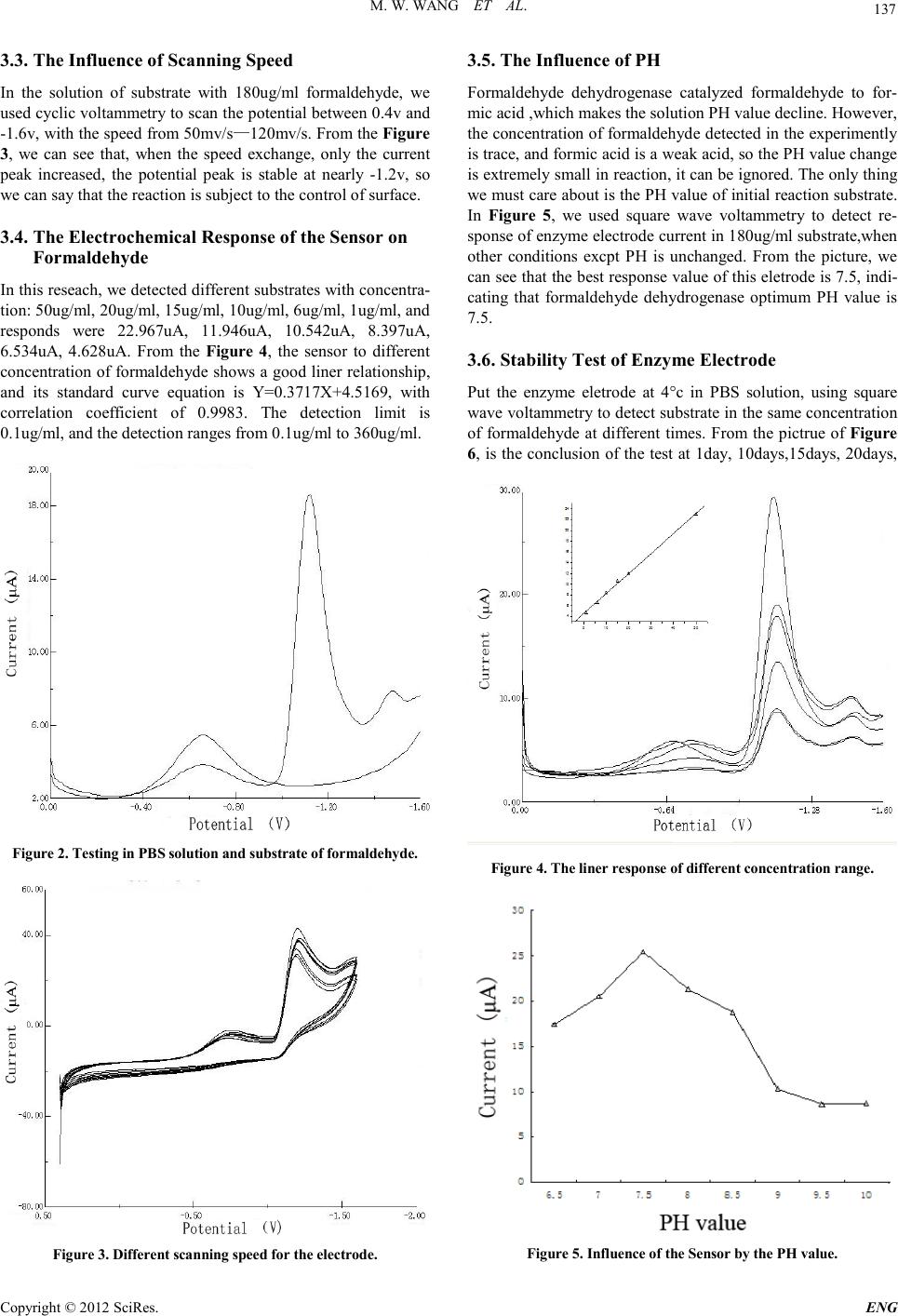 M. W. WANG ET AL. Copyright © 2012 SciRes. ENG 137 3.3. The Influence of Sc anning Spe e d In the solution of substrate with 180ug/ml formaldehyde, we used cyclic vol tammetr y to scan t he potential between 0.4v and -1.6v, with the speed from 50mv/s—120mv/s. From the Figure 3, we can see that, when the speed exchange, only the current peak increased, the potential peak is stable at nearly -1.2v, so we can sa y that the reaction is subj ect to the control of surface. 3.4. The Electrochemical Response of the Sensor on Formaldehyde In th is reseach, we detect ed different substrates with concentra- tion: 50ug/ml, 20ug/ml, 15ug/ml, 10ug/ml, 6ug/ml, 1ug/ml, and responds were 22.967uA, 11.946uA, 10.542uA, 8.397uA, 6.534uA, 4.628uA. From the Fig ure 4, the sensor to different concentration of formaldehyde shows a good liner relationship, and its standard curve equation is Y=0.3717X+4.5169, with correlation coefficient of 0.9983 . The detection limit is 0.1ug/ml, and the detection ranges from 0.1ug/ml to 360ug/ml. Figure 2. Testing in PBS solution and substrate of formaldehyde. Figure 3. Different scanning speed for the electrode. 3.5. The Influence of PH Formaldehyde dehydrogenase catalyzed formaldehyde to for- mic acid ,wh ich makes the solu tion PH value decline. However, the concen tration of for maldehyde det ected in the experi mentl y is trace, an d formic acid is a wea k acid , so th e P H valu e chan ge is extre mely small in reaction, it can be ignored . The only thing we must care about is the PH value of initial reaction substrate. In Figure 5, we used square wave voltammetry to detect re- sponse of enzyme electrode current in 180ug/ml substrate,when other conditions excpt PH is unchanged. From the picture, we can see th at the best respon se value of this eletr ode is 7.5, indi- cating that formaldehyde dehydrogenase optimum PH value is 7.5. 3.6. Stability Test of Enzyme Electrode Put the enzyme eletrode at 4°c in PBS solution, using square wave volt ammetry to d etect su bstrat e in th e same concent ratio n of formaldehyde at different times. From the pictrue of Figure 6, is the conclusion of the test at 1day, 10days,15days, 20days, Figure 4. The liner response of different concentration range. Figure 5. I nfluence of the Sensor by the PH value .  M. W. WANG ET AL. Copyright © 2012 SciRes. ENG 138 Figure 6. Testing at different times for stability. 25days, 30days, 40days, 45days, we can see that:the enzyme electrode at 1day, 10days, 15days is no obvious differece for testing the same substrate, but after 20days, the current is ob- vious reduced and has deviated from the standard curve, so the retention time of this electrode in 4°c environment is 15 days. 4. Conclusions Using polypyrrole as matrix, with the principle of electrostatic adsorption, we make the biosensor modified by enzyme for deteacting formaldehyde. In the biosensor,carbon nanotubes and formaldehyde dehydrogenase are immobilized on the sur- face of carbon eletrode, and nafion solution capped to prevent leakage of carbon nanotubes and enzyme. The sensor with for- maldehyde dehydrogenase modified is simple and easy ,with a good linear range, and also have practical value. However, there is some room for improvement. If we can fix NAD+ and glutathione with formaldehyde dehydrogenase on the surface of electrode, we don’t need to add them for test each time. The purity of enzyme solution is also need to improve, which may increase t he response range of the substr ate concentr ation. 5. Acknowledgements The authors would like to thank the Ministry of Science and Technology of the People’s Republic of China (2009GJA10047), Tianjin Municipal Science and Technology Commission (09ZCZDSFO4200), Tianjin Municipal Education Commission (SB20080035) and the Tianjin University of Science and Technology to support the work. REFERENCES [1] JIANG Zhongfa, YUAN Wenjun, ZHANG Benyan. Progress of study on the relationship between Indoor air formaldehyde Pollution and Leukemia[J] , Journal of Environment and Health, 2008,25(3): 276-278 (in Chinese). [2] ZHAI, Jinxia, ZHANG Chuanmu, ZHANG Peng. Acute effects of formaldehyde in anatomy laboratory on student health[J]. Journal of Environment and Health, 2008,25(2): 141-143 (in Chinese). [3] Yang J,Zhang B,et a1.Cloning and Expression of Pseudo- monas fluorescens 26-2 lipase gene in Pichla paaoris and cha- racterizing for transosterification.Appl Biochem Biotechnol, 2008,159(2):355-365. [4] Mitsubayashi K, NishioG, SawaiM, A bio-sniffer stick with FALDH (formaldehyde dehydrogenase)for convenient analysis of gaseous formaldehyde[J] . Sensors and ActuatorsB, 2008, 130( 1 ) : 32~ 37. [5] Boukerma K,Piquemal J Y,Chehimi M M,et al.Synthesis and interfacial properties of montmorillonite/polypyrrolenano- composites[J].Polym.,2006,47:569-576. [6] R en X Z,Zha o Q,Liu J H,et al.Prepa ration of polypyrrole nanop-articles in reverse micelle anditsapplication to glucose biosensor[J].Journal of Nan osc ienc e and Nanot echn olog y, doi:10.1166/jnn.2007(2):141. [7] Wang S G,Zhang Q,Wang R L,et al.A Novel multi-walled car- bonnanotube-based biosensor for glucose detection[J].Biochem BiophysRes Commun,2003,3 11(3):572 ~576. [8] ZHANG Shu-ping, ZHAO Yan, MAJie1, LIUXiao-hui, WANGMeng-fei, LIUWei. Study on performance of layer-by-layer self-assembled electrodein detection of thiocho- line[J].Modern Chemical Industry,2011,Jan,31(1):88~92 [9] William G.Gutheil, Elvin Kasimoglu, and Permila C.Nicholson. Induction of Glutathione-Dependent Formaldehyde Dehydroge- nase Activity in Escherichia coli and Hemophilus influenza[J]. Biochemical and biophysical research communications 238, 693--696 (1997) [10] William G.Gutheil,Barton Holmquist,and Bert L.Vallee. Purifi- cation, Characterization, and Partial Sequence of the Gluta- thione-Dependent Formaldehyde Dehydrogenase from Esche- richia coli: A Class I11 Alcohol[J]. Biochemistry 1992, 31, 475-481. [11] Shi yintao,Yuan ruo,Wang na, The biosensor based on polythio- nine Nano Au and horseradish peroxidase modified for detecting Hydrogen peroxide[J], Southwest China Normal Universi- ty,2006,31,3. [12] hou guoqing, The biosensor based on polyvinylpyrrolidone/Nano Au and horseradish peroxidase modified for detecting Hydrogen peroxide[J], S outhwest China Normal University,2010,36,4. [13] M. Ben Ali, M. Gonchar, G. Gayda, S. Paryzhak, M.A. Maaref, N. Jaffrezic-R enault , Y. Korp an . Forma ldehyde-sensitive sensor based on recombinant formaldehydedehydrogenase using capa- cit anc e versu s volt age mea su remen ts[ J] . Biosen sor s and Bioelec- tronics 22 (2007) 2790–2795 [14] Kohji Mitsubayashi, Genki Nishio, Masayuki Sawai, Elito Ka- zawa . A biochemical sniffer-chip f or conveni ent analysis of ga- seousformaldehyde from timber materials[J]. Microchim Acta (2008) 160: 427–433. [15] Lilach Bareket, Ada Rephaeli, Gili Berkovitch, Abraham Nu- delman, Judith Rishpon. Carbon nanotubes based electrochemi- cal biosen sor for detec tion of formaldeh yde rel eased from a ca n- cer cell line treated with formaldehyde-releasing anticancer pro- drugs[J]. Bioelectrochemistry 77 (2010) 94–99. [16] Yar oslav I. Korpan & Olexand r O. Soldatki n & Olga F. Sosovs- ka & Halyna M. Klepach . Formaldehyde-sensiti ve conductome- tric sensors b ased on commerc ial an d recombi nant formaldehyde dehydrogenase[J]. M ic r o chim Acta (2 01 0) 170:33 7–344. |

