Paper Menu >>
Journal Menu >>
 Engineering, 2012, 5, 129-134 doi:10.4236/eng.2012.410B034 Published Online October 2012 (http://www.SciRP.org/journal/eng) Copyright © 2012 SciRes. ENG Fast Determination of Vitamin B2 Based on Molecularly Imprinted Electrochemical Sensor Peidong X u, C hangsheng Qia o, Shufeng Y ang, Lijuan Liu, Mingwei Wang and Jua nkun Zhang* Key Laboratory of Industrial Microbiology, Ministry of Education, College of Bioengineering, Tianjin Key Laborat ory of Indust ry Microbi ology, C ollege of Bioengi neerin g, Tianjin University of Sci ence & Techn ology, TUST, Tianjin, P.R.China Email: zhangjk@tust.edu.cn Received 2012 ABSTRACT Under the condition of weak acidity of pH 5.2, a sensitive vitamin B2 electrochemical sensor based on molecularly imprinted non- conducting polymer of o-aminophenol by potentiostatic polymerization in the presence of template(vitamin B2) on a glassy carbon electrod e was p rep ared , an d i ts p erfor mance was studied. The sensor exhibited good sensitivity and selectivity to VB2. The detection limit went down to 2.3851nM, and a linear relationship between the current incremental and the concentration was found in the range of 10~120nM. And the sensor could use in detection of VB2 real sample for a long time and show good reproducibility. The aver- age recovery rate to VB2 was 98.41%. Keywords: Vitamin B2; Molecularly Imprinted Technique; Potentiostatic Polymerization; O-aminophenol; Drug Determination 1. Introduction Vitamin B2(VB2), also named riboflavin(RF), is B-group wa- ter-soluble vitamin. Its structure[1] is exhibited in Figure 1. VB2 is stable in acidic aqueous solution and its isoelectric point(PI) is 6. Human beings as well as all animals requires a constant supply of VB2 for the optimal maintenance of large number of metabolic processes requiring the flavin coenzymes, such as flavin mononucleotide(FMN) and flavin adenine di- nucletide(FAD)[2]. FMN and FAD are important cofactor of dehydrogenase in vivo. But VB2 can not be synthesized in vivo, only be obtained from vegetables, fruit, health care food and drug etc. What is worse, man and all animals have little ability to store VB2 and excess VB2 will be urinated out of body. Hence, VB2 should be intake and supplemented in time to avoid shortage. Recently, many counterfeit vitamin drugs (in- cluding veterinary medicine) that mainly consist of not enough quantity frequently appear on market. Thus, determination of VB2 is very significant in monitoring the amount of VB2 in vitamin drugs. Figure 1. Chemical structure of VB2. Currently, different methods and techniques have been used to determine concentration of VB2, including chemilumines- cence[ 3-4], chromatography[5-7], voltammetry[8], fluor escence [9], flame atomic absorption spectrometry[10], and combina- tion of these methods[11-13]. But these methods are not only sophisticated but nonspecific, worse still, some expensive and broad-scale equipments are needed. Molecularly imprinted electrochemical sensor, however, based on “molecular memo- ry”, combining the advantages of molecularly imprinted me- thod(MIM) with electrochemical measurement technique, but has not been reported to used in determination of VB2 yet. The synthesis of molecularly imprinted polymers(MIPs) and artificial recognition sites are key and core content in MIM. They are establ ished by chemical and electro chemical meth ods. These sites tailor-formed in situ by copolymerization of func- tional monomers and cross-linkers around the template. The print molecules are subsequently removed from the polymer, living accessi ble complementary binding cavity[14]. According to the interaction between template and polymer, MIM can be divided into covalent imprinting and noncovalent imprinting. Noncovalent interaction consists of hydrogen bond, electrostat- ic force, metal ion coordination interaction, Van der Waals force etc. Selection of functional monomer plays an essential role in preparing sensitive film in MIM. O-aminophenol(oAP), with important functional groups of –OH and –NH2, has been used to prepare kinds of sensitive films of biosensor[15]. In contrast to conducting polymers(polyaniline, polypyrrole, poly(3, 4-ethylenedioxythiophene) etc.), the electrosynthesis of non- conducting polymers, take PoAP as an example, is self-limited and inevitably ceased when the polymer film on the electrode grows thick enough to resist electron transfer between the elec- trode and monomer molecules[16]. Moreover, electrochemical polymerization is very easy to achieve i n electrolyte mixed with  P. D. XU ET AL. Copyright © 2012 SciRes. ENG 130 functional monomer and template. The thickness of films could be controlled precisely by adusting current density and goes down to nanometers scale[17]. PoAP film was selected in this study because of its relative cheapness, easy availability and preparation. Therefore, in this paper, VB2 molecularly imprinted electro- chemical sensor was prepared through electropolymerizaton of oAP with VB2 under pH 5.2. Meanwhile, the effects of percen- tage of oAP on polymerization were investigated by UV-vis spectrophotometry, and elution time of template, performance of this sensor, etc. were also investigated in detail. 2. Experimenta Materials and Methods 1) Chemicals and reagents O-aminophenol(oAP) was from Tianjin recovers the fine chemical research institute after appropriate recrystallization wherever necessary. Perchloric acid was purchased from Alad- din(Shanghai, China). Riboflavin(RF or VB2), ascorbic ac- id(VC), vitamin B6(VB6) came from Tianjin Ruijinte Chemical Co., Ltd. Flavin mononucleotide(FMN) was from Beijing dingguo changsheng biotech Co. Ltd. Acetic acid, sodium hy- droxide, potassium hexacyanoferrate, sodium phosphate dib asic dodecahydrate, sodium dihydrogen phosphate, sulfuric acid and other reagents were of analytical reagent grade. All solutions were prepared with deionized water(conductivity≤0.06μS/cm). Phosphate buffer solution(PBS) with various pH values and concentration were prepared by mixing standard stock solutions of NaH2PO4 and Na2HPO4 and adjusting the pH with NaOH or HCl. Acetate buffer solution(HAc-NaHAc, pH 5.0) was made by mixing HAc and NaOH. 2) Apparatus All electrochemical measurements were performed on LK2005A Electrochemistry Workstation (LANLIKE, Tianjin, China). A standard tree-electrode system, a glassy carbon(GC) electrode(3 mm in diameter) or its modified electrode, a Ag/AgCl with saturated KCl solution and a Pt001 disk elec- trode(2 mm in diameter) were used as working electrode, ref- erence electrode and counter electrode, respectively. TU-1810 UV-Vis Spectrofluorimeter(PERSEE, Beijing, China), FA2004A Electronic Analytical Balance(JIN GTI AN, Sh angh ai, China), SB3200DT Ultrasonic Cleaning Machine(SCIENTZ, Ningbo, China) and PHS3BW pH meter(LIDA, Shanghai, China) were utilized in this study. 3) Pretreatment of glassy carbon electrode Prior to modification, GC electrode was polished with 0.1μm and 0.05μm alumina slurry successively. After rinsed with deionized water, the electrode was treated in Piranha solu- tion(30% H2O2 mixed with H2SO4 at the volume rate of 3 to 7 ) for 15min, followed by rinsing and sonication in 1:1(V/V) HNO3 aqueous solution, 10%(W/V) NaOH solution, ethanol and acetone for 10min, respectively. Repeated above process triplicately. And then the three-electrode system was put into a single-compartment cell with 0.2M H2SO4 solution, cyclic scaning from potential of -0. 5 to 1.5V several cycles at th e rate of 0.1V/s to achieve steady state for activation. 4) Preparation of VB2 molecularly imprinted polymers modified electrode[18] VB2 molecularly imprinted polymers modified elec- trode(VB2-/PoAP/GCE) was prepared by traditional tree-electrode system. A oAP stock solution(0.1M) was pre- pared by dissolving the oAP in 0.5M HClO4 solution. A VB2 stock solution(1mM) was prepared by dissolving the VB2 in a 1%(V/V) acetic acid aqueous solution at refrigerator(4℃). A 10mL volume of polymerization solution was prepared by mixing 8 mL of oAP and 2 mL of VB2 and adjusting the pH to 5.2 with 1M NaOH solution, and then standing in dark place for 20min to prereaction. After that, three electrodes was in- serted into this mixed solution. Polymerization was performed by potentiostatic polymerization technology. The parameters were set as follows: potential 0.65V, time 1200s, sampling time interval 0.1125s. After polymerization, the modified electrod was rinsed by deio nized water and immersed in 0.8M H2SO4 for 8h to remove template molecules. Nonmolecularly im- printed modified electrode(PoAP/GCE) was prepared by the same proced u re in th e ab sence o f V B2 , 2mL of 1%(V / V ) aceti c acid in stead , for co mpariso n. When not in use th e elect rode was stored in 0.01M pH 7.0 PBS at room temperature(RT). 5) Electrochem ical measurements All electrochemical measurements were performed in 10mL single-compartment cell with 4mM K3[Fe(CN)6] as electro- chemical probe at RT and potential range from -0.1V to 0.6V. All parameters of cyclic voltammetry(CV) were scan rates of 0.05V/s. All parameters of di fferenti al pulse vo ltammetry(DP V) were potential incremental of 0.004V, pulse amplitude of 0.05V, pulse width of 0.06V. All parameters of square wave voltam- metry(SWV) were potential incremental of 0.004V, square-wave frequency of 15Hz, square-wave amplitude of 0.025V. All potentials refered to Ag/AgCl with saturated KCl solution. The tested solutions were deoxygenated by high pure nitrogen bubbling for 20min just before experiments and the nitrogen atmosphere was kept over the cell during all mea- surement s . 3. Results and Discussion 3.1. Current-time curve of forming VB2 imprinted membrane electrode Figure 2 i s Current-time(I -t) curve of copolymerization of oAP and VB 2 on GC electrode. As shown in Figure 2, at the begin- nin g of electrochemical polymeri zation (0s) the value o f current decreases sharply from 80μA which indicates the fast formation of nonconducting polymer. As increasing of electrolysis time the current decreases gradually slow, and then down to a plat- form. Finally the current decreases close to zero. This is the result of electrochemical oxidation of oAP to form PoAP on GC electrode, which is demonstrated that a layer of dense po- lymer membrane blocking electrons transferring from bulk solu tion to sur face of electrod e is formed and VB2 molecu lar is freezed in PoAP at the same time. Thi s thanks to “self-limited” electrodeposition process of nonconductive PoAP film[16] 3.2. Selection of Polymerization Time Here three modified electrode were prepared in 600s, 1200s and 1800s, respectively. CV was performed in PBS with 4mM 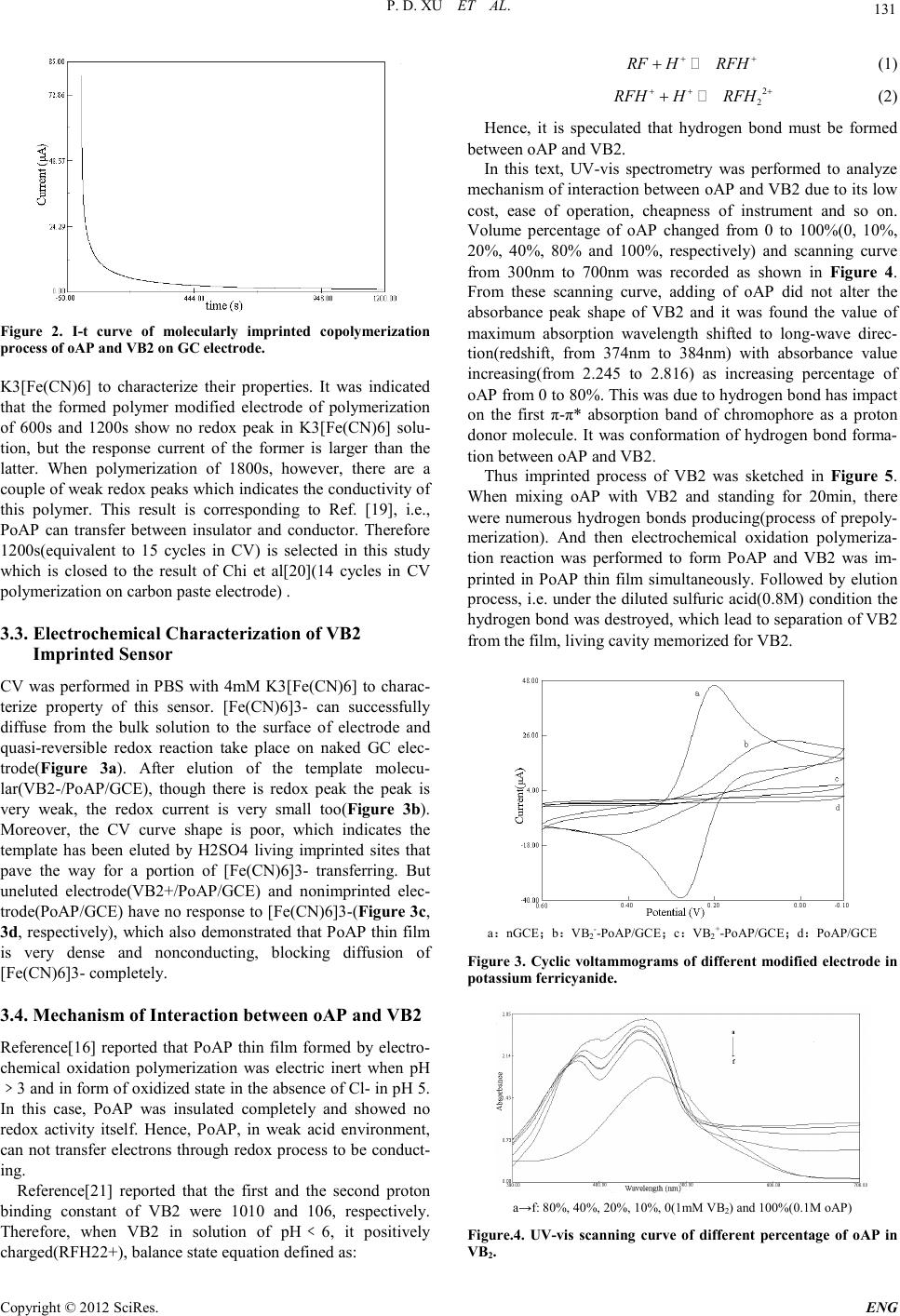 P. D. XU ET AL. Copyright © 2012 SciRes. E NG 131 Figure 2. I-t curve of molecularly imprinted copolymerization proc es s o f oA P an d VB2 on GC electr od e. K3[Fe(CN)6] to characterize their properties. It was indicated that the formed polymer modified electrode of polymerization of 600s and 1200s show no redox peak in K3[Fe(CN)6] solu- tion, but the response current of the former is larger than the latter. When polymerization of 1800s, however, there are a couple of weak redox peaks which indicates the conductivity of this polymer. This result is corresponding to Ref. [19], i.e., PoAP can transfer between insulator and conductor. Therefore 1200s(equivalent to 15 cycles in CV) is selected in this study which is closed to the result of Chi et al[20](14 cycles in CV pol ymerizati on on carbon paste electrode) . 3.3. Electrochemical Characterization of VB2 Imprinted Se nsor CV was performed in PBS with 4mM K3[Fe(CN)6] to charac- terize property of this sensor. [Fe(CN)6]3- can successfully diffuse from the bulk solution to the surface of electrode and quasi-reversible redox reaction take place on naked GC elec- trode(Fig ure 3a). After elution of the template molecu- lar(VB2-/PoAP/GCE), though there is redox peak the peak is very weak, the redox current is very small too(Fig ure 3b). Moreover, the CV curve shape is poor, which indicates the template has been eluted by H2SO4 living imprinted sites that pave the way for a portion of [Fe(CN)6]3- transferring. But uneluted electrode(VB2+/PoAP/GCE) and nonimprinted elec- trode(PoAP/GCE) have no response to [Fe(CN)6]3-(Figure 3c, 3d, respectively), which also demonstrated that PoAP thin film is very dense and nonconducting, blocking diffusion of [Fe(CN)6]3- compl etely. 3.4. Mechanism of Interaction between o A P and VB2 Reference[16] reported that PoAP thin film formed by electro- chemical oxidation polymerization was electric inert when pH ﹥3 and in form of oxidized state in the absence of Cl- in pH 5. In this case, PoAP was insulated completely and showed no redox activity itself. Hence, PoAP, in weak acid environment, can not transfer electrons through redox process to be conduct- ing. Reference[21] reported that the first and the second proton binding constant of VB2 were 1010 and 106, respectively. Therefore, when VB2 in solution of pH﹤6, it positively charged(RFH22+), b alance state equation defined as : RF HRFH ++ + (1) 2 2 RFH HRFH ++ + + (2) Hence, it is speculated that hydrogen bond must be formed between oAP and VB2. In this text, UV-vis spectrometry was performed to analyze mechanism of interaction between oAP and VB2 due to its low cost, ease of operation, cheapness of instrument and so on. Volume percentage of oAP changed from 0 to 100%(0, 10%, 20%, 40%, 80% and 100%, respectively) and scanning curve from 300nm to 700nm was recorded as shown in Figure 4. From these scanning curve, adding of oAP did not alter the absorbance peak shape of VB2 and it was found the value of maximum absorption wavelength shifted to long-wave direc- tion(redshift, from 374nm to 384nm) with absorbance value increasing(from 2.245 to 2.816) as increasing percentage of oAP from 0 to 80%. This was due to hydrogen bond has impact on the first π-π* absorption band of chromophore as a proton donor molecule. It was conformation of hydrogen bond forma- tion between oAP and VB2. Thus imprinted process of VB2 was sketched in Figure 5. When mixing oAP with VB2 and standing for 20min, there were numerous hydrogen bonds producing(process of prepoly- merization). And then electrochemical oxidation polymeriza- tion reaction was performed to form PoAP and VB2 was im- printed in PoAP thin film simultaneously. Followed by elution process, i.e. under the diluted sulfuric acid(0.8M) condition the hydrogen bond was destroyed, which lead to separation of VB2 from the film, living cavity memorized for VB2. a:nGCE;b:VB2--PoAP/GCE;c:VB2+-PoAP/GCE;d:PoAP/GCE Figure 3. Cyclic voltammograms of different modified electrode in potassium ferricyanide. a→f: 80%, 40%, 20%, 10%, 0(1mM VB2) and 100%(0.1M oAP) Figure.4. UV-vis scanning curve of different percentage of oAP in VB2.  P. D. XU ET AL. Copyright © 2012 SciRes. ENG 132 3.5. Selection of Elution Time Diluted strong acid solution is a powerful tool to destroy the hydrogen bond. 0.8M H2SO4 was used in this study and its elution kinetics was also studied. It was indicated that the re- duced peak current incremental value(ΔI) increased with dilu- tion time at the range from 0 to 80min. Up to 140min ΔI achieved a platform. After elution of 480min(8h) ΔI was almost unch anged which in dicated elution came to equilib rium. Hence 8h of elution was selected in this study. 3.6. Eval uat ion of the Imprinting Effe ct PoAP/GCE and VB2+-PoAP/GCE had no response to target molecule(VB2) in 0.2M pH 5.0 HAc-NaHAc with 1mM VB2 and 4mM K3[Fe(CN)6](Figure 5d, 5e). VB2--PoAP/GCE had good response to VB2 and the cathode peak current to target molecule decreased from 14.183μA to 12.786μA (Figure 6b, 6c). Compared to nGCE(Figure 6a) respons e current decreased significantly which demonstrated VB2 molecule binded to im- printed cavity by hydrogen bond and closed the empty site, blocking [Fe(CN)6]3- diffused from bulk solution to the surface of electrode. Thus concentration of [Fe(CN)6]3- on the surface of electrode decreased significantly, leading to reduced current decreased signific antly. PP: Prepolymerization; ECP: Electrochemical Polymerization; E: Elution Figure 5. Schematic illustra tion of imprinted process of VB2 o n GC electrode. a: nGCE; b: before rebinding of VB2; c: after rebinding of VB2 d: PoAP/GCE;e: VB 2+-PoAP/GCE Figure 6. DPV curve of different modified electrode in potassium ferricyanide with VB2. 3.7. Drawing of Calibration Curve of VB2 Rebinding SWV with high sensitivity was performed to draw calibration curve of VB2 rebinding as shown in Figure 7. The curve showed nonlinear overall from 20nM to 80μM, and the curve tended to steady at 80μM with increasing concentration of VB2, which indicated there was saturation effect of the sensor res- ponding to VB2. To obtain good linear relationship between the concentration and ΔI, low VB2 concentration was selected to do rebianding test again. The result was shown in Figure 8. The linear response range of this imprinted sensor to VB2 concen- tration was from 10nM to 120nM with a correlation coefficient of 0.9989(n=7). The linear regression equation was ΔI=-0.03381[VB2]+15.76 94. The detect ion limit was calcu lated according to equation[22] as follows: 3SD LOD B = (3) where SD and B were standard deviation and slope of linear regression equ ation, r es pectively. LOD of VB2 was 2.3851nM. VB2 concentration from 20nM to 80μM(20nM, 40nM, 60nM, 80nM, 20μM, 40μM, 60μM and 80μM, respectively ) Figure 7 Relationship between VB2 concentration and current of cathode. VB2 concentration from 10nM to 120nM(10nM, 20nM, 40nM, 60nM, 100nM, 110nM, and 120nM, respectively ). Inset: SWV testing curve of dif ferent concentration of VB2 Figure 8. Calibration curve of VB2 rebi nding to imprinted sensor. 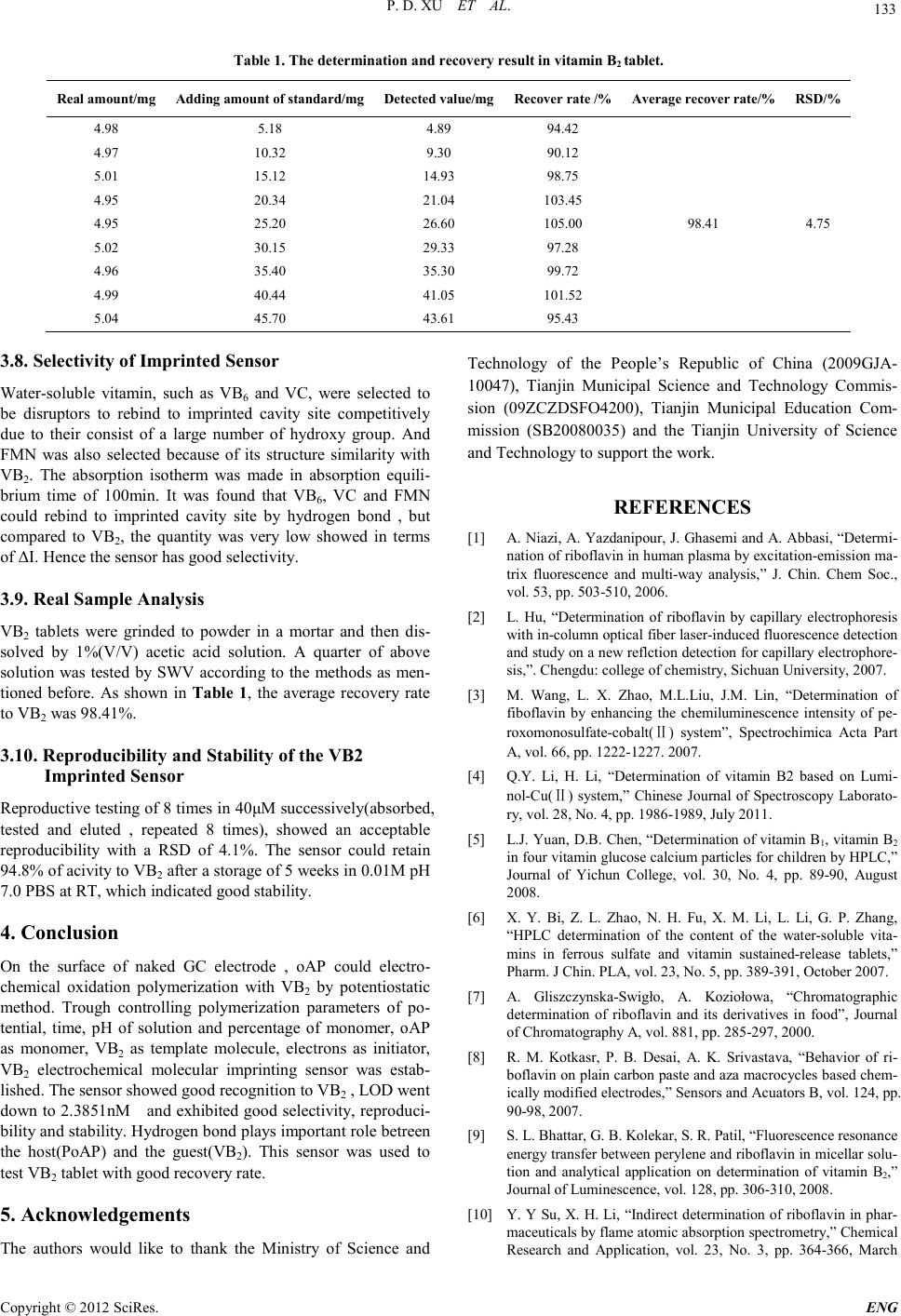 P. D. XU ET AL. Copyright © 2012 SciRes. E NG 133 Table 1. The determination and recovery result in vitamin B2 tablet. Real amount/mg Adding a mount o f st an dard/mg Detected valu e/mg Re cover rat e /% Average recover ra te/ % RSD/% 4.98 5.18 4 .89 94.42 4.97 10.32 9.30 90.12 5.01 15.12 14.93 98.75 4.95 20.34 21.04 103.45 4.95 25.20 26.60 105.00 98.41 4.75 5.02 30.15 29.33 97.28 4.96 35.40 35.30 99.72 4.99 40.44 41.05 101.52 5.04 45.70 43.61 95.43 3.8. Selectivity of Imprinted Se nsor Water-soluble vitamin, such as VB6 and VC, were selected to be disruptors to rebind to imprinted cavity site competitively due to their consist of a large number of hydroxy group. And FMN was also selected because of its structure similarity with VB2. The absorption isotherm was made in absorption equili- brium time of 100min. It was found that VB6, VC and FMN could rebind to imprinted cavity site by hydrogen bond , but compared to VB2, the quantity was very low showed in terms of ΔI. Hence the sensor has good selectivity. 3.9. Real Sample Analysis VB2 tablets were grinded to powder in a mortar and then dis- solved by 1%(V/V) acetic acid solution. A quarter of above solution was tested by SWV according to the methods as men- tioned before. As shown in Table 1, the average recovery rate to VB2 was 98.41%. 3.10. Reproducibility and St ability of the V B2 Imprinted Se nsor Reproductive testing of 8 times in 40μM successively(absorbed, tested and eluted , repeated 8 times), showed an acceptable reproducibility with a RSD of 4.1%. The sensor could retain 94.8% of acivity to VB2 after a storage of 5 weeks i n 0. 01M pH 7.0 PBS at RT, which indicated good stability. 4. Conclusion On the surface of naked GC electrode , oAP could electro- chemical oxidation polymerization with VB2 by potentiostatic method. Trough controlling polymerization parameters of po- tential, time, pH of solution and percentage of monomer, oAP as monomer, VB2 as template molecule, electrons as initiator, VB2 electrochemical molecular imprinting sensor was estab- lished. The sensor showed good recognition to VB2 , LOD went down to 2.3851nM and exhibited good selectivity, reproduci- bility and stability. Hydrogen bond plays important role betreen the host(PoAP) and the guest(VB2). This sensor was used to test VB2 tablet with good recover y rate. 5. Acknowledgements The authors would like to thank the Ministry of Science and Technology of the People’s Republic of China (2009GJA- 10047), Tianjin Municipal Science and Technology Commis- sion (09ZCZDSFO4200), Tianjin Municipal Education Com- mission (SB20080035) and the Tianjin University of Science and Technology to support the work. REFERENCES [1] A. Ni azi, A. Ya zdanip our, J. Gh asemi and A. Abbas i, “Determ i- nati on of ribofla vin in hum an plasma by excitat ion-emis sion ma- trix fluorescence and multi-way analysis,” J. Chin. Chem Soc., vol. 53, pp. 503-510, 2006. [2] L. Hu, “Determination of riboflavin by capillary electrophoresis with in-column optic al fib er laser-i ndu ced flu oresc enc e det ecti on and study on a new reflction detection for capillary electrophore- sis,”. Chen gdu: college of chemis try, Sic huan University, 2007. [3] M. Wang, L. X. Zhao, M.L.Liu, J.M. Lin, “Determination of fiboflavin by enhancing the chemiluminescence intensity of pe- roxomonosulfate-cobalt(Ⅱ) system”, Spectrochimica Acta Part A, vol. 66, pp. 1222-1227. 2007. [4] Q.Y. Li, H. Li, “Determination of vitamin B2 based on Lumi- nol-Cu(Ⅱ) system,” Chinese Journal of Spectroscopy Laborato- ry, vol. 2 8, No. 4, pp. 1986-1989, July 2011. [5] L.J. Yuan, D.B. Chen, “Determination of vitamin B1, vita min B2 in four vitamin glucose calcium particles for children by HPLC,” Journal of Yichun College, vol. 30, No. 4, pp. 89-90, August 2008. [6] X. Y. Bi, Z. L. Zhao, N. H. Fu, X. M. Li, L. Li, G. P. Zhang, “HPLC determination of the content of the water-soluble vita- mins in ferrous sulfate and vitamin sustained-release tablets,” Pharm. J Chin. PLA, vol. 23, No. 5, pp. 389-391, October 2007. [7] A. Gliszczynska-Swigło, A. Koziołowa, “Chromatographic determination of riboflavin and its derivatives in food”, Journal of Chrom atograp hy A, vol. 881, pp. 285-297, 2000. [8] R. M. Kotkasr, P. B. Desai, A. K. Srivastava, “Behavior of ri- boflavin on p lain carbon paste and aza macroc ycles based chem- ically modi fied electrod es ,” Sensors and Acuators B, vol. 124, pp. 90-98, 2007. [9] S. L. Bhattar, G. B. Kolekar, S. R. Patil, “Fluorescence resonance energ y tra nsf er bet ween p er ylene a nd r ib oflavi n in m icellar s olu- tion and analytical application on determination of vitamin B2,” Journ al of Lumin escence, vol. 128, pp. 306-310, 2008. [10] Y. Y Su, X. H. Li, “Indirect determination of riboflavin in phar- maceuticals by flame atomic absorption spectrometry,” Chemical Research and Application, vol. 23, No. 3, pp. 364-366, March  P. D. XU ET AL. Copyright © 2012 SciRes. ENG 134 2011. [11] A. K. Su, C. H. Lin, “Determination of riboflavin in urine by capillary electrophoresis-blue light emitting diode-induced fluo- rescence detection combined with a stacking technique,” Journal of Chrom atograp hy B, vol. 785, pp. 39-46, 2003. [12] M. Z. Ángel Ríos, “Supercritical fluid extraction as on-line clean-up technique for determination of riboflavin vitamins in food samples by capillary electrophoresis with fluorimetric de- tection,” Electrophoresis, vol. 29, pp. 3213-32 19, 2008. [13] M. Aranda, G. Morlock, “Simultaneous determination of ribofla- vin, pyridoxine, nicotinamide, caffeine and taurine in energy drinks by planar chromatography-multiple detection with con- firmation by elect rospray ionization mass spectrometry,” Journal of Chrom atograp hy A, vol. 1131, pp. 253-260, 2006. [14] S. Pradhan, M. Boopathi, O. Kumar, A. Baghel, P. Pandey, T.H. Mahato, B. Singh, R. Vijayaraghavan, “Molecularly imprinted nanopatterns for the recognition of biological warfare agent ri- cin, ” Biosensors and Bi oelectronics, vol. 25, pp. 592-598, 2009. [15] D. W. Pan, J. H. Chen, S. Z. Yao, W. Y. Tao, and L. H. Nie, “An amperometric glucose biosensor based on glucose oxidase im- mobilized in electropolymerized poly(o-aminophenol) and car- bon nanotubes composite film on a gold electrod,” Analytical Science, vol. 21, pp. 367-371, April 2005. [16] D. Hu, C. Peng, and G.Z. Chen, “Electrodeposition of noncon- ducting polymers: roles of carbon nanotubes in the process and products,” ACS NANO, vol. 4, No. 7, pp. 4274-4282. 2010. [17] Y.Q. Miao, J.R. Chen, X.H. Wu, “Using electropolymerized non-conducting polymers to develop enzyme amperometric bio- sensors,” Trends in Biotechnology, vol. 22, issue. 5, pp. 227-231, May 2004. [18] W. A. Wu, Z. F. Yin, J. R. Wei, M. D. Gu o, “A sensi tiv e mitox- antron e sens or b ased on molecu la rly impr int ed electrop ol ymer o f o-aminophenol,” Journal of Analytical Science, vol. 24, No. 6, pp. 681-684, December 2008. |

