Paper Menu >>
Journal Menu >>
 Engineering, 2012, 5, 110-113 doi:10.4236/eng.2012.410B028 Published Online October 2012 (http://www.SciRP.org/journal/eng) Copyright © 2012 SciRes. ENG Does Making Method of Alginate Hydrogel Influence the Chondrogenic Differ entiation of Human Mesenchymal Stem Cells? Jessica Schiavi1, Naceur Charif2, Natalia de Isla2, D anièle Bensoussan2, Jean-François Stoltz2, Nadia Benkirane-Jessel1, Céline Husel st e in2 1INSERM-UMR, Faculty of medicine, Strasbourg University, Strasbourg, France 2Université de Lorraine, CNRS-UMR– Campus Santé – Biopôle, Faculty of medicine, Lorraine University, Vandœuvre-lès-Nancy, France Email: Celine.Huselstein@univ-lorraine.fr Received 2012 ABSTRACT To overco me cartila ge inju ry, strat egies have b een d eveloped in the last fe w years b ased on tissu e engineerin g to rebu ild the defects. Cartilage en gin eerin g is pri ncip ally based o n three main b iolo gical facto rs: cells (n ative cell s (cho ndrocytes) or a more primitive ones as mesenchymal stem cells), scaffolds and functionalization factors (growth factors, mechanical stimulation and/or hypoxia). Carti- lage tissue engineering strategies generally result in homogeneous tissue structures with little resemblance to native zonal o rganiza- tion of articular cartilage. The main objective of our work concerns the buildup of complex biomaterials aimed at reconstructing bio- logical tissue with three dimensional cells construction for mimicking cartilage architecture. Ou r strategy is based on structures for- mation by simple and progressive spraying of mixed alginate hydrogel and human mesenchymal stem cells (hMSC). In this work, the comportment of cells and more precisely their chondrogenic differentiation potential is compared to a traditional making process: the mold. We report here that spraying method allowed to product a scaffold with hMSC that confer a favorable environment for neocar- tilage construction. Keywords: Component: Cartilage Tissu e Engin eering; So dium Alginate; Sprayed method; Molded Method 1. Introduction Healthy cartilage is a complex tissue with well-defined func- tions allowing principally harmonious movements of the arti- culations over the whole life. Acute or repetitive blunt trauma and excessive impact loading can cause irreversible alterations in articular cartilage matrix. The lack of blood vessels and the inability of chondrocytes (cartilage cells) to repair significantly tissue defects limit the response of cartilage injury. Tissue en- ginee ring aims at repairing or replacing damaged or diseased tissue which present a limited self-repair cap acit y like carti lag e. Cells, scaffold materials or both form the basis of this approach, [tritz1, 2]. For cartilage tissue engineering, the use of mature cell types such as chondrocytes is associated with several drawbacks. The limited availability, donor site morbidity, de- differentiation and limited proliferative capacity urged re- searchers to study other cell types, particularly mesenchymal stem cells, [3]. Hydrogels provide advantages as their capacity of shaping, supporting chondrocytic phenotype, transducing mechanical loads to cells and their biodegradability, [4, 5]. Alginate hydrogel can be composed with different proteins, GAG (hyaluronic acid), natural or synthetic polymer to mimic tissue composition, have specific mechanical properties or in- ducing differentiation, [6]. Whereas, most studies using these scaffolds obtain homogenous structure without zonal variation as cartilage in vivo, [7, 8]. Recently, investigations have consi- dered cartilage organization in order to create stratified tissue engineering which attempts a layered design, [5, 9-13]. How- ever, up to now, few methodologies efficiently synthesizing stratified structures including viable specific cells interacting with a biofunctionalized environment has been proposed. An easily applicable technique that is able to deposit multiple cell types in 3-D without overt cellular damage is probably cell spraying [14]. The goal of the present work is based on structures formation by simple and progressive spraying of mixed alginate hydrogel and human mesenchymal stem cells (hMSC) and to compare their chondrogenic differentiation potential to a traditional making process: the mold. The differentiation potential of hu- man mesench ymal stem cells was characterized parti cularly for their interest for cartilage engineering. 2. Materials and Methods 2.1. Extraction and Culture of Mesenc hymal Ste m Cells Mesenchymal Stem Cells from human donors were isolated from bone marrow obtained from total hip or knee replacement surgery. Bone marrow was aspirated and diluted in HBSS. Then, mononuclear cells were counted and the suspension was seeded at 50 000 mononuclear cells/cm2 with co mplete medium (Dulbecco's Modified Eagles Medium low glucose supple- mented with 1 0% F etal Bovin e Serum ( Gib co, Fran ce), P enicil- lin at 100 U/mL /Streptomycin at 100 μg/mL (P/S), Amphoteri- cin B (Amp) at 2.5 μg/mL, 2 mM of Glutamine, and 1 ng/mL of FGF). hMSC were cultivated up to confluence, passage 0 (P0), 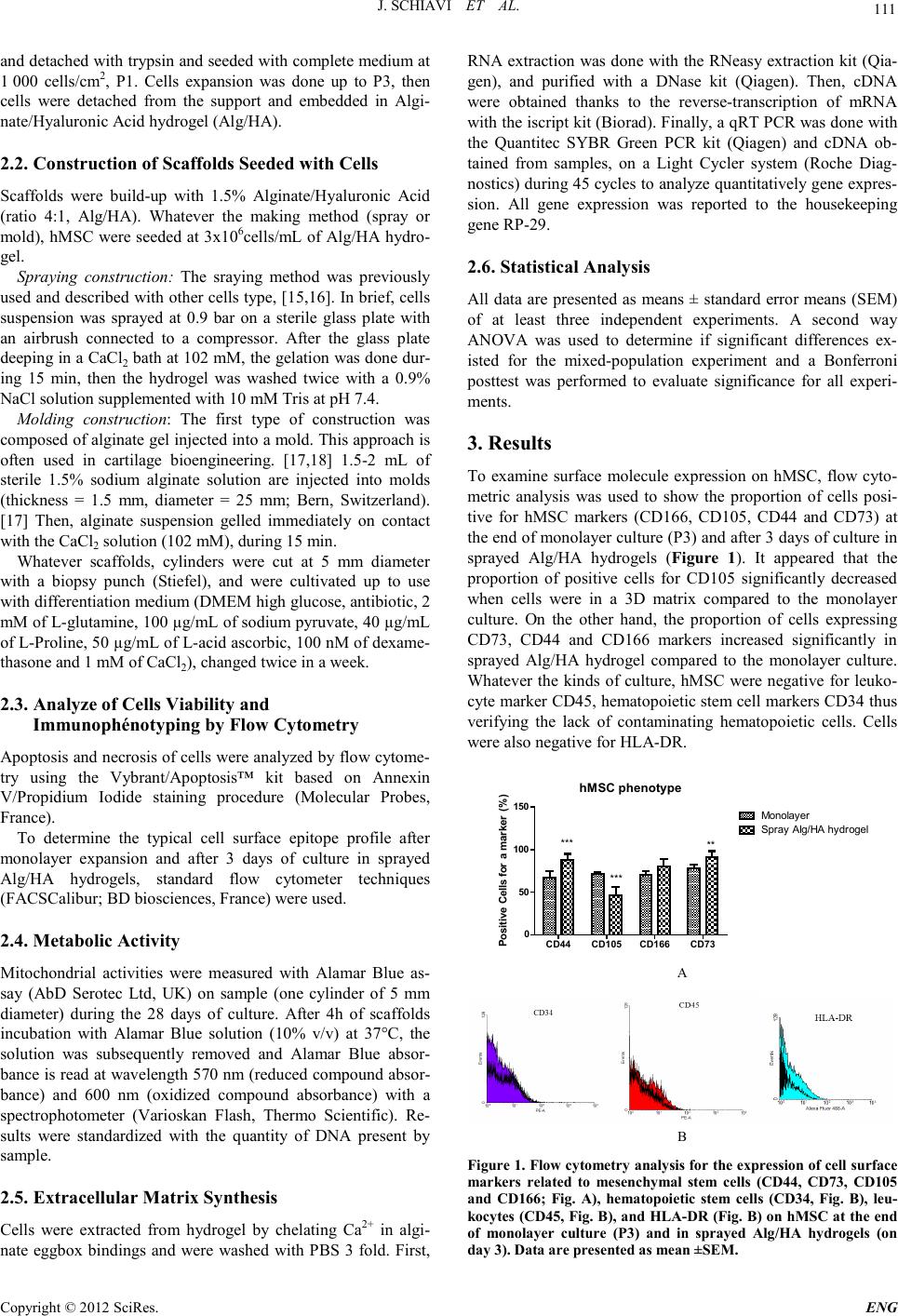 J. SC H IAVI ET AL. Copyright © 2012 SciRes. E NG 111 and detached with trypsin and seeded with complete mediu m at 1 000 cells/cm2, P1. Cells expansion was done up to P3, then cells were detached from the support and embedded in Algi- nate/Hyaluronic Acid hydrogel (Alg/HA). 2.2. Construction of Scaf folds Seeded with Cells Scaffolds were build-up with 1.5% Alginate/Hyaluronic Acid (ratio 4:1, Alg/HA). Whatever the making method (spray or mold), hMSC were seeded at 3x106cells/mL of Alg/HA hydro- gel. Spraying construction: The sraying method was previously used and described with other cells type, [15,16]. In brief, cel ls suspension was sprayed at 0.9 bar on a sterile glass plate with an airbrush connected to a compressor. After the glass plate deepi ng in a CaCl2 bath at 102 mM, the gelation was done dur- ing 15 min, then the hydrogel was washed twice with a 0.9% NaCl solution supplemented with 10 mM Tris at pH 7.4. Molding construction: The first type of construction was composed of alginate gel injected into a mold. This approach is often used in cartilage bioengineering. [17,18] 1.5-2 mL of sterile 1.5% sodium alginate solution are injected into molds (thickness = 1.5 mm, diameter = 25 mm; Bern, Switzerland). [17] Then, alginate suspension gelled immediately on contact with the CaCl2 solution (102 mM), during 15 min. Whatever scaffolds, cylinders were cut at 5 mm diameter with a biopsy punch (Stiefel), and were cultivated up to use with differentiation medium (DMEM high glucose, antibiotic, 2 mM o f L -glutamine, 100 µg/mL of sodium pyruvate, 40 µg/mL of L-Proline, 50 µg/mL of L-acid ascorbic, 100 nM of dexame- thasone and 1 mM of CaCl2), changed twice in a week. 2.3. Analyze of Cells Viabilit y and Immunophénotyping by Flow Cytometry Apopt osis and necr osis of cells were anal yzed by flow c yto me- try using the Vybrant/Apoptosis™ kit based on Annexin V/Propidium Iodide staining procedure (Molecular Probes, France). To determine the typical cell surface epitope profile after monolayer expansion and after 3 days of culture in sprayed Alg/HA hydrogels, standard flow cytometer techniques (FACSCalibur; BD biosciences , France) were used. 2.4. Metabo lic A c t ivity Mitochondrial activities were measured with Alamar Blue as- say (AbD Serotec Ltd, UK) on sample (one cylinder of 5 mm diameter) during the 28 days of culture. After 4h of scaffolds incubation with Alamar Blue solution (10% v/v) at 37°C, the solution was subsequently removed and Alamar Blue absor- bance is read at wavelength 570 nm (reduced compound absor- bance) and 600 nm (oxidized compound absorbance) with a spectrophotometer (Varioskan Flash, Thermo Scientific). Re- sults were standardized with the quantity of DNA present by samp le . 2.5. Extracellular Mat rix Synthesis Cells were extracted from hydrogel by chelating Ca2+ in algi- nate eggbox bindings and were washed with PBS 3 fold. First, RNA extractio n was done with the RNeasy extr action kit (Qia- gen), and purified with a DNase kit (Qiagen). Then, cDNA were obtained thanks to the reverse-transcription of mRNA with the iscript kit (Biorad). Finally, a qRT PCR was done with the Quantitec SYBR Green PCR kit (Qiagen) and cDNA ob- tained from samples, on a Light Cycler system (Roche Diag- nost ics) during 45 cycles to analyze quantitat ively gene expres- sion. All gene expression was reported to the housekeeping gene RP-29. 2.6. Statistical Analysis All data are presented as means ± standard error means (SEM) of at least three independent experiments. A second way ANOVA was used to determine if significant differences ex- isted for the mixed-population experiment and a Bonferroni posttest was performed to evaluate significance for all experi- ments. 3. Results To examine surface molecule expression on hMSC, flow cyto- metric analysis was used to show the proportion of cells posi- tive for hMSC markers (CD166, CD105, CD44 and CD73) at the end of monolayer culture (P3) and after 3 days of culture in sprayed Alg/HA hydrogels (Figure 1). It appeared that the proportion of positive cells for CD105 significantly decreased when cells were in a 3D matrix compared to the monolayer culture. On the other hand, the proportion of cells expressing CD73, CD44 and CD166 markers increased significantly in sprayed Alg/HA hydrogel compared to the monolayer culture. Whatever the kinds of culture, hMSC were negative for leuko- cyte marker CD45 , hematop oieti c stem cell markers CD34 thus verifying the lack of contaminating hematopoietic cells. Cells were also negative for HLA-DR. hMSC phenoty pe CD44 CD105 CD166 CD73 0 50 100 150 Monolayer Spr ay A lg/HA hy drogel *** *** ** Positive Cells for a marker (%) A B Figure 1. Flow cytometry analysis for the expression of cell surface markers related to mesenchymal stem cells (CD44, CD73, CD105 and CD166; Fig. A), hematopoietic stem cells (CD34, Fig. B), leu- kocytes (CD45, Fig. B), and HLA-DR (Fig. B) on hMSC at the end of monolayer culture (P3) and in sprayed Alg/HA hydrogels (on day 3). Data are presented as mean ±SEM. 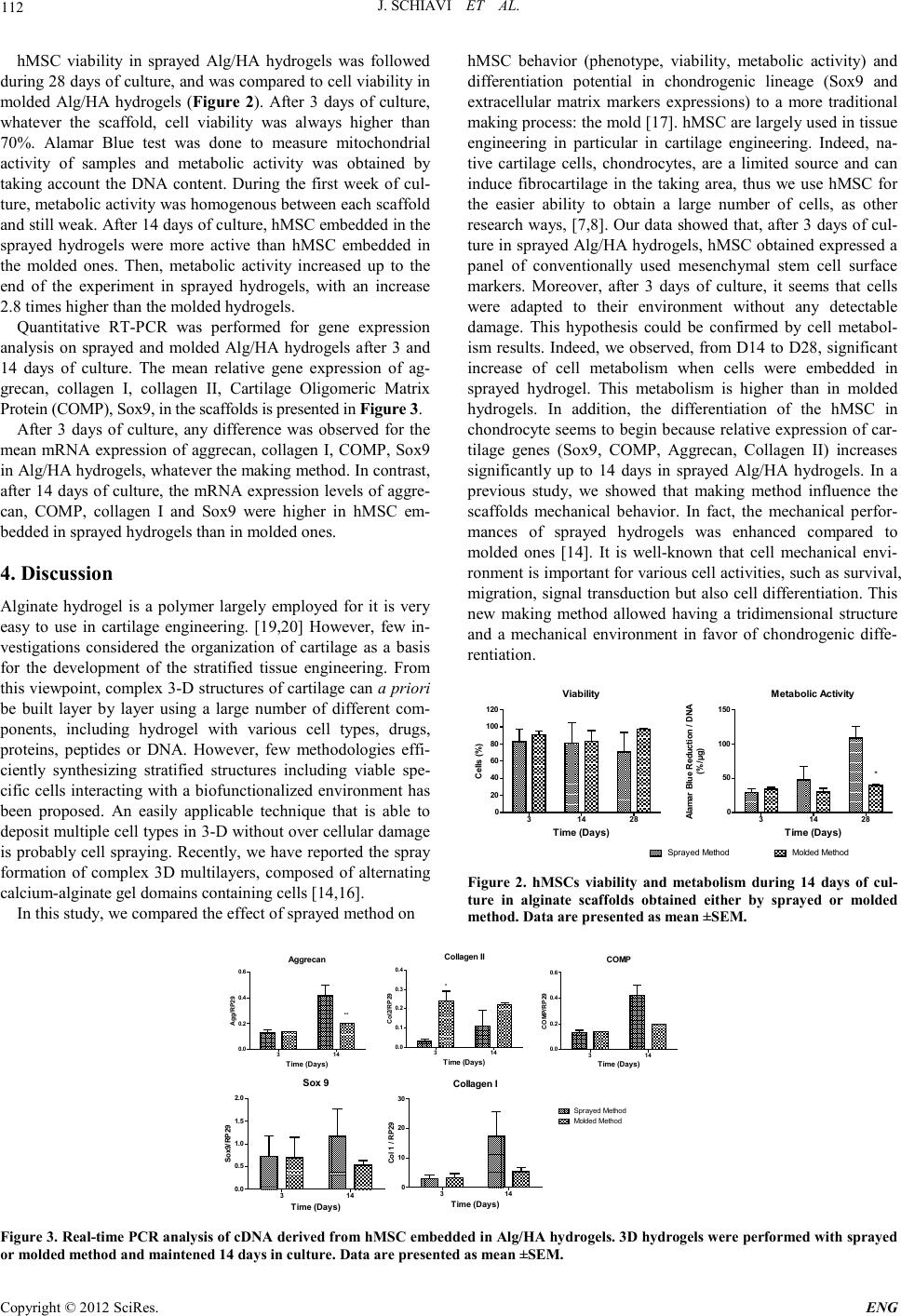 J. SC H IAVI ET AL. Copyright © 2012 SciRes. ENG 112 hMSC viability in sprayed Alg/HA hydrogels was followed during 28 days of culture, and was compared to cell viability in molded Alg/HA hydrogels (Figure 2). After 3 days of culture, whatever the scaffold, cell viability was always higher than 70%. Alamar Blue test was done to measure mitochondrial activity of samples and metabolic activity was obtained by taking account the DNA content. During the first week of cul- ture, metabol ic acti vit y was h omogen ou s b etween each sca ffold and still weak. After 14 days of culture, hMSC embedded in the sprayed hydrogels were more active than hMSC embedded in the molded ones. Then, metabolic activity increased up to the end of the experiment in sprayed hydrogels, with an increase 2.8 times higher than the molded hydrogels. Quantitative RT-PCR was performed for gene expression analysis on sprayed and molded Alg/HA hydrogels after 3 and 14 days of culture. The mean relative gene expression of ag- grecan, collagen I, collagen II, Cartilage Oligomeric Matrix Protein (CO MP), Sox9, i n the s c af folds is pr es e nte d in Figu re 3. After 3 days of culture, any difference was observed for the mean mRNA expression of aggrecan, collagen I, COMP, Sox9 in Alg/HA hydrogels, whatever the making method. In contrast, after 14 days of culture, the mRNA expression levels of ag gre- can, COMP, collagen I and Sox9 were higher in hMSC em- bedded in sprayed hydrogels than in molded ones. 4. Discussion Alginate hydrogel is a polymer largely employed for it is very easy to use in cartilage engineering. [19,20] However, few in- vestigations considered the organization of cartilage as a basis for the development of the stratified tissue engineering. From this viewpoint, complex 3-D structu res of cartilage can a priori be built layer by layer using a large number of different com- ponents, including hydrogel with various cell types, drugs, proteins, peptides or DNA. However, few methodologies effi- ciently synthesizing stratified structures including viable spe- cific cells interacting with a biofunctionalized environment has been proposed. An easily applicable technique that is able to deposit multiple cell types in 3-D without over cellular damage is prob ably cell spraying. Recen tly, we have repo rted the spray formation of complex 3D multilayers, composed of alternating calcium-alginate gel d omains contain ing cells [14,16]. In this study, we compared the effect of sprayed method on hMSC behavior (phenotype, viability, metabolic activity) and differentiation potential in chondrogenic lineage (Sox9 and extracellular matrix markers expressions) to a more traditional making process: the mold [17]. hMS C are l argel y used in ti ssu e engineering in particular in cartilage engineering. Indeed, na- tive cartilage cells, chondrocytes, are a limited source and can induce fibrocartilage in the taking area, thus we use hMSC for the easier ability to obtain a large number of cells, as other research wa ys, [7,8]. Ou r data showed that, after 3 days of cul- ture in sprayed Alg/HA hydrogels, hMSC obtained expressed a panel of conventionally used mesenchymal stem cell surface markers. Moreover, after 3 days of culture, it seems that cells were adapted to their environment without any detectable damage. This hypothesis could be confirmed by cell metabol- ism results. Indeed, we observed, from D14 to D28, significant increase of cell metabolism when cells were embedded in sprayed hydrogel. This metabolism is higher than in molded hydrogels. In addition, the differentiation of the hMSC in chond rocyte seems to begin because relative e xpression o f car- tilage genes (Sox9, COMP, Aggrecan, Collagen II) increases significantly up to 14 days in sprayed Alg/HA hydrogels. In a previous study, we showed that making method influence the scaffolds mechanical behavior. In fact, the mechanical perfor- mances of sprayed hydrogels was enhanced compared to molded ones [14]. It is well-known that cell mechanical envi- ronment is important for various cell activities, such as survival, migration, signal transduction but also cell differentiation. This new making method allowed having a tridimensional structure and a mechanical environment in favor of chondrogenic diffe- rentiation. Viab ility 314 28 0 20 40 60 80 100 120 Time (D ays) Cells (%) Metaboli c Activit y 314 28 0 50 100 150 Molded MethodSprayed Method * Time (D ays) Alamar Blue Re duction / DNA (%/µ g) Figure 2. hMSCs viability and metabolism during 14 days of cul- ture in alginate scaffolds obtained either by sprayed or molded method. Data are presented as mean ±SEM. Aggrecan 314 0.0 0.2 0.4 0.6 ** Time (D a ys) Agg/RP29 Collagen II 314 0.0 0.1 0.2 0.3 0.4 * Time ( D ays ) Col2/RP29 COMP 314 0.0 0.2 0.4 0.6 Time ( D ays ) COMP/RP29 Collagen I 314 0 10 20 30 Sprayed Method Molded Method Time (D ays) Col 1 / RP29 Sox 9 314 0.0 0.5 1.0 1.5 2.0 Time (Days) Sox9/RP29 Figure 3. Real-time PCR analysis of cDNA derived from hMSC embedded in Alg/HA hydrogels. 3D hydrogels were performed with sprayed or molded method and maintened 14 days in culture. Data are presented as mean ±SEM.  J. SC H IAVI ET AL. Copyright © 2012 SciRes. E NG 113 The proposed way will allow to control from the surface to th e in-depth of the distribution of the different needed elements (matrix and cells). This strategy is important for chondrogene- sis induction of hMSC without growth factor, [8], it will permit also building up a scaffold and gives configuration to mimic cartilage structu re. 5. Acknowledgements This work was supported by the "Lorraine region" grant. N. Jessel is indebted to CHU de Nancy, Hôpital Central, ortho- pedic surgery (Contrat d’interface INSERM-CHU). REFERENCES [1] C. Chung and J. A. Burdick, "Engineering cartilage tissue," Adv Drug Deliv Rev, vol. 60, pp. 243-62, 2008. [2] R. Langer and J. P. Vacanti, "Tissue engineering," Science, vol. 260, pp. 920-6, 199 3. [3] L. D. Solorio, A. S. Fu, R. Hernández-Irizarry, and E. Alsberg, "Chondrogenic differentiation of human mesenchymal stem cell aggregates via controlled release of TGF-beta1 from incorpo- rated polymer microspheres," Journal of Biomedical Materials Rese ar ch Part A, vol. 92A, pp. 1139-1144, 2009. [4] E. Fragonas, M. Valente, M. Pozzi-Mucelli, R. Toffanin, R. Rizzo, F. Silvestri, and F. Vittur, "Articular cartilage repair in rabbits by using suspensions of allogenic chondrocytes in algi- nate," Biomaterials, vol. 21, pp. 795-801, 2000. [5] [5] E. Han , W. C. B ae, N. D. Hs ieh-Bonassera, V. W. Wong, B. L. Schumacher, S. Gortz, K. Masuda, W. D. Bugbee, and R. L. Sah, "Shaped, stratified, sc affold-free grafts for articu la r ca rtilage defects," Clin Orthop Relat Res, vol. 466, pp . 1912-20, 2008. [6] N. S. Hwang, S. Varghese, H. J. Lee, P. Theprungsirikul, A. Canver, B. Sharma, and J. Elisseeff, "Response of zonal chon- drocytes to extracellular matrix-hydrogels," FEBS Lett, vol. 581, pp. 4172-8, 2007. [7] K. S. Stok, G. Lisignoli, S. Cristino, A. Facchini, and R. Muller, "Mechano-functional assessment of human mesenchymal stem cells grown in three-dimensional hyaluronan-based scaffolds for car tila ge ti ssu e eng in eerin g," J Biomed Mater Res A, vol. 93 , p p. 37-45, Apr 2009. [8] A. Derfoul, G. L. Perkins, D. J. Hall, and R. S. Tuan , "Gluco cor- ticoids promote chondrogenic differentiation of adult human mesenchymal stem cells by enhancing expression of cartilage extracellular matrix genes," Stem Cells, vol. 24, pp. 1487-95 , Jun 2006. [9] C. S. Lee, J. P. Gleghorn, N. Won Choi, M. Cabodi, A. D. Stroock, and L. J. Bonassar, "Integration of layered chondro- cyte -seeded alginate hydrogel scaffolds," Biomaterials, vol. 28, pp. 2987-93, 2007. [10] K. W. Ng, C. C. B. Wang, R. L. Mauck, T.-A. N. Kelly, N. O. Chahine, K. D. Costa, G. A. Ateshian, and C. T. Hung, "A layered agarose approach to fabricate depth-dependent inhomo- geneity in chondrocyte-seeded constructs," Journal of Orthopa e- dic Research, vol. 23, pp. 134-141, 2005. [11] B. A. Harley, A. K. Lynn, Z. Wissner-Gross, W. Bonfield, I. V. Yann a s, an d L. J. Gibs on, "Desi gn of a mu ltipha se ost eochondral scaffold III: Fabrication of layered scaffolds with continuous in- terfaces," J Biomed Mater Res A, vol. 92, pp. 1078-93, Mar 1. [12] T. J. Klein, S. C. Rizzi, J. C. Reichert, N. Georgi, J. Malda, W. Schuurman, R. W. Crawford, and D. W. Hutmacher, "Strategies for zonal ca rti lage repa ir u sing h ydrogels," Macromol Biosci, vol. 9, pp. 1049-58, Nov 10 2009. [13] T. J. Klein, B. L. Schumacher, T. A. Schmidt, K. W. Li, M. S. Voegtline, K. Masuda, E. J. Thonar, and R. L. Sah, "Tissue en- gineering of stratified articular cartilage from chondrocyte sub- populations," Osteoarthritis Cartilage, vol. 11, pp. 595 -602 , Aug 2003. [14] J. Tritz, R. Rahouadj, N. d. Isla, N. Charif, A. Pinzano, D. Mai- na rd, D. Ben souss an, P. Net ter, J.-F. St olt z, N. Benkirane-Jessel, and C. Huselstein., "Designing a three-dimensional alginate hy- drogel by spraying method for cartilage tissue engineering," Soft Matter, vol. 6, pp. 5165-5174, 2010. [15] H. Mjahed, C. Porcel, B. Senger, A. Chassepot, P. Netter, P. Gillet, G. Decher, J.-C. Voegel, P. Schaaf, N. Benkirane-Jessel, and F. Bou lmedai s, "Micro-stratified architectures based on suc- cessive stacking of alginate gel layers and po ly(L -lysine)–hyaluronic acid multilayer films aimed at tissue engineering," Soft matter, vol. 4, pp. 1422-1429, 2008. [16] J. Tritz-Schiavi, N. Charif, C. Henrionnet, N. de Isla, D. Bens- oussan, J. Magdalou, N. Benkirane-Jessel, J. F. Stoltz, and C. Huselstein, "Original approach for cartilage tissue engineering with mesenchymal stem cells," Biomed Mater Eng, vol. 20, pp. 167-74, 2010. [17] M. Wong, M. Siegrist, X. Wang, and E. Hunziker, "Development of mechanically stable alginate/chondrocyte constructs: effects of guluronic acid content and matrix synthesis," J Orthop Res, vol. 19, pp. 493-9, 2001. [18] S. C. N. Chang, J. A. Rowley, G. Tobias, N. G. Genes, A. K. Roy, D. J. Mooney, C. A. Vacanti, and L. J. Bonassar, "Injection molding of chondrocyte/alginate constructs in the shape of facial implants," Journal of Biomedical Materials Research, vol. 55, pp . 503-511, 2001. [19] G. M. Williams, T. J. Klein, and R. L. Sah, "Cell density alters matrix accumulation in two distinct fractions and the mechanical integrity of alginate-chondrocyte constructs," Acta Biomater, vol. 1, pp. 6 25-33, 2005. [20] Y. Wang, N. de Isla, C. Huselstein, B. Wang, P. Netter, J. F. Stolt z, and S. Muller, "E ffect of algin ate cult ure and m echanical stimulation on cartilaginous matrix synthesis of rat dedifferen- tiated chondrocytes," Biomed Mater Eng, vol. 18, pp. S47-54, 2008. |

