 Advances in Bioscience and Biotechnology, 2013, 4, 949-957 ABB http://dx.doi.org/10.4236/abb.2013.410126 Published Online October 2013 (http://www.scirp.org/journal/abb/) Failure of hCG/LH receptors to stimulate the transmembrane effector adenylyl cyclase in human endometrium L. Bernardini1, I. Moretti-Rojas2, M. Brush2, F. J. Rojas3, J. P. Balmaceda4 1Department of Obstetrics and Gynaecology, Ospedale Sant’Andrea, La Spezia, Italy 2Hitachi Chemical Research Center, Irvine, USA 3Worldwide Medical Corporation, Irvine, USA 4Clinica Las Condes, Santiago, Chile Email: ostgin-sarzana@libero.it Received 12 July 2013; revised 12 August 2013; accepted 15 September 2013 Copyright © 2013 L. Bernardini et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The functional significance of the endometrial hCG/ LH receptors has been related to a rapid release of prostaglandins. However, as compared to gonads and myometrium, in-endometrium mechanisms of trans- membrane signalling of the hCG/LH receptors are probably not conventional and remain unclear. Here we investigated, in vivo, the potential of hCG to in- teract with, and stimulate the membrane effector en- zyme, adenylyl cyclase (AC), in human endometrium. Hormonal and nonhormonal activation of AC was tested in membrane fractions prepared from endome- trial biopsies obtained from patients undergoing eval- ua tio n cycles for hormone replacement therapy (HR T) and controlled ovarian hyperstimulation (COH). AC activity was determined by the direct conversion of the substrate ATP into cAMP under unstimulated conditions and in the presence of the non-hormonal activators guanyl nucleotide and forskolin. Also AC activity was tested in the presence of hCG under con- ditions allowing maximal enzyme stimulation. Iso- proterenol and prostaglandin E2 (PGE2) were in- cluded for comparison. Immunoblot analyses demon- strated the presence of hCG/LH receptors and Gsα protein and other members of the G protein family in the membrane fractions. Endometrial membranes also exhibited high levels of AC activity compared to luteal membranes used as control. Stimulation by GMP-P(NH)P alone was 196 ± 63 (n = 8) (pmol/mg/ min ± SD). Neither hCG nor isoproterenol showed stimulation of endometrial AC (210 ± 65, and 197 ± 53, respectively; n = 66 assays). But PGE2 stimulated the enzyme system signif icantly (264 ± 63, p < 0.05; n = 66 assays). These data show that membrane frac- tions from human endometrium express all the AC system components, namely, hCG/LH receptors, Gsα protein and AC; however, hCG does not stimulate the endometrial AC system. Our data indicate that, in gr eat contrast to gonadal receptors, en do me t ri al h CG / LH receptors are not coupled to the transmembrane AC effector. The well known release of eicosanoids in response to hCG suggests that these receptors are functional in human endometrium but throughout a signalling system different from AC. This enzyme is certainly coupled to and directly activated by eicosa- noids an d o ther embr yonic sign als. Keywords: hCG/LH Receptors; Human Endometrium; Membrane Signal Transduction; Adenylyl Cyclase 1. INTRODUCTION Following our original studies on hCG/LH receptors, G proteins and adenyl cyclase enzyme characterization in human endometrium [1,2], a number of clinical and mo- lecular biology studies have increasingly been published on this topic. Detection of non-gonadal hCG/LH recep- tors has been reported in many reproductive and non- reproductive human tissues including uterus, fallopian tubes, placenta, lymphocytes of pregnant women, adrenal, mammary, prostate glands, and even skin and brain [3- 7]. Current information is now available to candidate the hCG binding hormone and its cognate receptor to be, at least in primates and humans, the main biochemical mar- kers and key regulators of the entire embryo-maternal dialogue from the start until the end of the pregnancy [8-10]. Accordingly, there is general evidence that hCG regulates the endometrial blood flow through vasoactive molecules (PGE2 and VEGF) and controls in combina- OPEN ACCESS  L. Bernardini et al. / Advances in Bioscience and Biotechnology 4 (2013) 949-957 950 tion with other embryonic signals (interleukin 1) the de- cidual transformation of the stroma and the invasion process [11-13]. Despite this, the functions of these ex- tragonadal hCG/LH receptors, under normal and physio- logical conditions have yet to be established. In particu- lar, how these receptors generate transmembrane signal- ling and whether these mechanisms are similar to those described for gonadal hCG/LH receptors are controver- sial. In gonadal tissues, it is well known that the actions of hCG and LH are initiated by a specific binding to the membrane-bound hCG/LH receptors, followed by cou- pling of the receptors to GTP-binding stimulatory (Gsα) protein and activation of the adenylyl cyclase (AC), the transmembrane effector enzyme responsible for the in- tracellular synthesis of cyclic AMP (cAMP) [14]. In the uterus the presence of hCG/LH receptors has been shown in all parts including myometrium, large, small blood vessels and endometrium. During pregnancy they are active and favour vasodilatation and uterine tone relaxa- tion by coupling to probably different signal transduction pathways associated with prostaglandins and cAMP production, respectively [15,16]. Immunohystochemical studies show that endothelial cells contain more recep- tors than vascular smooth muscle and both cell types in vessels of smaller diameter contain more hCG/LH re- ceptors than larger vessels [6,15]. Whether these recep- tors could behave differently according to the type of specific cellular tissue where they are expressed it is, at present, unknown. Similar considerations hold true for endometrium: where and to which exact signal transduc- tion the hCG/LH receptors are coupled, are the subject of controversy. Available information basically relies on in vitro studies of human endometrial cells or in vivo stud- ies on the baboon uterus [17-20]. This notwithstanding, all the investigators agree that whenever the endome- trium is exposed to hCG a consistent and constant acti- vation of the COX2 enzyme is derived along with an increased eicosanoids biosynthesis [17,21-23]. Up to date, there are no in vivo data in human endometrium to support that the hCG/LH receptors are or not coupled to the adenylyl cyclase effector system. We report here never published data from a study designed to gain new insights on the physiological mechanisms involving hCG/LH receptors in human endometrial tissue. Specifi- cally we investigated the transmembrane receptor-ade- nylyl cyclase system in membrane fractions obtained from human endometrium and evaluated the capacity of hCG/LH receptors to interact with and directly stimulate the enzyme system. We will comment in the discussion why we believe these data are of interest and capable to explain missing points of the complex transduction sig- nals initiated by the embryonic hCG at the time of im- plantation. These data also allow a reasonable interpreta- tion of the premature stromal maturation of endometrium apparently associated with reduced implantation rates during cycles of controlled ovarian stimulation when an exogenous injection of hCG is administered. 2. MATERIAL AND METHODS 2.1. Animals Luteal cells membrane fractions were harvested from ovaries of adult female rats maintained in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Collection of rat corpora lutea was performed after inducing superovulation [24]. The protocol allows to achieving a rather homogenous population of corpora lutea within the ovary. 2.2. Ethics of Human Tissues Collection Endometrial specimens (n = 22) were performed by means of biopsy from patients (n = 6) undergoing evalu- ation cycles for hormone replacement (HR) therapy (mean patients age ± SD: 41.4 ± 1.6) and from women (n = 4) undergoing controlled ovarian hyperstymulation (COH) cycles for oocyte-donation (mean patients age ± SD: 26.6 ± 0.5). From each patient, 2 biopsies were ob- tained in subsequent dates of the same cycle. For hor- mone replacement therapy cycles this occurred on the last day of oestrogen administration (day 0) and on day plus 3 (n = 2) or 6 (n = 2) or 9 (n = 2) of progesterone (P) supplementation. For controlled ovarian hyperstymula- tion cycles this occurred on the day of transvaginal oo- cytes aspiration (day 2) and 3 (n = 1) or 5 (n = 1) or 8 (n = 2) days later (days 5, 7 and 10 respectively). A part of each tissue sample obtained was reserved for histological examination and dated according to Noyes et al. (1950) [25]. Hormonal replacement therapy was achieved by sequential and combined administration of oestradiol and progesterone. 17- oestradiol was given per os at in- creasing dosage (2 - 6 mg) for a period of 14.3 ± 0.5 days. At this time, concomitant administration of natural pro- gesterone (100 mg i.m., daily) was started. During eval- uation cycles, oestradiol and progesterone serum levels were measured by immunoassay on cycle day 20 (day plus 6 of progesterone supplementation) and resulted 367 ± 124 pg/ml and 22 ± 5.0 ng/ml, respectively. LH serum levels were measured by an immunoradiometric assay in patients undergoing evaluation cycles of hormone re- placement therapy. These patients were expected to pre- sent different levels of circulating LH (3 patients were down-regulated with GnRH to suppress the interference of any gonadal function while other 3 were premature ovarian failure patients). Controlled ovarian hyperstimu- lation was achieved by a combination protocol of LHRH- analogue plus FSH-HMG as previously published [26]. In experiments concerning measurement of adenylyl cyclase activity, corpora lutea obtained from ovaries of 4 women undergoing exploratory laparotomies for benign Copyright © 2013 SciRes. OPEN ACCESS  L. Bernardini et al. / Advances in Bioscience and Biotechnology 4 (2013) 949-957 951 gynaecological conditions were used as controls. 2.3. Membrane Preparation and Detection of hCG/LH Receptors and G Proteins Preparation of membrane fractions for determination of receptor-adenylyl cyclase system was carried out ac- cording to procedures previously published in our labo- ratory [27]. Briefly, endometrial tissue was flushed with phosphate-buffered saline (PBS) and weighed. The tissue was minced and homogenised in a small Dounce ho- mogenizer and homogenate centrifuged at 10,000 × g for 45 min, at 4˚C. Resulting pellet was then resuspended in 5 parts of 27% (vol/wt) sucrose in 1 mM EDTA and 10 mM Tris-HCL, pH 7.5. hCG/LH-receptors and GTP-binding proteins [(Gs , Gi1, 2 , Gi3 and common sub-unit)] were determi- nated by immunoblot analysis as reported previously [1]. Briefly, protein content in the homogenates was deter- mined by the BCA protein assay (Pierce Chemical Co., Rockford, IL). Proteins were precipitated by adding 2.5 volumes of acetone, incubated for 1 hour at −80˚C in a freezer, and then centrifuged at 70,000 × g for 20 min- utes at 4˚C. The supernatant was then removed, and the pellet resuspended in sample buffer. Same procedure was used to prepare control tissue (rat corpus luteum). After extracting proteins in presence of proteinase inhibitors, the proteins were separated on discontinuous sodium do- decyl sulphate-polyacrilamide gel electrophoresis (12%) under reducing conditions. Same experiments were per- formed under no reducing conditions (7.5% gel). Differ- ent types of molecular weight markers were used de- pending on the experiment (this is specified in the figure legend). The migrated proteins were then transferred to Immobilon-P transfer membranes (Millipore Corp., Bed- ford, MA) using a transfer unit. After blocking the mem- branes for 1 hour with 2% non-fat dry milk Tris-buffered saline, we incubated the blots overnight at 4˚C with dif- ferent dilutions of polyclonal first antibodies. Receptors were detected by 1:10,000 dilution of polyclonal anti- bodies to synthetic fragments of rat luteal hCG/LH re- ceptors [anti-PCR II-(1-11) and anti-LHR-(15-38)], gen- erously provided by Dr. P. Roche (Mayo Clinic, Roch- ester, MN). The G proteins were detected by 1:5000 di- lution of polyclonal antibodies to fragments of rat Gs , Gi1/2 , Gi3 and common (DuPont, Boston, MA). The blots were washed and treated with anti-rabbit im- munoglobulin G horseradish peroxidase conjugate. Rat and human corpus luteum membranes were used as posi- tive controls. The molecular sizes of proteins were de- termined by running standard marker proteins in an ad- jacent lane. An enhanced chemiluminescence detection system (ECL kit; Amersham, Arlington Heights, IL) was used to detect bands. Autoradiographs were then scanned with a gel digitiser (Ultraviolet Products, Cambridge, England) to obtain a semiquantitative evaluation of pro- teins levels. 2.4. Adenylyl Cyclase Assay Optimal experimental conditions to determine expression of membrane-bound adenylyl cyclase and coupling of the enzyme system to hormonal receptors have been de- scribed elsewhere [28,29]. Quantitative determination of adenylyl cyclase activity was performed by the direct conversion of the substrate ATP into the reaction product, cAMP. Briefly, aliquots (10 μl) of membrane particle preparation (10 μg protein) were assayed in duplicate for adenylyl cyclase activity. The final volume of 50 μl con- tained: [ -32]ATP (150 cpm/pmol), 0.5 mM ATP, 5.0 mM MgCl2, 1.0 mM EDTA, 1.0 mM [3H]cAMP (15,000 cpm), 25 mM Tris-HCL, pH 7.5, and a nucleoside tri- phosphate-regenerating system consisting of 20 mM creatine phosphate, 0.2 mg/ml creatine kinase and 0.02 mg/ml myokinase. Incubations were performed at 32˚C for 10 min. Termination of adenylyl cyclase activity was accomplished by addition of 100 μl of a “stop” solution that contained 40 mM ATP, 10 mM cAMP and 1% so- dium dodecyl sulfate. Formed [ -32P]cAMP and [3H]cAMP (used as recovery marker) were isolated and separated from [ -32P]ATP by double chromatography using Dowex 50 columns and alumina columns accord- ing to a procedure previously described [28] and were collected in scintillation fluid for beta counting. Results were expressed in picomoles of cAMP generated/mg protein/minute. Adenylyl cyclase activities detected in membranes prepared from corpora lutea were used as internal controls in all assays performed. 2.5. Statistics and Other Procedures To minimise contamination with guanine nucleotide-like endogenous compounds that can result in high basal AC activities, the components of the nucleoside triphosphate- regenerating system were subjected to purification as previously reported [29]. The results are presented as the mean ± SD of two independent experiments using sepa- rated batches of membranes, each carried out in triplicate. Statistical significance between experimental groups was evaluated using Student’s t test. 3. RESULTS 3.1. Presence of hCG/LH Receptors and G Proteins in Human Endometrium Immunoblot analysis demonstrated the presence of hCG/ LH receptors and the GTP-binding stimulatory (Gs) pro- tein in the endometrial membranes obtained from both hormone replacement cycles and stimulated cycles. The receptor antibody immunoreacted with a major protein migrating at about 68 kDa (Figure 1). The band-inten- Copyright © 2013 SciRes. OPEN ACCESS 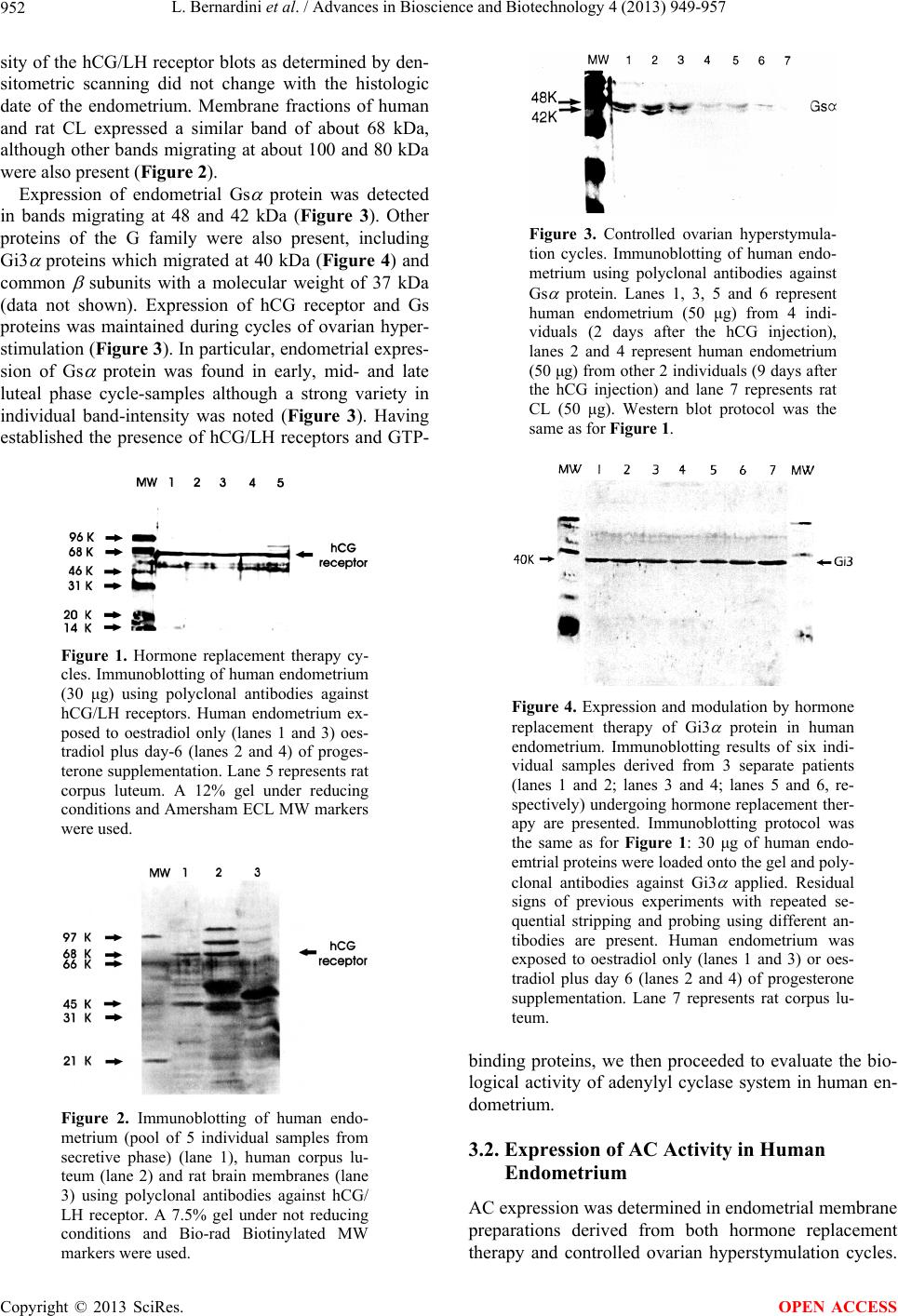 L. Bernardini et al. / Advances in Bioscience and Biotechnology 4 (2013) 949-957 952 sity of the hCG/LH receptor blots as determined by den- sitometric scanning did not change with the histologic date of the endometrium. Membrane fractions of human and rat CL expressed a similar band of about 68 kDa, although other bands migrating at about 100 and 80 kDa were also present (Figure 2 ). Expression of endometrial Gs protein was detected in bands migrating at 48 and 42 kDa (Figure 3). Other proteins of the G family were also present, including Gi3 proteins which migrated at 40 kDa (Figure 4) and common subunits with a molecular weight of 37 kDa (data not shown). Expression of hCG receptor and Gs proteins was maintained during cycles of ovarian hyper- stimulation (Figure 3). In particular, endometrial expres- sion of Gs protein was found in early, mid- and late luteal phase cycle-samples although a strong variety in individual band-intensity was noted (Figure 3). Having established the presence of hCG/LH receptors and GTP- Figure 1. Hormone replacement therapy cy- cles. Immunoblotting of human endometrium (30 μg) using polyclonal antibodies against hCG/LH receptors. Human endometrium ex- posed to oestradiol only (lanes 1 and 3) oes- tradiol plus day-6 (lanes 2 and 4) of proges- terone supplementation. Lane 5 represents rat corpus luteum. A 12% gel under reducing conditions and Amersham ECL MW markers were used. Figure 2. Immunoblotting of human endo- metrium (pool of 5 individual samples from secretive phase) (lane 1), human corpus lu- teum (lane 2) and rat brain membranes (lane 3) using polyclonal antibodies against hCG/ LH receptor. A 7.5% gel under not reducing conditions and Bio-rad Biotinylated MW markers were used. Figure 3. Controlled ovarian hyperstymula- tion cycles. Immunoblotting of human endo- metrium using polyclonal antibodies against Gs protein. Lanes 1, 3, 5 and 6 represent human endometrium (50 μg) from 4 indi- viduals (2 days after the hCG injection), lanes 2 and 4 represent human endometrium (50 μg) from other 2 individuals (9 days after the hCG injection) and lane 7 represents rat CL (50 μg). Western blot protocol was the same as for Figure 1. Figure 4. Expression and modulation by hormone replacement therapy of Gi3 protein in human endometrium. Immunoblotting results of six indi- vidual samples derived from 3 separate patients (lanes 1 and 2; lanes 3 and 4; lanes 5 and 6, re- spectively) undergoing hormone replacement ther- apy are presented. Immunoblotting protocol was the same as for Figure 1: 30 μg of human endo- emtrial proteins were loaded onto the gel and poly- clonal antibodies against Gi3 applied. Residual signs of previous experiments with repeated se- quential stripping and probing using different an- tibodies are present. Human endometrium was exposed to oestradiol only (lanes 1 and 3) or oes- tradiol plus day 6 (lanes 2 and 4) of progesterone supplementation. Lane 7 represents rat corpus lu- teum. binding proteins, we then proceeded to evaluate the bio- logical activity of adenylyl cyclase system in human en- dometrium. 3.2. Expression of AC Activity in Human Endometrium AC expression was determined in endometrial membrane preparations derived from both hormone replacement therapy and controlled ovarian hyperstymulation cycles. Copyright © 2013 SciRes. OPEN ACCESS 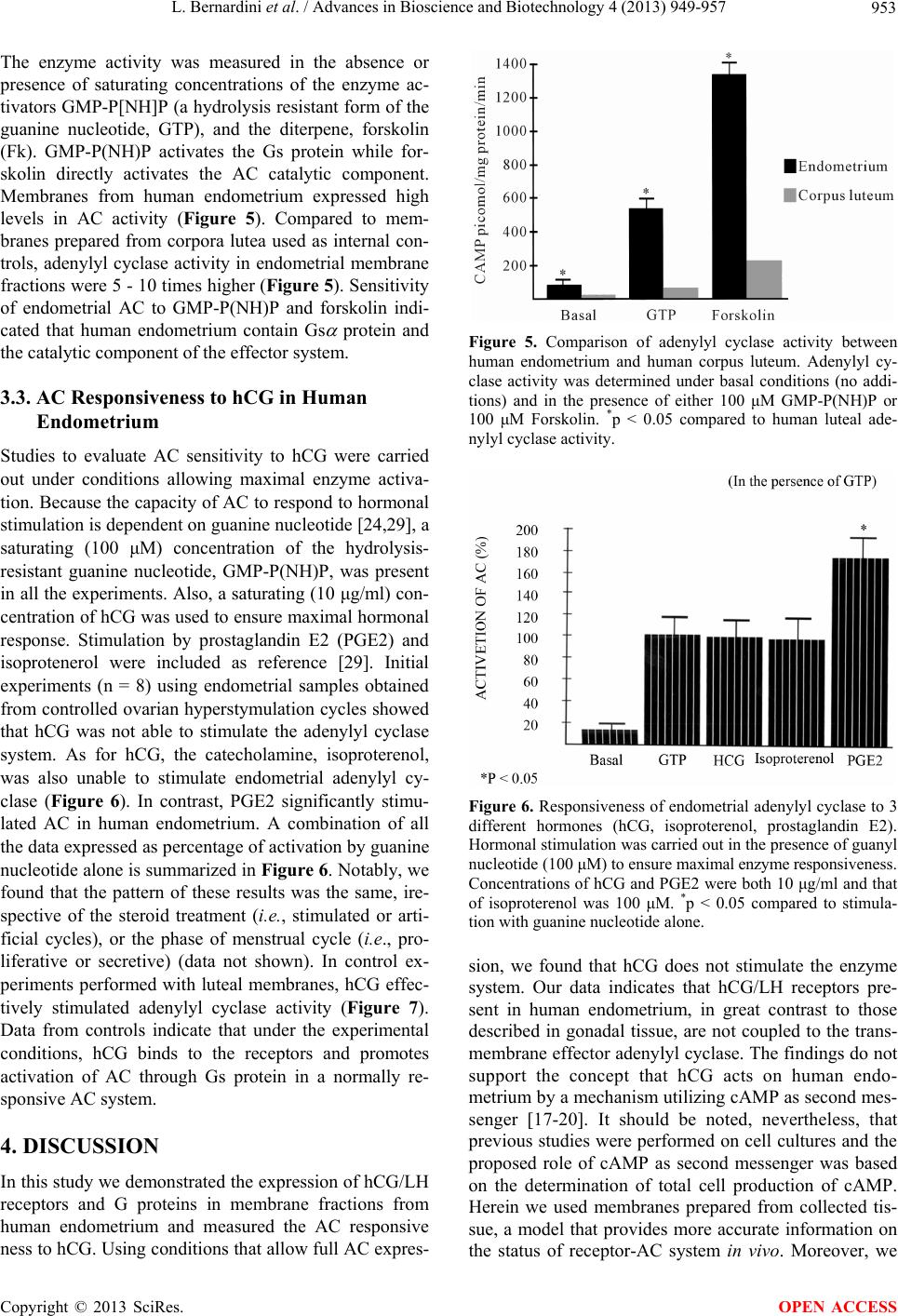 L. Bernardini et al. / Advances in Bioscience and Biotechnology 4 (2013) 949-957 953 The enzyme activity was measured in the absence or presence of saturating concentrations of the enzyme ac- tivators GMP-P[NH]P (a hydrolysis resistant form of the guanine nucleotide, GTP), and the diterpene, forskolin (Fk). GMP-P(NH)P activates the Gs protein while for- skolin directly activates the AC catalytic component. Membranes from human endometrium expressed high levels in AC activity (Figure 5). Compared to mem- branes prepared from corpora lutea used as internal con- trols, adenylyl cyclase activity in endometrial membrane fractions were 5 - 10 times higher (Figure 5). Sensitivity of endometrial AC to GMP-P(NH)P and forskolin indi- cated that human endometrium contain Gs protein and the catalytic component of the effector system. 3.3. AC Responsiveness to hCG in Human Endometrium Studies to evaluate AC sensitivity to hCG were carried out under conditions allowing maximal enzyme activa- tion. Because the capacity of AC to respond to hormonal stimulation is dependent on guanine nucleotide [24,29], a saturating (100 μM) concentration of the hydrolysis- resistant guanine nucleotide, GMP-P(NH)P, was present in all the experiments. Also, a saturating (10 μg/ml) con- centration of hCG was used to ensure maximal hormonal response. Stimulation by prostaglandin E2 (PGE2) and isoprotenerol were included as reference [29]. Initial experiments (n = 8) using endometrial samples obtained from controlled ovarian hyperstymulation cycles showed that hCG was not able to stimulate the adenylyl cyclase system. As for hCG, the catecholamine, isoproterenol, was also unable to stimulate endometrial adenylyl cy- clase (Figure 6). In contrast, PGE2 significantly stimu- lated AC in human endometrium. A combination of all the data expressed as percentage of activation by guanine nucleotide alone is summarized in Figure 6. Notably, we found that the pattern of these results was the same, ire- spective of the steroid treatment (i.e., stimulated or arti- ficial cycles), or the phase of menstrual cycle (i.e., pro- liferative or secretive) (data not shown). In control ex- periments performed with luteal membranes, hCG effec- tively stimulated adenylyl cyclase activity (Figure 7). Data from controls indicate that under the experimental conditions, hCG binds to the receptors and promotes activation of AC through Gs protein in a normally re- sponsive AC system. 4. DISCUSSION In this study we demonstrated the expression of hCG/LH receptors and G proteins in membrane fractions from human endometrium and measured the AC responsive ness to hCG. Using conditions that allow full AC expres- Figure 5. Comparison of adenylyl cyclase activity between human endometrium and human corpus luteum. Adenylyl cy- clase activity was determined under basal conditions (no addi- tions) and in the presence of either 100 μM GMP-P(NH)P or 100 μM Forskolin. *p < 0.05 compared to human luteal ade- nylyl cyclase activity. Figure 6. Responsiveness of endometrial adenylyl cyclase to 3 different hormones (hCG, isoproterenol, prostaglandin E2). Hormonal stimulation was carried out in the presence of guanyl nucleotide (100 μM) to ensure maximal enzyme responsiveness. Concentrations of hCG and PGE2 were both 10 μg/ml and that of isoproterenol was 100 μM. *p < 0.05 compared to stimula- tion with guanine nucleotide alone. sion, we found that hCG does not stimulate the enzyme system. Our data indicates that hCG/LH receptors pre- sent in human endometrium, in great contrast to those described in gonadal tissue, are not coupled to the trans- membrane effector adenylyl cyclase. The findings do not support the concept that hCG acts on human endo- metrium by a mechanism utilizing cAMP as second mes- senger [17-20]. It should be noted, nevertheless, that previous studies were performed on cell cultures and the proposed role of cAMP as second messenger was based on the determination of total cell production of cAMP. Herein we used membranes prepared from collected tis- sue, a model that provides more accurate information on the status of receptor-AC system in vivo. Moreover, we Copyright © 2013 SciRes. OPEN ACCESS 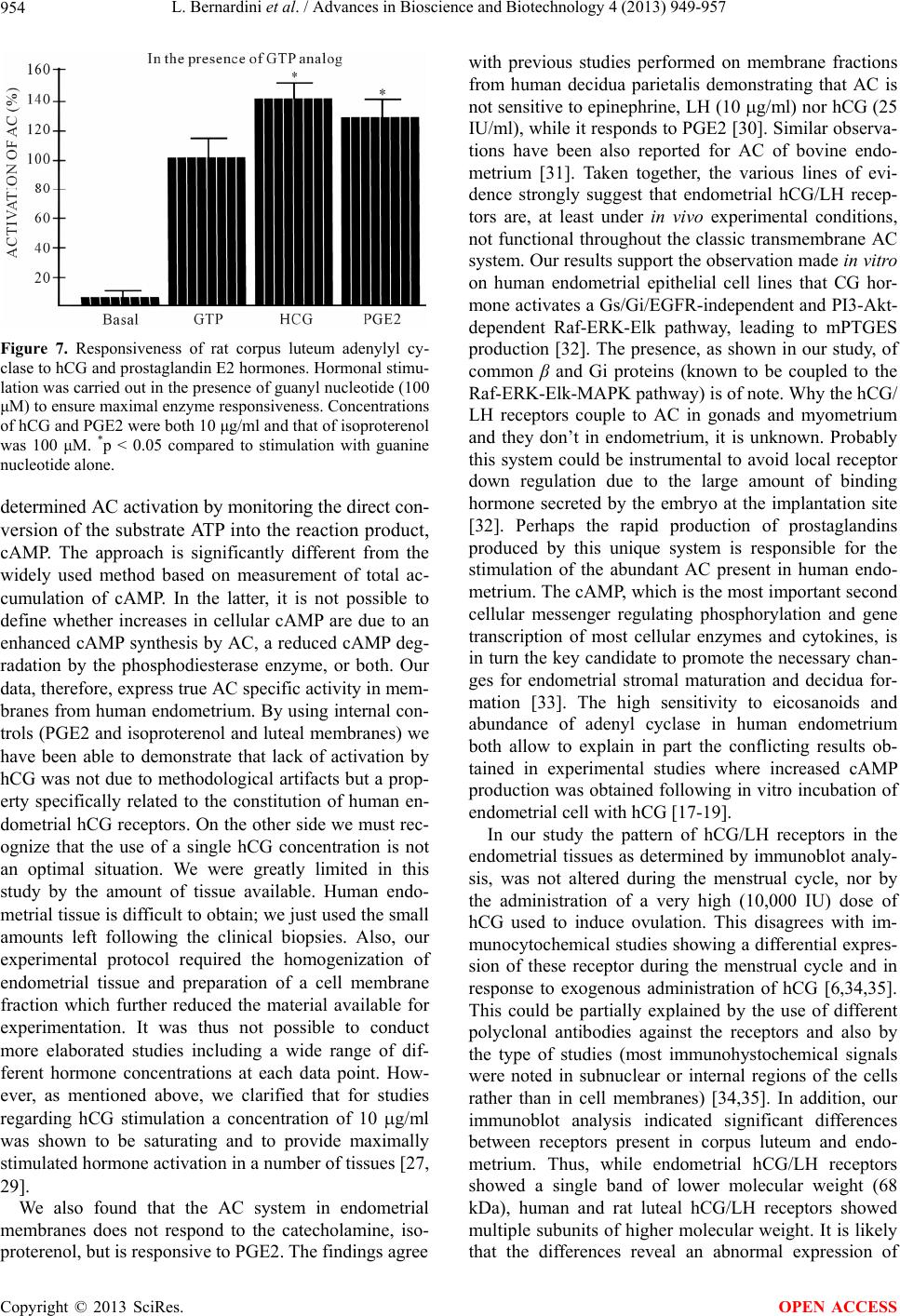 L. Bernardini et al. / Advances in Bioscience and Biotechnology 4 (2013) 949-957 954 Figure 7. Responsiveness of rat corpus luteum adenylyl cy- clase to hCG and prostaglandin E2 hormones. Hormonal stimu- lation was carried out in the presence of guanyl nucleotide (100 μM) to ensure maximal enzyme responsiveness. Concentrations of hCG and PGE2 were both 10 μg/ml and that of isoproterenol was 100 μM. *p < 0.05 compared to stimulation with guanine nucleotide alone. determined AC activation by monitoring the direct con- version of the substrate ATP into the reaction product, cAMP. The approach is significantly different from the widely used method based on measurement of total ac- cumulation of cAMP. In the latter, it is not possible to define whether increases in cellular cAMP are due to an enhanced cAMP synthesis by AC, a reduced cAMP deg- radation by the phosphodiesterase enzyme, or both. Our data, therefore, express true AC specific activity in mem- branes from human endometrium. By using internal con- trols (PGE2 and isoproterenol and luteal membranes) we have been able to demonstrate that lack of activation by hCG was not due to methodological artifacts but a prop- erty specifically related to the constitution of human en- dometrial hCG receptors. On the other side we must rec- ognize that the use of a single hCG concentration is not an optimal situation. We were greatly limited in this study by the amount of tissue available. Human endo- metrial tissue is difficult to obtain; we just used the small amounts left following the clinical biopsies. Also, our experimental protocol required the homogenization of endometrial tissue and preparation of a cell membrane fraction which further reduced the material available for experimentation. It was thus not possible to conduct more elaborated studies including a wide range of dif- ferent hormone concentrations at each data point. How- ever, as mentioned above, we clarified that for studies regarding hCG stimulation a concentration of 10 g/ml was shown to be saturating and to provide maximally stimulated hormone activation in a number of tissues [27, 29]. We also found that the AC system in endometrial membranes does not respond to the catecholamine, iso- proterenol, but is responsive to PGE2. The findings agree with previous studies performed on membrane fractions from human decidua parietalis demonstrating that AC is not sensitive to epinephrine, LH (10 g/ml) nor hCG (25 IU/ml), while it responds to PGE2 [30]. Similar observa- tions have been also reported for AC of bovine endo- metrium [31]. Taken together, the various lines of evi- dence strongly suggest that endometrial hCG/LH recep- tors are, at least under in vivo experimental conditions, not functional throughout the classic transmembrane AC system. Our results support the observation made in vitro on human endometrial epithelial cell lines that CG hor- mone activates a Gs/Gi/EGFR-independent and PI3-Akt- dependent Raf-ERK-Elk pathway, leading to mPTGES production [32]. The presence, as shown in our study, of common β and Gi proteins (known to be coupled to the Raf-ERK-Elk-MAPK pathway) is of note. Why the hCG/ LH receptors couple to AC in gonads and myometrium and they don’t in endometrium, it is unknown. Probably this system could be instrumental to avoid local receptor down regulation due to the large amount of binding hormone secreted by the embryo at the implantation site [32]. Perhaps the rapid production of prostaglandins produced by this unique system is responsible for the stimulation of the abundant AC present in human endo- metrium. The cAMP, which is the most important second cellular messenger regulating phosphorylation and gene transcription of most cellular enzymes and cytokines, is in turn the key candidate to promote the necessary chan- ges for endometrial stromal maturation and decidua for- mation [33]. The high sensitivity to eicosanoids and abundance of adenyl cyclase in human endometrium both allow to explain in part the conflicting results ob- tained in experimental studies where increased cAMP production was obtained following in vitro incubation of endometrial cell with hCG [17-19]. In our study the pattern of hCG/LH receptors in the endometrial tissues as determined by immunoblot analy- sis, was not altered during the menstrual cycle, nor by the administration of a very high (10,000 IU) dose of hCG used to induce ovulation. This disagrees with im- munocytochemical studies showing a differential expres- sion of these receptor during the menstrual cycle and in response to exogenous administration of hCG [6,34,35]. This could be partially explained by the use of different polyclonal antibodies against the receptors and also by the type of studies (most immunohystochemical signals were noted in subnuclear or internal regions of the cells rather than in cell membranes) [34,35]. In addition, our immunoblot analysis indicated significant differences between receptors present in corpus luteum and endo- metrium. Thus, while endometrial hCG/LH receptors showed a single band of lower molecular weight (68 kDa), human and rat luteal hCG/LH receptors showed multiple subunits of higher molecular weight. It is likely that the differences reveal an abnormal expression of Copyright © 2013 SciRes. OPEN ACCESS  L. Bernardini et al. / Advances in Bioscience and Biotechnology 4 (2013) 949-957 955 endometrial hCG/LH receptors. Studies from our labora- tory as well as from others indicate that the endometrial hCG/LH receptors may represent an incomplete, non- mature form of receptor protein derived from an abnor- mally truncated mRNA transcript. Accordingly, we have found that compared to gonadal receptors, the endo- metrial hCG/LH receptor genes express a normal trans- membrane region, but an abnormal extracellular region [36]. Similar observations have been reported by Stewart et al. [37] who detected presence of mRNA for the highly conserved transmembrane portion of the hCG re- ceptor but not for the extracellular ligand-binding portion that recognizes and specifically binds hCG/LH. These forms of receptors may be due to a difference in the al- ternative splicing process during gene expression. Ineffi- cient LHR maturation is a natural condition in rat tissues and there is a clear age-, tissue-, and sex-dependent va- riation in the relative amount of mature receptors to im- mature ones [38]. At the current time we know that the interaction be- tween hCG ligand and hCG/LH receptor undergo an high degree of variability due to the presence, in nature, of multiple variants of hCG molecule having different gly- cosylation [10]. Therefore, in experimental studies, the use of purified or recombinant hCG may impact on the results [10,39]. The issue is even more complicated by the natural presence of receptors at different status of maturity (splice variants) [38,40] and mechanisms used by these to transfer signals into the cell. Most G protein- coupled receptors can couple to several G proteins within a single cell and several cells express more than one subtype of a particular receptor each potentially able to stimulate different G proteins. It is well established that common βγ subunits of G proteins play a major role in mediating signal transmission to Ras and ERK/MAPK cascades but quantitative regulation of this pathway can also proceed through cooperation of Gαq/11 and Gαi sig- nals [41]. Cooperative signaling by multiple G proteins might represent a novel concept implicated in the regula- tion of different cellular responses induced by G protein- coupled-receptors [41]. Conflicting literature data can be explained by this and other considerations including whether studies are in vivo or in vitro, type and source of cells (epithelial, stromal, cloned cancer cell lines, mono- cytes) [7,42], additions to culture media (insulin vs ster- oids), timing and way of the end product measurement (i.e., cAMP) [43], and also primers set used for PCR or polyclonal antibodies used for western blotting or im- munohistochemistry studies. 5. CONCLUSION In conclusion we believe that hCG, the main embryonic signal of embryo implantation, binds to quite special en- dometrial receptors frequently undergoing natural post- trascription (splice variants) and post-translation changes [40,44]. These receptors respond to hCG with prostag- landins release by means of an uncommon signal trans- duction pathway. Prostaglandins are certainly coupled to AC in cell membranes of endometrium (glandular and stromal cells). The endometrium is particularly rich in AC and besides eicosanaoids this enzyme is ready to be activated and directly coupled to receptors for also other embryonic signals (i.e., interleukin 1) [13]. In any case, this allows intracellular accumulation of enormous amounts of cAMP which is the internal messenger re- sponsible for the activation of all enzymes and cytokines necessary to promote the condition of adequate endo- metrial receptivity (initiation of neo-angiogenesis and decidua process). Our opinion agrees with the notion that early in the luteal phase a complex molecular machinery is present in human endometrium ready to over-respond to initial amounts hCG secreted by the embryo [45]. On this regard other investigators have demonstrated by immuno-histochemical studies a change in hCG/LH re- ceptors expression during the menstrual cycle similar to that reported by us for Gsα and AC during the very first days of progesterone supplementation using HRT cycles [2]. This short time window of endometrial receptivity- potential disappears during the course of luteal phase and it is probably anticipated and disrupted whenever exter- nal stimulation of the receptors or enzymes is altered by high dosages of exogenous hCG injection (as it is done during cycles of COH). This implies that during COH gonadotropin hormones may induce a release of endo- metrial cAMP with a subsequent acceleration of the process of stromal maturation and impairment of uterine receptivity. We have previously reported modification of AC function associated with precise histological dating of endometrium [2] and recent clinical and molecular studies support this contention [34,46-49]. REFERENCES [1] Bernardini, L., Moretti-Rojas, I., Brush, M., Rojas, F.J. and Balmaceda, J.P. (1995) Status of hCG/LH receptor and G proteins in human endometrium during artificial cycles of hormone replacement therapy. Journal of the Society for Gynecology Investigation, 2, 630-635. http://dx.doi.org/10.1016/1071-5576(95)00010-C [2] Bernardini, L., Moretti-Rojas, I., Brush, M., Rojas, F.J. and Balmaceda, J.P. (1999) Changes in expression of adenylyl cyclase activity in human endometrium during hormone replacement therapy and controlled ovarian hy- perstymulation. Molecular Human Reproduction, 5, 955. http://dx.doi.org/10.1093/molehr/5.10.955 [3] Reshef, E., Lei, Z.M. and Rao, C.V. (1990) The presence of gonadotropin receptors in nonpregnant human uterus, human placenta, fetal membranes, and decidua. Journal of Clinical Endocrinology and Metabolism, 70, 421-430. http://dx.doi.org/10.1210/jcem-70-2-421 Copyright © 2013 SciRes. OPEN ACCESS  L. Bernardini et al. / Advances in Bioscience and Biotechnology 4 (2013) 949-957 956 [4] Huang, Z.H., Lei, Z.M. and Rao, C.V. (1995) Immor- taized anterior pituitary alpha T3 gonadotropes contain functional luteinizing hormone/human chorionic gonad- otropin receptors. Molecular Cell Endocrinology, 114, 217-222. http://dx.doi.org/10.1016/0303-7207(95)03635-K [5] Tao, Y.X., Lei, Z.M. and Rao, C.V. (1997) The presence of luteinizing hormone/human chorionic gonadotropin re- ceptors in lactating rat mammary glands. Life Science, 60, 1297-303. http://dx.doi.org/10.1016/S0024-3205(97)00073-8 [6] Toth, P., Li, X. and Rao, C.V. (1994) Expression of func- tional human chorionic gonadotropin/human luteinizing hormone receptor gene in human uterine arteries. Journal of Clinical Endocrinology and Metabolism, 79, 307-315. http://dx.doi.org/10.1210/jc.79.1.307 [7] Kosaka, K., Fujiwara, H., Tatsumi, K., et al. (2002) Hu- man chorionic gonadotropin (HCG) activates monocytes to produce interleukin-8 via a different pathway from lu- teizing hormone/HCG receptor system. Journal of Clini- cal Endocrinology and Metabolism, 87, 5199-5208. http://dx.doi.org/10.1210/jc.2002-020341 [8] Rao, C.V. (2006) Physiological and pathological rele- vance of human uterine LH/hCG receptors. Journal of the Society for Gynecologic Investigation, 13, 77-78. http://dx.doi.org/10.1016/j.jsgi.2005.12.005 [9] Banerjee, P. and Fazleabaz, A.T. (2010) Endometrial re- sponses to embryonic signals in the primate. The Interna- tional Journal of Developmental Biology, 54, 295-302. http://dx.doi.org/10.1387/ijdb.082829pb [10] Cole, L. (2010) Biological functions of hCG and hCG- related molecules. Reproductive Biology and Endocri- nology, 8, 102. http://dx.doi.org/10.1186/1477-7827-8-102 [11] Cameo, P., Srisupap, S., Strakova, Z. and Fazleabas, A.T. (2004) Chorionic gonadotropin and uterine dialogue in the primate. Reproductive Biology and Endocrinology, 2, 50. http://dx.doi.org/10.1186/1477-7827-2-50 [12] Zygmunt, M., Herr, F., Keller-Schoenwetter, S., et al. (2002) Characterization of human cghorionic gonadotro- pin as a novel angiogenic factor. The Journal of Clinical Endocrinology & Metabolism, 87, 5290-5296. http://dx.doi.org/10.1210/jc.2002-020642 [13] Strakova, Z., Mavrogianis, P., Meng, X., et al. (2005) In vitro infusion of interleukin-1B and chorionic gonadotro- pin induces endometrial changes that mimic early preg- nancy events in the baboon. Endocrinology, 146, 4097- 4104. http://dx.doi.org/10.1210/en.2005-0380 [14] Segaloff, D.L. and Ascoli, M. (1993) The lutropin/chorio- gonadotropin receptor ... 4 years later. Endocrine Reviews, 14, 324-347. [15] Toth, P., Lukacs, H., Gimes, G., et al. (2001) Clinical importance of vascular LH/hCG receptors—A review. Reproductive Biology, 1, 5-11. [16] Phillps, R.J., Bailey, J., Robson, S.C. and Europe-Finner, G.N. (2002) Differential expression of the adenyl cyclase- stimulatory guanosine thriphosphate-binding protein Gsα in the human myometrium during pregnancy and labor involves transcriptional regulation by cyclic adenosine 3’,5’-monophosphate and binding of phosphorylated nu- clear proteins to multiple GC boxes within the promoter. The Journal of Clinical Endocrinology & Metabolism, 87, 5675-5685. [17] Zhou, X.L., Lei, Z.M. and Rao, C.V. (1999) Treatment of human endometrial gland epithelial cells with chorionic gonadotropin/luteinizing hormone increases the expres- sion of the cyclooxygenase-2 gene. The Journal of Clini- cal Endocrinology & Metabolism, 84, 3364-3377. http://dx.doi.org/10.1210/jc.84.9.3364 [18] Tang, B. and Gurpide, E. (1993) Direct effect of gonad- otropins on decidualization of human endometrial stromal cells. Journal of Biochemistry and Molecular Biology, 47, 115-121. [19] Chatterjee, A., Jana, N.R. and Bhattacharya, S. (1997) Stimulation of cyclic AMP, 17-beta-oestradiol and protein synthesis by human chorionic gonadotrophin in human endometrial cells. Human Reproduction, 12, 1903-1908. http://dx.doi.org/10.1093/humrep/12.9.1903 [20] Fazleabas, A.T., Donnelly, K.M., Srinivasan, S., Fortman, J.D. and Miller, J.B. (1999) Modulation of the baboon (Papio anubis) uterine endometrium by chorionic gonad- otrophin during the period of uterine receptivity. Pro- ceedings of the National Academy of Sciences of the Uni- ted States of America, 96, 2543-2548. http://dx.doi.org/10.1073/pnas.96.5.2543 [21] Han, S.W., Lei, Z.M. and Rao, C.V. (1996) Up-regulation of cyclooxygenase-2 gene expression by chorionic go- nadotropin during the differentiation of human endo- metrial stromal cells into decidua. Endocrinology, 137, 1791-1797. http://dx.doi.org/10.1210/en.137.5.1791 [22] Banerjee, P., Cameo, P., Srisuparp, S., Strakova, Z. and Fazleabas, A.T. (2007) A novel MAPK pathway activated by chorionic gonadotropin in primate endometrial epithe- lial cells. Biology of Reproduction, 77, 135-136. [23] Sales, K.J., Grant, V., Catalano, R.D. and Jabbour, H.N. (2011) Chorionic gonadotrophin regulates CXCR4 ex- pression in human endometrium via E-series prostanoid receptor 2 signalling to PI3K-ERK I/2: Implications for fetal-maternal crosstalk for embryo implantation. Mo- lecular Human Reproduction, 17, 22-32. http://dx.doi.org/10.1093/molehr/gaq069 [24] Birnbaumer, L., Yang, P.C. and Hunzicker-Dunn, M. (1976) Adenylyl cyclase activity in ovarian tissues: Ho- mogenization and condition of assay in Graafian follicles and corpora lutea of rabbits, rats, and pigs: Regulation by ATP, and some comparative properties. Endocrinology, 99, 163. http://dx.doi.org/10.1210/endo-99-1-163 [25] Noyes, R.W., Hertig, A.T. and Rock, J. (1950) Dating the endometrial biopsy. Fertility and Sterility, 1, 3-25. [26] Bernardini, L., Alam, V., Asch, R.H. and Balmaceda, J.P. (1993) Pregnancy and implantation rates in hormonal re- placement versus stimulated cycles. Human Reproduc- tion, 8, 1983-1941. [27] Rojas, F.J., Moretti-Rojas, I., Balamceda, J.P. and Asch, R.H. (1991) The role of the adenylyl cyclase system in the regulation of corpus luteum function in the human and in nonhuman primates. Steroids, 56, 252-257. http://dx.doi.org/10.1016/0039-128X(91)90043-U Copyright © 2013 SciRes. OPEN ACCESS  L. Bernardini et al. / Advances in Bioscience and Biotechnology 4 (2013) 949-957 Copyright © 2013 SciRes. 957 OPEN ACCESS [28] Rojas, F.J. and Ciridon, J.W. (1996) Evidence that hor- mone receptors couple and activate a common signal transducer adenylyl cyclase in corpus luteum. Fertility and Sterility, 65, 275-279. [29] Rojas, F.J., Moretti-Rojas, I.M., Balmaceda, J.P. and Asch, R.H. (1989) Changes in adenylyl cyclase activity of the human and non-human primate during the menstrual cy- cle and pregnancy. Journal of Clinical Endocrinology and Metabolism, 68, 379-385. http://dx.doi.org/10.1210/jcem-68-2-379 [30] Whitsett, J.A. and Johnson, C.L. (1979) Adenylate cy- clase from human decidua parietalis. American Journal of Obstetrics and Gynecology, 133, 479. [31] Bhalla, R.C., Sanborn, R.M. and Korenman, S.G. (1972) Hormonal interaction in the uterus: Inhibition of isopro- terenol-induced accumulation of adenosine 3’,5’-cyclic monophosphate by oxytocin and prostaglandins. Pro- ceedings of the National Academy of Science, 69, 3761. http://dx.doi.org/10.1073/pnas.69.12.3761 [32] Banerjee, P., Sapru, K., Strakova, Z. and Fazleabas, A.T. (2009) Chorionic gonadotropin regulates prostaglandin E synthase via a phosphatidylinositol 3-kinase-extracellular regulatory kinase pathway in a human endometrial epi- thelial cell line: Implications for endometrial responses for embryo implantation. Endocrinology, 150, 4326- 4337. http://dx.doi.org/10.1210/en.2009-0394 [33] Gellersen, B. and Brosens, J. (2003) Cyclic AMP and progesterone receptor cross-talk in human endometrium: A decidualizing affair. Journal of Endocrinology, 178, 357-372. http://dx.doi.org/10.1677/joe.0.1780357 [34] Evans, J. and Salamonsen, L.A. (2013) Too much of a good thing? Experimental evidence suggests prolonged exposure to hCG is detrimental to endometrial receptiv- ity. Human Reproduction, 28, 1610-1619. http://dx.doi.org/10.1093/humrep/det055 [35] Jee, B.C., Suh, C.S. and Kim, S.H. (2011) Endometrial expression of hCG/LH receptor in infertile women with repeated implantation failure. Journal of Women’s Medi- cine, 4, 6-10. http://dx.doi.org/10.5468/jwm.2011.4.1.6 [36] Singh, A., Moretti-Rojas, I., Brush, M., Rojas, F., et al. (1996) Gene expression of hCG/LH receptors in human endometrium: Evidence for an abnormal extracellular do- main. Journal of the Society for Gynecologic Investiga- tion, 3, 166A. [37] Stewart, E.A., Sahakian, M., Rhoades, A., Van Voorhis, B.J. and Nowak, R.A. (1999) Messenger ribonucleic acid for the gonadal luteinizing hormone/human chorionic gonadotropin receptor is not present in human endo- metrium. Fertility and Sterility, 71, 368-372. http://dx.doi.org/10.5468/jwm.2011.4.1.6 [38] Apaja, P.M., Tuusa, J.T., Pietila, E.M., Rajaniemi, H.J. and Petaja-Repo, U.E. (2006) Luteinizing hormone re- ceptor ectodomain splice variant misroutes the full-length receptor into a subcompartment of the endoplasmic re- ticulum. Molecular Biology of the Cell, 17, 2243-2255. http://dx.doi.org/10.1091/mbc.E05-09-0875 [39] Banerjee, P. and Fazleabas, A.T. (2011) Extragonadal ac- tions of chorionic gonadotropin. Reviews in Endocrine and Metabolic Disorders, 12, 323-332. http://dx.doi.org/10.1007/s11154-011-9193-1 [40] Apaja, P.M., Aatsinki, J.T., Rajaniemi, H.J. and Petaja- Repo, U.E. (2005) Expression of the mature luteinizing hormone receptor in rodent urogenital and adrenal tissues is developmentally regulated at a posttranslational level. Endocrinology, 146, 3224-3232. [41] Blaukat, A., Barac, A., Cross, M.J., Offermanns, S. and Dikic, I. (2000) G protein-coupled receptor-mediated mi- togen-activated protein kinase activation through coop- eration of Gαq and Gαi signals. Molecular and Cellular Biology, 20, 6837-6848. http://dx.doi.org/10.1128/MCB.20.18.6837-6848.2000 [42] Berndt, S., Blacher, S., Perrier d’Hauterive, S., et al. (2009) Chorionic gonadotropin stimulation of angiogene- sis and pericyte recruitment. Journal of Clinical Endo- crinology & Metabolism, 94, 4567-4574. http://dx.doi.org/10.1210/jc.2009-0443 [43] Viswanath, G., Chatterjee, S. and Roy, P. (2007) Assess- ment of luteinizing hormone receptor function in an en- dometrial cancer cell line Ishikawa cells in response to human chorionic gonadotrophin (hCG). Molecular and Cellular Endocrinology, 272, 14-21. http://dx.doi.org/10.1016/j.mce.2007.04.006 [44] Licht, P., Russu, V. and Wildt, L. (2001) On the role of human chorionic gonadotropin (hCG) in the embryo-en- dometrial microenviroment: implications for differentia- tion and implantation. Seminars in Reproductive Medi- cine, 19, 37-42. http://dx.doi.org/10.1055/s-2001-13909 [45] Sherwin, J.R.A., Sharkey, A.M., Cameo, P., et al. (2007) Identification of novel genes regulated by chorionic go- nadotropin in baboon endometrium during the window of implantation. Endocrinology, 148, 618-626. http://dx.doi.org/10.1210/en.2006-0832 [46] Fanchin, R., Frydman, R., Peltier, E. and de Ziegler, D. (2001) Human chorionic gonadotropin: does it affect hu- man endometrial morphology in vivo? Seminars in Re- productive Medicine, 19, 31-35. [47] Prapas, N., Tavaniotou, A., Panagiotidis, Y., et al. (2009) Low-dose human chorionic gonadotropin during the pro- liferative phase may adversely affect endometrial recap- tivity in oocyte recipients. Gynecological Endocrinology, 25, 53-59. http://dx.doi.org/10.1080/09513590802360769 [48] Fatemi, H.M., Kyrou, D., Bourgain, C., et al. (2010) Cry- opreserved-thawed human embryo transfer: Spontaneous natural cycle is superior to human chorionic gonadotro- pin-induced natural cycle. Fertility and Sterility, 94, 2054-2058. http://dx.doi.org/10.1016/j.fertnstert.2009.11.036 [49] Kyrou, D., Kolibianakis, E.M., Fatemi, H.M., et al. (2012) Spontaneous triggering of ovulation versus hCG admini- stration in patients undergoing IUI: A prospective ran- domized study. Reproductive Biomedicine Online, 25, 278-283. http://dx.doi.org/10.1016/j.rbmo.2012.05.005
|