 American Journal of Plant Sciences, 2013, 4, 2031-2038 http://dx.doi.org/10.4236/ajps.2013.410254 Published Online October 2013 (http://www.scirp.org/journal/ajps) 2031 Terpene Composition of Three Species of Gymnosperms from Vietnam Do N. Dai1, Tran D. Thang2*, Isiaka A. Ogunwande3* 1Faculty of Biology, Vinh University, Vinh City, Vietnam; 2Faculty of Chemistry, Vinh University, Vinh City, Vietnam; 3Natural Products Research Unit, Department of Chemistry, Faculty of Science, Lagos State University, Lagos, Nigeria. Email: *thangtd@vinhuni.edu.vn, *isiaka.ogunwande@lasu.edu.ng Received June 15th, 2013; revised July 15th, 2013; accepted August 19th, 2013 Copyright © 2013 Do N. Dai et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT In the present investigation, we studied new essential oil contents and compositions of three individual plants from Cu- pressaceae family cultivated in Vietnam. The air-dried plants were hydrodistilled and the oils analysed by Gas chroma- tography (GC) and Gas chromatography couples with Mass spectrometry (GC-MS). The components were identified by comparison of their retention indices on HP-5 MS capillary column with literature reference and MS pattern with au- thentic library’s collection. -Pinene (36.0%), α-cedrol (18.3%) and thujopsene (5.7%) were the main constituents of Cupressus tonkiensis Silba. Monoterpenes were the quantitative significant compounds of Sabina chinensis (L.) An- toine. These are bornyl acetate (19.5%), sabinene (17.3%) and -pinene (15.8%). Moreover, the main compounds of Thuja orientalis L., were identified as -pinene (34.1%), α-cedrol (16.5%), β-caryophyllene (5.4%) and β-selinene (5.2%). The chemotaxonomy implication of these results was also discussed. Keywords: Cupressus tonkiensis; Sabina chinensis; Thuja orientalis; Bornyl Acetate; α-Cedrol; -Pinene; Sabinene 1. Introduction The genus Cupressus is one of several genera within the family Cupressaceae that has the common name cypress. It is considered a polyphyletic group. Based on genetic and morphological analysis, the Cupressus genus is found in the Cupressoideae subfamily [1]. As currently treated, these cypresses are native to scattered localities in mainly warm temperate regions in the Northern Hemisphere, in- cluding western North America, Central America, north- west Africa, the Middle East, the Himalayas, southern China and northern Vietnam [2]. Cupressus tonkinensis Silba., is an evergreen, medium-sized tree, up to 15 - 25 cm in height. Bark is grey brown with longitudinal fis- sures. Leaves are scaly, closely inserted on twigs. Cones are unisexual, grouped on a stalk. Male cone is subglo- bular. This is an endangered species in Vietnam, only found in a narrow area of the Central Region [3]. Little is known about the chemical constituents and biological po- tential of this plant. Leaves and stems of C. tonkinensis produce monoterpene-rich oils whose composition is α-pinene (23.1%), sabinene (21.0%) and terpinen-4-ol (14.4%) in the leaf while, α-pinene (42.5%), myrcene (10.2%) and cedrol (9.0%) were identified in the stem [4]. Another investigation identified sabinene (29.34%), α-pi- nene (25.4%), 4-terpineol (13.91%) and -terpinene (5.5%) as major compounds of its leaf oil [3]. Sabina chinensis (L.) Antoine is an evergreen tree that grows up to 20 m high. The leaves are basal while the yellow flowers are unisexual [5]. S. chinensis is one of the important species used in Chinese garden and has a long history. It has many uses and more than 100 culti- vars. The first report on the main constituents of S. chi- nensis identified sabinene (46.73%), α-pinene (17. 03%), terpinen-4-ol (5.34%) and limonene (4.48%) as major con- stituents [6]. Another analysis [7] reported an abundance of α-pinene (21.1%), sabinene (18.2%) and limonene (9.2%) in the oil. Cui et al. [8] demonstrated that the main substances in volatile oil S. chinensis were bornyl acetate (38.1%), α-phellandrene (24.0%) and p-menth-l-en-4-o1 (12.4%). Another report [9] described the main composi- tion of S. chinensis to be sabinene (20.99%), limonene (19.78%) and bornyl acetate (11.68%). The oil composition of two other species in the genus has been documented. The major compounds of S. vir- giniana [10] from Japan were limonene (32.9%), safrole (23.0%), asarone (15.9%) and α-pinene (5.2%). S. chi- *Corresponding author. Copyright © 2013 SciRes. AJPS  Terpene Composition of Three Species of Gymnosperms from Vietnam 2032 nensis cv. Kaizuca leaf oil, as reported by Cui et al. [10] contained bornyl acetate (46.5%), limonene (30.0%) and β-pinene (7.9%), while limonene (24.56%) and β-myr- cene (8.04%) are the prominent compounds from sample analysed by Hao et al. [9]. The volatile oil from S. chi- nensis had greater effects of bacteriostasis and air decon- tamination [7,8,11]. It is also indicated that constituents such as bornyl acetate, phellandrene, limonene and alpha- pinene and beta-pinene could also contribute to the anti- microbial activity of the volatile oils [11]. Thuja occidentalis (syn. Platycladus orientalis (L.) Franco or Biota orientalis (L.) Endl.) is an evergreen co- niferous tree, in the cypress family Cupressaceae, which is native to the northeast of the United States and the southeast of Canada, but widely cultivated as an orna- mental plant. T. occidentalis has fan-like branches and scaly leaves. It is only a small tree, growing to a height of 10 - 20 m. The bark is red-brown, furrowed and peels in narrow, longitudinal strips. The cones are slender, yel- low-green ripening brown, 10 - 15 mm broad, with 6 - 8 overlapping scales. The branches may take root if the tree falls. Plants are susceptible to attacks by honey fun- gus [12]. Plants are monoecious, male catkins being pro- duced at the tips of branches and female cones at the base [12]. The essential oil from the leaf of P. orientalis is an important natural product which is used in fragrance, air freshener, deodorizer, insectifuge, and aromatherapy. The essential oil composition of the leaf of T. orien- talis was investigated in Xuzhou, China [13], where its main components were α-pinene (40.2%), 3-carene (13.2%), and cedrol (9.3%). The data reported from Iran [14-16] showed that the main constituents were α-pinene (15.0% - 30.0%), 3-carene (10.5% - 21.7%), and cedrol (6.1% - 20.3%). Previous studies in China [17-20] had revealed that the main constituents were α-pinene (8.4% - 24.9%), 3-carene (2.6% - 12.2%), and cedrol (0.7% - 31.4%). In a recent report [21], a population of T. orientalis afforded oils whose major compounds were α-pinene (37.6% - 68.1%), cedrol (3.4% - 18.1%), 3-carene (0.1% - 29.4%), β-caryophyllene (2.0% - 10.2%) and α-caryophyllene (1.7% - 7.1%) while a sample contained cedrol (15.0%), 3-carene (12.2%) and β-caryophyllene (8.0%), yet an- other sample had cedrol (19.2%), β-caryophyllene (14.3%) and α-caryophyllene (11.3%) while the last sample con- tained cedrol (23.7%), terpinenyl acetate (19.4%), 3-ca- rene (10.6%) and α-pinene (10.2%). α- and β-Thujone, fenchone, camphor and sabinene, as well as the diterpen- es beyerene and rimuene were the main compounds in the work of Tsiri et al. [22] and Chizzola et al. [23]. In their own investigation, Guleria et al. [24] identified α- pinene (29.2%), -3-carene (20.1%), α-cedrol (9.8%), ca- ryophyllene (7.5%), α-humulene (5.6%) and limonene (5.4%) in the oil of the plant. The dominant constituents in essential oil of T. orientalis from most localities are a- pinene, cedrol, and 3-carene. The previous studies also reveal a large variation in the essential oil composition of T. orientalis. In the present paper, we report the essential oil compo- sitions of three gymnosperms namely Cupressus tonki- nensis Silba., Sabina chinensis (L.) Antoine and Thuja occidentalis L., from Vietnam. This is part of our exten- sive research on the volatile composition of Vietnamese flora as they are made available [25]. 2. Materials and Methods 2.1. Plants Collection Leaves of C. tonkiensis were collected from Huu Lien Natural reserve, Lang Son Province, Vietnam, in August 2010. The leaves of S. chinensis and T. orientalis were collected from Nghe An Province in August 2011. Vou- cher specimens DND 721, DND 194 and DND 196, re- spectively have been deposited at the Botany Museum, Vinh University, Vietnam. Plant samples were air-dried prior to extraction. 2.2. Isolation of the Volatile Oils 0.5 kg of air-dried sample of each species was shredded and their oils obtained by hydrodistillation for 4 h at nor- mal pressure, according to the Vietnamese Pharmacopo- eia [26]. The plant samples yielded a low content of es- sential oils: 0.20%, 0.18% and 0.20% (v/w; respectively for C. tonkiensis, S. chinensis and T. orientalis). Oil sam- ples were light yellow colored. 2.3. Gas Chromatography (GC) Analysis Gas chromatography (GC) analysis was performed on an Agilent Technologies HP 6890 Plus Gas chromatograph equipped with a FID and fitted with HP-Wax and HP- 5MS columns (both 30 m × 0.25 mm, film thickness 0.25 m, Agilent Technology). The analytical conditions were: carrier gas H2 (1 mL/min), injector temperature (PTV) 250˚C, detector temperature 260˚C, column temperature programmed from 40˚C (2 min hold) to 220˚C (10 min hold) at 4˚C/min. Samples were injected by splitting and the split ratio was 10:1. The volume injected was 1.0 L. Inlet pressure was 6.1 kPa. 2.4. Gas Chromatography/Mass Spectrometry Analysis/(GC-MS) An Agilent Technologies HP 6890N Plus Chromato- graph fitted with a fused silica capillary HP-5 MS col- umn (30 m × 0.25 mm, film thickness 0.25 m) and in- terfaced with a mass spectrometer HP 5973 MSD was used for the GC/MS analysis, under the same conditions Copyright © 2013 SciRes. AJPS 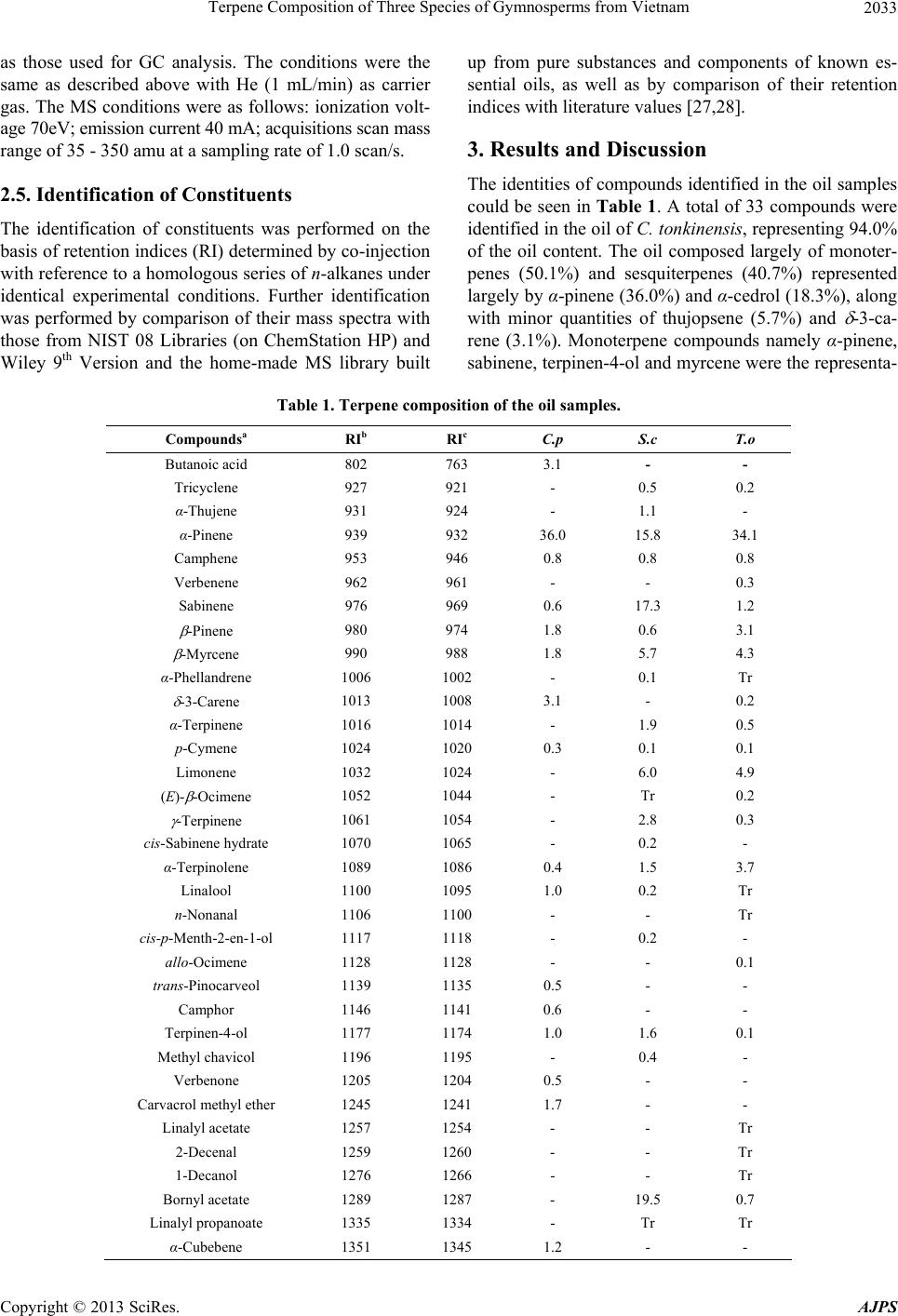 Terpene Composition of Three Species of Gymnosperms from Vietnam Copyright © 2013 SciRes. AJPS 2033 as those used for GC analysis. The conditions were the same as described above with He (1 mL/min) as carrier gas. The MS conditions were as follows: ionization volt- age 70eV; emission current 40 mA; acquisitions scan mass range of 35 - 350 amu at a sampling rate of 1.0 scan/s. 2.5. Identification of Constituents The identification of constituents was performed on the basis of retention indices (RI) determined by co-injection with reference to a homologous series of n-alkanes under identical experimental conditions. Further identification was performed by comparison of their mass spectra with those from NIST 08 Libraries (on ChemStation HP) and Wiley 9th Version and the home-made MS library built up from pure substances and components of known es- sential oils, as well as by comparison of their retention indices with literature values [27,28]. 3. Results and Discussion The identities of compounds identified in the oil samples could be seen in Table 1. A total of 33 compounds were identified in the oil of C. tonkinensis, representing 94.0% of the oil content. The oil composed largely of monoter- penes (50.1%) and sesquiterpenes (40.7%) represented largely by α-pinene (36.0%) and α-cedrol (18.3%), along with minor quantities of thujopsene (5.7%) and -3-ca- rene (3.1%). Monoterpene compounds namely α-pinene, sabinene, terpinen-4-ol and myrcene were the representa- Table 1. Terpene composition of the oil samples. Compoundsa RIb RIc C.p S.c T.o Butanoic acid 802 763 3.1 - - Tricyclene 927 921 - 0.5 0.2 α-Thujene 931 924 - 1.1 - α-Pinene 939 932 36.0 15.8 34.1 Camphene 953 946 0.8 0.8 0.8 Verbenene 962 961 - - 0.3 Sabinene 976 969 0.6 17.3 1.2 -Pinene 980 974 1.8 0.6 3.1 -Myrcene 990 988 1.8 5.7 4.3 α-Phellandrene 1006 1002 - 0.1 Tr -3-Carene 1013 1008 3.1 - 0.2 α-Terpinene 1016 1014 - 1.9 0.5 p-Cymene 1024 1020 0.3 0.1 0.1 Limonene 1032 1024 - 6.0 4.9 (E)- -Ocimene 1052 1044 - Tr 0.2 -Terpinene 1061 1054 - 2.8 0.3 cis-Sabinene hydrate 1070 1065 - 0.2 - α-Terpinolene 1089 1086 0.4 1.5 3.7 Linalool 1100 1095 1.0 0.2 Tr n-Nonanal 1106 1100 - - Tr cis-p-Menth-2-en-1-ol 1117 1118 - 0.2 - allo-Ocimene 1128 1128 - - 0.1 trans-Pinocarveol 1139 1135 0.5 - - Camphor 1146 1141 0.6 - - Terpinen-4-ol 1177 1174 1.0 1.6 0.1 Methyl chavicol 1196 1195 - 0.4 - Verbenone 1205 1204 0.5 - - Carvacrol methyl ether 1245 1241 1.7 - - Linalyl acetate 1257 1254 - - Tr 2-Decenal 1259 1260 - - Tr 1-Decanol 1276 1266 - - Tr Bornyl acetate 1289 1287 - 19.5 0.7 Linalyl propanoate 1335 1334 - Tr Tr α-Cubebene 1351 1345 1.2 - - 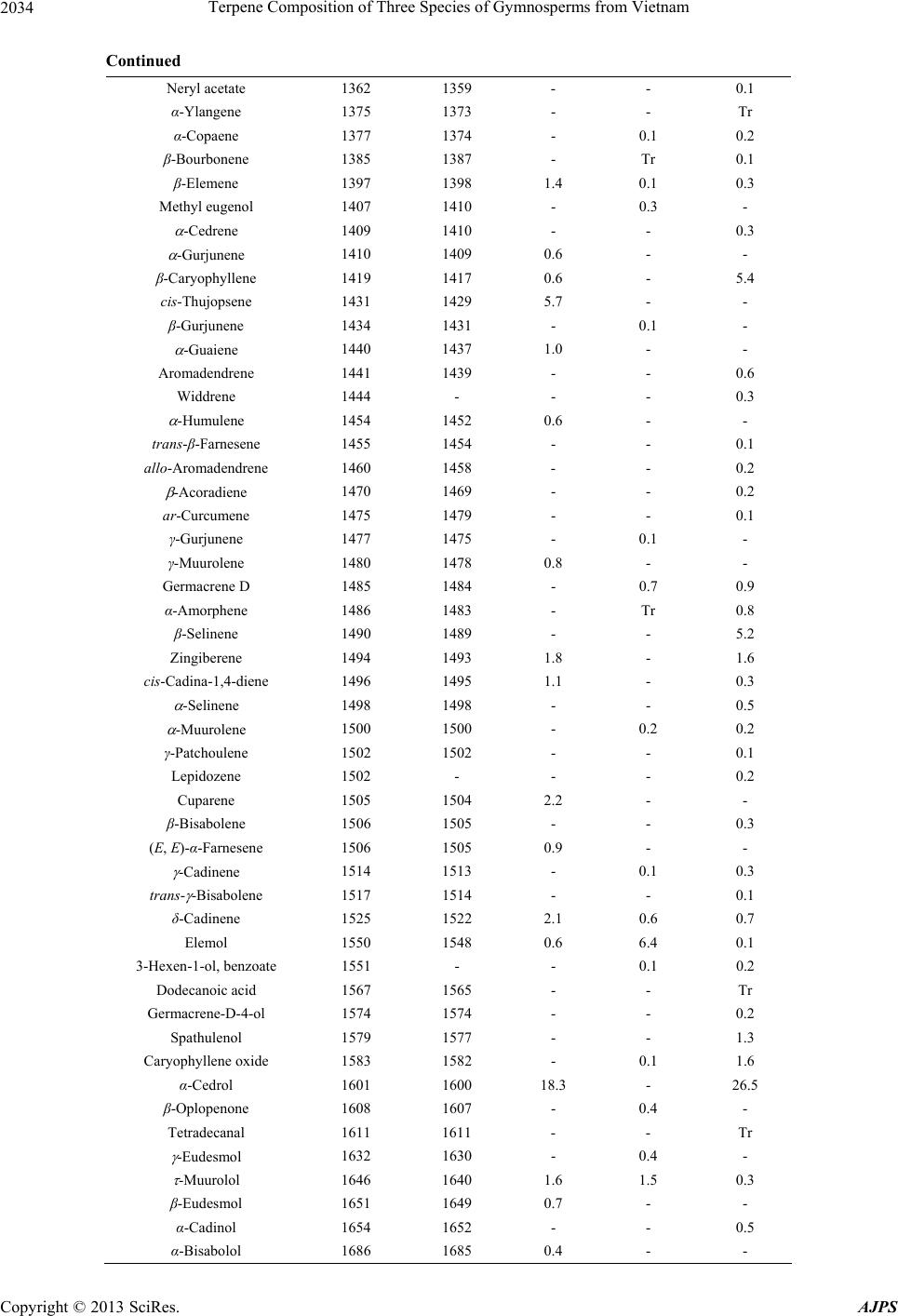 Terpene Composition of Three Species of Gymnosperms from Vietnam 2034 Continued Neryl acetate 1362 1359 - - 0.1 α-Ylangene 1375 1373 - - Tr α-Copaene 1377 1374 - 0.1 0.2 β-Bourbonene 1385 1387 - Tr 0.1 β-Elemene 1397 1398 1.4 0.1 0.3 Methyl eugenol 1407 1410 - 0.3 - -Cedrene 1409 1410 - - 0.3 -Gurjunene 1410 1409 0.6 - - β-Caryophyllene 1419 1417 0.6 - 5.4 cis-Thujopsene 1431 1429 5.7 - - β-Gurjunene 1434 1431 - 0.1 - -Guaiene 1440 1437 1.0 - - Aromadendrene 1441 1439 - - 0.6 Widdrene 1444 - - - 0.3 -Humulene 1454 1452 0.6 - - trans-β-Farnesene 1455 1454 - - 0.1 allo-Aromadendrene 1460 1458 - - 0.2 -Acoradiene 1470 1469 - - 0.2 ar-Curcumene 1475 1479 - - 0.1 γ-Gurjunene 1477 1475 - 0.1 - γ-Muurolene 1480 1478 0.8 - - Germacrene D 1485 1484 - 0.7 0.9 α-Amorphene 1486 1483 - Tr 0.8 β-Selinene 1490 1489 - - 5.2 Zingiberene 1494 1493 1.8 - 1.6 cis-Cadina-1,4-diene 1496 1495 1.1 - 0.3 -Selinene 1498 1498 - - 0.5 -Muurolene 1500 1500 - 0.2 0.2 γ-Patchoulene 1502 1502 - - 0.1 Lepidozene 1502 - - - 0.2 Cuparene 1505 1504 2.2 - - β-Bisabolene 1506 1505 - - 0.3 (E, E)-α-Farnesene 1506 1505 0.9 - - -Cadinene 1514 1513 - 0.1 0.3 trans- -Bisabolene 1517 1514 - - 0.1 δ-Cadinene 1525 1522 2.1 0.6 0.7 Elemol 1550 1548 0.6 6.4 0.1 3-Hexen-1-ol, benzoate 1551 - - 0.1 0.2 Dodecanoic acid 1567 1565 - - Tr Germacrene-D-4-ol 1574 1574 - - 0.2 Spathulenol 1579 1577 - - 1.3 Caryophyllene oxide 1583 1582 - 0.1 1.6 α-Cedrol 1601 1600 18.3 - 26.5 β-Oplopenone 1608 1607 - 0.4 - Tetradecanal 1611 1611 - - Tr -Eudesmol 1632 1630 - 0.4 - -Muurolol 1646 1640 1.6 1.5 0.3 β-Eudesmol 1651 1649 0.7 - - α-Cadinol 1654 1652 - - 0.5 α-Bisabolol 1686 1685 0.4 - - Copyright © 2013 SciRes. AJPS  Terpene Composition of Three Species of Gymnosperms from Vietnam Copyright © 2013 SciRes. AJPS 2035 Continued Franesold 1718 - - - 0.1 Benzyl benzoate 1760 1759 - Tr - Tetradecanoic acid 1770 - - - -Tr 1,2-Benzenedicarboxylic acid 1917 1917 - 1.0 2.9 16-Hexadecanolide 1928 - - - 0.2 ent-Pimara-8(14),15-diene 1939 - - 0.3 - Hexadecanoic acid 1970 1959 - Tr Tr Abietatriene 2050 2055 - 0.7 0.2 Phytol 2125 1942 - - Tr Totarol 2314 2314 - 0.1 - cis-Ferruginol 2371 2370 - 0.1 0.1 (Z)-9-Octadecenamide 2398 2396 - - 0.1 Total 94.0 90.0 98.4 Monoterpene hydrocarbons 44.8 53.6 54.2 Oxygenated monoterpenes 5.3 23.5 0.9 Sesquiterpene hydrocarbons 20.0 2.0 19.2 Oxygenated sesq u iterpenes 20.7 8.8 20.6 Diterpenes - 1.2 0.1 Non-terpenes 3.1 1.1 3.4 aElution order on HP-5MS capillary column; bRetention indices on HP-5MS capillary column; cLiterature Retention indi- ces (see Experimental); dCorrect isomer not identified; - Not identified and not present in Literature; Tr, Trace amount, <0.1%; C. p = Cupressus tonkinensis; S. c = Sabina chinensis; T. o = Thuja orientalis. tive compounds of previous studies on C. tonkinensis [3, 4]. The α-pinene content of this oil competes favourably with previous studies while the contents of other mono- terpenes above are significantly lower. Cedrol was pre- viously present in the stem oil but not in the leaf [4]. A total of 45 compounds could be identified from the leaf oil of S. chinensis, representing 90.0% of the oil con- tent. Also, monoterpenoids (77.1%) were in abundance in the oil. The major constituents among the monoter- penes were bornyl acetate (19.5%), sabinene (17.3%) and α-pinene (15.8%). The large amount of α-pinene and sa- binene is in agreement with previous reports [6-9], but differs from its lower contents of tricyclene, β-myrcene, α-phellandrene, limonene and p-menth-l-en-4-o1. Ses- quiterpenes are less common (10.8%) represents mainly by elemol (6.4%) and -muurolol (1.5%) as compounds above 1%. There seems to be homogeneity in the oil composition of S. chinensis. Bornyl acetate was among the main compounds of S. chinensis cv. Kaizuca [10]. Limonene is a common compound of the oil of the genus Sabina [6-10]. Monoterpenes (55.1%) and sesquiterpenes (39.8%) are classes of compounds identified in the 69 constituents of the oil of T. orientalis. The main compounds were α- pinene (34.1%) and α-cedrol (16.5%). Other significant compounds were β-caryophyllene (5.4%), β-selinene (5.2%), limonene (4.9%) and α-terpinene (3.7%). The predominant compounds were α-pinene and cedrol. This result was similar to those previously reported [13-21,24, 29,30-32], which revealed that the main constituents were a-pinene (15.0% - 68.1%), cedrol (3.4% - 39.06%) and 3-carene (0.1% - 34.55%). However, the content of 3-carene in this sample is too low when compared with previous studies (Table 2). Other compounds such as β- caryophyllene, α-caryophyllene and terpinenyl acetate [21], α-and β-thujone, fenchone, sabinene, camphor, beyerene and rimuene [22,23], fenchol and β-santalene [32] and cedrene [19], that are characteristics of previous reports are either observed in low amounts (e.g. -3-carene, sabi- nene) or completely absent (e.g. α- and β-thujone, fen- chone, fenchol, terpinenyl acetate, β-santalene, α-caryo- phyllene, cedrene, beyerene and rimuene) in this present study (Tables 1 and 2). The previous studies also revealed a large variation in the essential oil composition of T. orientalis. Therefore, the oils of T. orientalis could be classified as follows: 1) group characterized by a predominant α-pinene, - 3-carene and cedrol [13-18,20,21,24,29 and this study] 2) group dominated by cedrol, -3-carene and β-cary- ophyllene [21] 3) group with contents of cedrol, β-caryophyllene and α-caryophyllene 4) α-pinene, -3-carene and sabinene group [14] 5 ) α-thujone, β-thujone and fenchone rich oil [22] 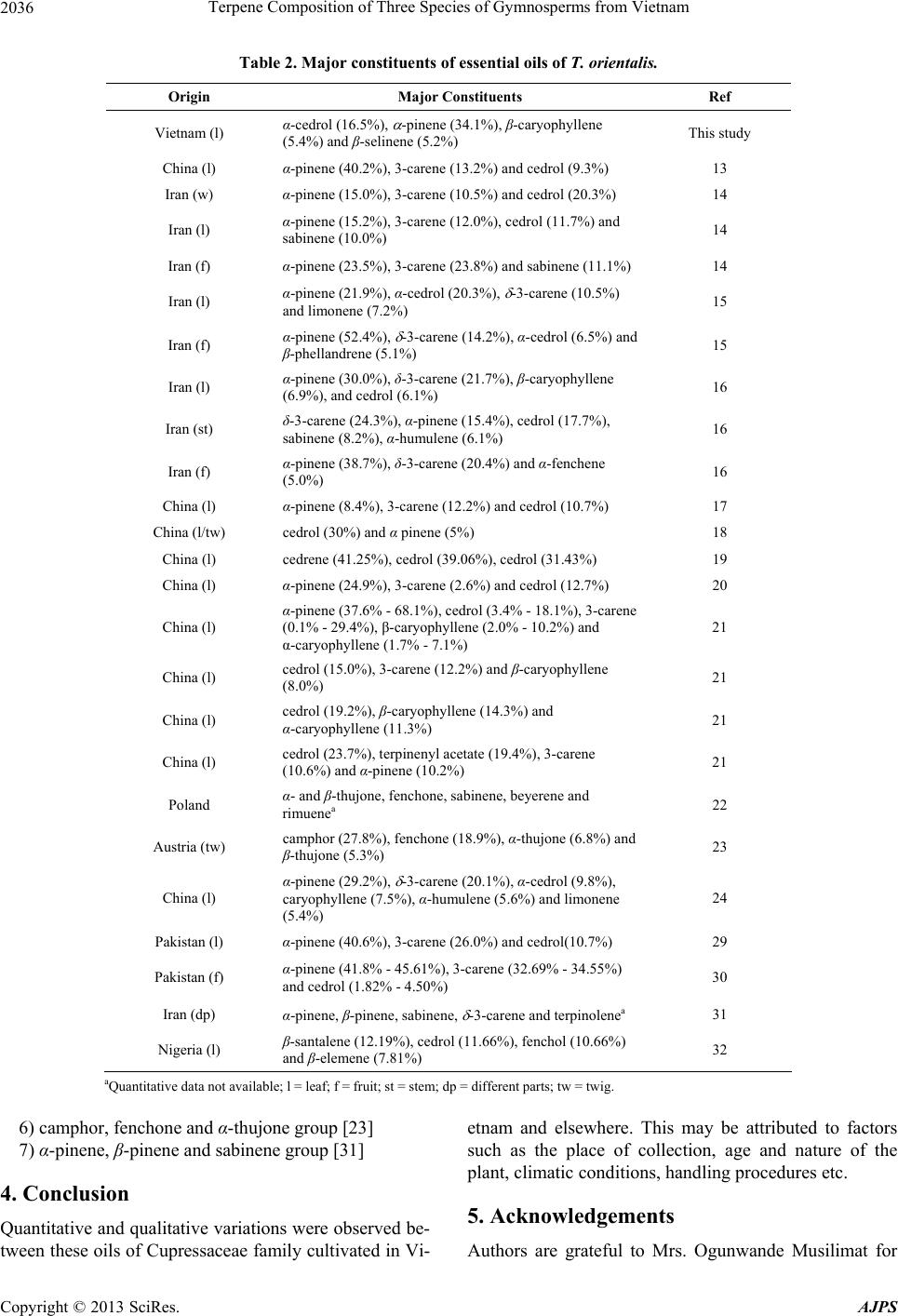 Terpene Composition of Three Species of Gymnosperms from Vietnam 2036 Table 2. Major constituents of essential oils of T. orientalis. Origin Major Constituents Ref Vietnam (l) α-cedrol (16.5%), -pinene (34.1%), β-caryophyllene (5.4%) and β-selinene (5.2%) This study China (l) α-pinene (40.2%), 3-carene (13.2%) and cedrol (9.3%) 13 Iran (w) α-pinene (15.0%), 3-carene (10.5%) and cedrol (20.3%) 14 Iran (l) α-pinene (15.2%), 3-carene (12.0%), cedrol (11.7%) and sabinene (10.0%) 14 Iran (f) α-pinene (23.5%), 3-carene (23.8%) and sabinene (11.1%) 14 Iran (l) α-pinene (21.9%), α-cedrol (20.3%), -3-carene (10.5%) and limonene (7.2%) 15 Iran (f) α-pinene (52.4%), -3-carene (14.2%), α-cedrol (6.5%) and β-phellandrene (5.1%) 15 Iran (l) α-pinene (30.0%), δ-3-carene (21.7%), β-caryophyllene (6.9%), and cedrol (6.1%) 16 Iran (st) δ-3-carene (24.3%), α-pinene (15.4%), cedrol (17.7%), sabinene (8.2%), α-humulene (6.1%) 16 Iran (f) α-pinene (38.7%), δ-3-carene (20.4%) and α-fenchene (5.0%) 16 China (l) α-pinene (8.4%), 3-carene (12.2%) and cedrol (10.7%) 17 China (l/tw) cedrol (30%) and α pinene (5%) 18 China (l) cedrene (41.25%), cedrol (39.06%), cedrol (31.43%) 19 China (l) α-pinene (24.9%), 3-carene (2.6%) and cedrol (12.7%) 20 China (l) α-pinene (37.6% - 68.1%), cedrol (3.4% - 18.1%), 3-carene (0.1% - 29.4%), β-caryophyllene (2.0% - 10.2%) and α-caryophyllene (1.7% - 7.1%) 21 China (l) cedrol (15.0%), 3-carene (12.2%) and β-caryophyllene (8.0%) 21 China (l) cedrol (19.2%), β-caryophyllene (14.3%) and α-caryophyllene (11.3%) 21 China (l) cedrol (23.7%), terpinenyl acetate (19.4%), 3-carene (10.6%) and α-pinene (10.2%) 21 Poland α- and β-thujone, fenchone, sabinene, beyerene and rimuenea 22 Austria (tw) camphor (27.8%), fenchone (18.9%), α-thujone (6.8%) and β-thujone (5.3%) 23 China (l) α-pinene (29.2%), -3-carene (20.1%), α-cedrol (9.8%), caryophyllene (7.5%), α-humulene (5.6%) and limonene (5.4%) 24 Pakistan (l) α-pinene (40.6%), 3-carene (26.0%) and cedrol(10.7%) 29 Pakistan (f) α-pinene (41.8% - 45.61%), 3-carene (32.69% - 34.55%) and cedrol (1.82% - 4.50%) 30 Iran (dp) α-pinene, β-pinene, sabinene, -3-carene and terpinolenea 31 Nigeria (l) β-santalene (12.19%), cedrol (11.66%), fenchol (10.66%) and β-elemene (7.81%) 32 aQuantitative data not available; l = leaf; f = fruit; st = stem; dp = different parts; tw = twig. 6) camphor, fenchone and α-thujone group [23] 7) α-pinene, β-pinene and sabinene group [31] 4. Conclusion Quantitative and qualitative variations were observed be- tween these oils of Cupressaceae family cultivated in Vi- etnam and elsewhere. This may be attributed to factors such as the place of collection, age and nature of the plant, climatic conditions, handling procedures etc. 5. Acknowledgements Authors are grateful to Mrs. Ogunwande Musilimat for Copyright © 2013 SciRes. AJPS  Terpene Composition of Three Species of Gymnosperms from Vietnam 2037 the typesetting of the manuscript. REFERENCES [1] J. Silba, “The Trans-Pacific Relationship of Cupressus in India and North America,” Journal of the International Conifer Preservation Society, Vol. 1, No. 1, 1994, pp. 23- 25. [2] K. Rushforth, “Notes on the Cupressaceae in Vietnam,” Vietnam Journal of Biology, Vol. 29, No. 1, 2007, pp. 32- 39. [3] T. H. Thai, N. T. Hien, D. T. Minh and P. V. The, “The Chemical Composition of Leaf Oil of Cupressus tonki- nensis Silba. in Huu Lien, Lang Son Province,” Vietnam Journal of Biology, Vol. 31, No. 1, 2009, pp. 74-79. [4] T. H. Thai, O. Bazzali, N. T. Hien, P. V. The, P. K. Loc, T. M. Hoi, F. Tomi, J. Casanova and A. Bighelli, “Che- mical Composition of Leaf and Stem Oils from Vietnam- ese Cupressus tonkinensis Silba,” Journal of Essential Oil Research, Vol. 25, No. 1, 2013, pp. 11-16. http://dx.doi.org/10.1080/10412905.2012.751056 [5] Y. Zhang and J. Ming, “Classification of Sabina chinensis (Linn.) Ant. Cultivars,” Journal of Hubei Agricultural College, Vol. 21, No. 1, 2001, pp. 25-28. [6] H. Y. Zilian, “Constituents of the Essential Oils from Sa- bina chinensis,” Natural Product Research and Develop- ment, Vol. 6, No. 1, 1990, pp. 36-39. [7] Y. Gao, Y.-J. Jin, H.-D. Li and H.-J. Chen, “Volatile Or- ganic Compounds and Their Roles in Bacteriostasis in Five Conifer Species,” Journal of Integrative Plant Biol- ogy, Vol. 47, No. 4, 2005, pp. 499-507. http://dx.doi.org/10.1111/j.1744-7909.2005.00081.x [8] Y.-Q. Cui, P. Nan and M.-H. Lin, “Main Volatile Com- ponents in the Leaves of Sabina chinensis L. Ant. and Sabina chinensis L. Ant. cv. Kaizuca and Their Effects on Bacteria,” Journal of Environment and Health, Vol. 21, No. 3, 2006. http://www.shvoong.com/medicine-and-health/1611504- main-volatile-components-leaves-sabina/#ixzz2RyT5slG7 [9] D. Hao, Y. Zhang, H. Dai and Y. Wang, “Analysis of Vo- latile Constituents in Leaves of Three Cypress Species by Gas Chromatography/Mass Spectrometry,” Chinese Jour- nal of Chromatography, Vol. 24, No. 2, 2006, pp. 185- 187. [10] S. A. Aboaba, I. A. Oladosu and I. A. Ogunwande, “Topi- cal Anti-Inflammatory and Chemical Composition of Es- sential Oil of Sabina virginiana L. Antoine (Cupressa- ceae),” Archives of Applied Science Research, Vol. 2, No. 2, 2010, pp. 1-6. [11] Y.-Q. Cui, X.-H. Cui, Y. Zhu, Q. Zhang and P. Nan, “Che- mical Composition and Antimicrobial Activity of Volatile Oil of Six Gymnosperm Species Leaves from Shanghai,” The 2nd International Conference on Bioinformatics and Biomedical Engineering (ICBBE), 2008, pp. 4573-4577. http://dx.doi.org/10.1109/ICBBE.2008.303 [12] V. D. Nguyen and T. N. Doan, “Medicinal Plants in Viet- nam,” Hanoi Publishing House, Hanoi, 2006, p. 234. [13] Y. D. Chen, S. X. Li, L. Yang, Z. Y. Jiang and N. H. Cui, “Comparative Study on Chemical Constituents of Essen- tial Oils from several parts of Platycladus orientlais,” Chemical Industry and Forest, Vol. 1, No. 1, 1984, pp. 1- 11. [14] M. K. Hassanzadeh, M. Rahimizadeh, B. S. Fazly Bazzaz, S. A. Emami and J. Assili, “Chemical and Antimicrobial Studies of Platycladus Orientalis Essential Oils,” Phar- maceutical Biology, Vol. 39, No. 5, 2001, pp. 388-390. http://dx.doi.org/10.1076/phbi.39.5.388.5894 [15] B. Nickavar, G. Amin and S. Parhami, “Volatile Consti- tuents of the Fruit and Leaf Oils of Thuja orientalis L. grown in Iran,” Z Naturforsch C, Vol. 58, No. 3-4, 2003, pp. 171-172. [16] S. Afsharypuor and B. Nayebzadeh, “Essential Oil Con- stituents of Young Stem, Leaf and Fruit of Platycladus orientalis (L.) Franco Grown in Isfahan (Iran),” Journal of Essential Oil Research, Vol. 21, No. 6, 2009, pp. 525- 528. http://dx.doi.org/10.1080/10412905.2009.9700235 [17] T. L. Liu, Q. Qiu, Y. Zhao, Y. M. Shen and J. Wang, “Study on Chemical Constituents of Essential Oil of Bi- ota orientlais by GC-MS,” Journal of Chinese Medicinal Materials, Vol. 23, No. 10, 2000, pp. 460-461. [18] G. Wei and S. Y. Wang, “The GC-MS Analysis for Vola- tile Constituents in Leaf/Twigs of Chinese Arborvitae,” Lishizhen Medicinal Materia Medica Research, Vol. 12, No. 1, 2001, pp. 18-19. [19] R. H. Hui, D. Y. Hou, X. Y. Liu, X. C. Li and H. L. Geng, “Analysis of Volatile Components from Leaf Twigs in Biota orientalis with Different Extraction Methods by Gas Chromatography-Mass Spectrometry,” Journal of Chinese Mass Spectrometry Society, Vol. 27, No. 3, 2006, pp. 226-231. [20] P. Li, L. S. Wang and H. Tang, “Determination of Chemi- cal Constituents of the Essential Oil from Cacumen Platy- cladi in Xinjiang by GC-MS,” Lishizhen Medicinal Mate- ria Medica Research, Vol. 17, No. 12, 2006, pp. 951-957. [21] H. Lei, Y. Wang, F. Liang, W. Su, Y. Feng, X. Guo and N. Wang, “Composition and Variability of Essential Oils of Platycladus orientalis Growing in China,” Biological and Systematic Ecology, Vol. 38, No. 9, 2010, pp. 1000- 1006. http://dx.doi.org/10.1016/j.bse.2010.09.018 [22] D. Tsiri, K. Graikou, L. Pobłocka-Olech, M. Krauze-Ba- ranowska, C. Spyropoulos and I. Chinou “Chemosyste- matic Value of the Essential Oil Composition of Thuja Species Cultivated in Poland-Antimicrobial Activity,” Mo- lecules, Vol. 19, No. 14, 2009, pp. 4707-4715. [23] R. Chizzola, W. Hochsteiner and S. Hajek, “GC Analysis of Essential Oils in the Rumen Fluid after Incubation of Thuja orientalis Twigs in the Rusitec System,” Research in Veterinary Science, Vol. 76, No. 1, 2004, pp. 77-82. http://dx.doi.org/10.1016/j.rvsc.2003.07.001 [24] S. Guleria, A. Kumar and A. K. Tiku, “Chemical Compo- sition and Fungitoxic Activity of Essential Oil of Thuja orientalis L. Grown in the North-Western Himalaya,” Zeit Naturforsch C, Vol. 63, No. 3-4, 2008, pp. 211-214. [25] D. N. Dai, T. D. Thang, L. T. M. Chau and I. A. Ogun- wande, “Chemical Constituents of the Root Essential Oils of Zingiber rubens and Zingiber zerumbet,” American Journal of Plant Sciences, Vol. 4, No. 1, 2013, pp. 7-10. Copyright © 2013 SciRes. AJPS  Terpene Composition of Three Species of Gymnosperms from Vietnam Copyright © 2013 SciRes. AJPS 2038 http://dx.doi.org/10.4236/ajps.2013.41002 [26] “Vietnamese Pharmacopoeia,” Medical Publishing House, Hanoi, 1997, p. 89. [27] R. P. Adams, “Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectrometry,” 4th Edition, Allured Publishing, Carol Stream, 2007. [28] D. Joulain and W. A. Koenig, “The Atlas of Spectral Data of Sesquiterpene,” E. B. Verlag, Hamburg, 1998. [29] M. Riaz, M. K. Rashid and F. M. Chaudary, “Volatile Constituents of the Leaves Pakistani Cumpressus sem- pervirens and Thuja orientalis,” Pakistan Journal of Sci- ence and Industrial Research, Vol. 42, No. 2, 1999, pp. 98-101. [30] M. Riaz, S. Qamar, M. K. Rashid and F. M. Chaudary, “Chemical Composition of Thuja orientalis L. Fruits at Different Stages of Maturity,” Pakistan Journal of Sci- ence and Industrial Research, Vol. 42, No. 4, 1999, pp. 188-191. [31] I. A. Ogunwande, N. O. Olawore, K. A. Adeleke and O. Ekundayo, “Composition of the Volatile Oil of Thuja ori- entalis L. from Nigeria,” Journal of Tropical Forest Re- sources, Vol. 19, No. 2, 2003, pp. 41-47. [32] S. A. Emami, J. Asili, M. Malekian and M. K. Hassanzade, “Antioxidant Effects of the Essential Oils of Different Parts of Platycladus orientalis L. (Franco) and Their Com- ponents,” Journal of Essential Oil Bearing Plants, Vol. 14, No. 3, 2011, pp. 103-107. http://dx.doi.org/10.1080/0972060X.2011.10643943
|