 J. Biomedical Science and Engineering, 2013, 6, 8-14 JBiSE http://dx.doi.org/10.4236/jbise.2013.610A2002 Published Online October 2013 (http://www.scirp.org/journal/jbise/) Dobesilate for dry age-related macular degeneration* Pedro Cuevas1#, Luis A. Outeiriño2, Carlos Azanza2, Javier Angulo1, Guillermo Giménez-Gallego3 1Departamento de Investigación, IRYCIS, Hospital Universitario Ramón y Cajal, Madrid, Spain 2Departamento de Oftalmología, Hospital de Día Pío XII, Madrid, Spain 3Departamento de Estructura y Función de Proteínas, Centro de Investigaciones Biológicas, CSIC, Madrid, Spain Email: #pedro.cuevas@hrc.es Received 24 March 2013; revised 29 August 2013; accepted 16 September 2013 Copyright © 2013 Pedro Cuevas et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT We have evaluated the effects of intravitreal dobesi- late, a synthetic fibroblast growth factor inhibitor, in patients with dry age-related macular degeneration, an inflammatory-related retinal disease without available treatment up to date. 36 eyes from 36 pa- tients with dry age-related macular degeneration were treated with a single intravitreal dobesilate in- jection. The end points were the improvement from baseline visual acuity and normalization of retinal histology at one month. Intravitreal dobesilate injec- tion resulted in a significant improvement in func- tional and anatomical outcomes at one month after injection. Our results suggest that intravitreal dobe- silate may increase the chance of visual acuity gain in dry age-related macular degeneration, even in cases with initial low vision. This study supports the find- ings of previously published case reports, regarding the short-term improvement in visual acuity by in- travitreal dobesilate injection in different degenera- tive retinal diseases. Keywords: Dry Age-Related Macular Degeneration; Fibroblast Growth Factor Inhibition; Intravitreal Dobesilate 1. INTRODUCTION Age-related macular degeneration (AMD) is the primary cause of blindness and visual disability in people aged over 50 and its prevalence increases exponentially after the age of 70 [1,2]. AMD is diagnosed as either wet (ne- ovascular) or dry (atrophic). Wet AMD is characterized by choroidal nevoascularization (CNV): the formation of hyperpermeable new blood vessels beneath the retinas that leak plasma and often bleed, leading to the forma- tion of scar tissue which can severely and irreversibly compromise visual acuity. Although it constitutes only 10% - 15% of all cases, wet AMD accounts for almost 80% of AMD-related blindness [3]. Dry AMD, in contrast, does not involve leaking ves- sels from choroidal vasculature. Dry AMD is character- ized by a well-defined constellation of clinical features, including drusen, pigment abnormalities/focal hyper- or hypo-pigmentation of the retinal pigment epithelium (RPE), and geographic atrophy (GA) of the macula. GA represents the atrophic late stage of dry AMD [1,2]. GA is characterized by roughly oval areas of hypopigmenta- tion and is usually the consequence of RPE cell loss. Loss of RPE cells, responsible for the overlying photo- receptors surviving, leads to the gradual degeneration of nearly photoreceptors, resulting in thinning of the retina. RPE degeneration leads to the death of photoreceptor cells causing irreversible vision loss. Since the natural evolution of dry AMD is toward a wet AMD condition, the holy grail of therapy for AMD is to avoid the devel- opment of CNV. Today, there are no approved treat- ments for dry AMD. Considering the significant medical, personal, social and economic costs of AMD, the need for novel thera- peutic and preventive strategies for AMD is pressing. Innovation in AMD pharmacotherapy, in turn, depends largely upon a thorough understanding of the molecular mechanisms underlying AMD pathogenesis. There is ample evidence that inflammation plays an important role in both dry and wet AMD [4-9]. Several studies have documented a significant association between in- flammatory diseases and upregulation of fibroblast growth factor (FGF) [10-16]. Recently, it has been pos- ited the importance of FGF in neuroinflammatory dis- eases such as AMD where inflammation is one of the main components of disease progression and FGF and its receptors (FGF/FGFR) are prominently expressed [17]. *Consent: obtained. Competing interest: the authors report no conflicts of interest in this work. #Corresponding author. OPEN ACCESS  P. Cuevas et al. / J. Biomedical Science and Engineering 6 (2013) 8-14 9 This study supports an important clinical interest for searching safe and efficient inhibitors of FGF/FGFR axis to treat retinal inflammatory diseases, including AMD. The aim of this study was to analyze treatment with a single dose of intravitreal dobesilate in patients who were diagnosed with dry age-related macular degenera- tion. 2. METHODS 2.1. Participants In this consecutive study, more than 200 patients with age-related macular degeneration who visited the Clínica Oftalmológica Hospital Pío XII of Madrid (Spain) be- tween January 2012 and October 2012, were screened, and 36 eyes from 36 patients with dry age-related macu- lar degeneration were included. Baseline data for all study patients is shown in Table 1. The study was ap- proved by the local institutional review board and in- formed consent was obtained from every patient for the intravitreal injection. The subjects were also provided information about the off-label use of dobesilate. 2.2. Inclusion/Exclusion Criteria Inclusion criteria were the presence of early, intermedi- ate or late stages of dry AMD. Eyes that met any of the following criteria were excluded from enrolment: 1) had severe disease that was judged by the treating investiga- tor as being unlikely to benefit from further therapy (such as those with central ischemia or macular scarring); 2) had vision loss from other coexisting ocular disease and 3) had undergone ocular surgical interventions within 6 months prior to study entry. 2.3. Procedures Examination at baseline included best corrected visual Table 1. Baseline characteristics of the patients. Sex Male 16 Female 20 Age Mean 70 ± 7 Intermediate phase of dry AMD Male 14 Female 16 Late phase of dry AMD (geographic atrophy) Male 2 Female 4 acuity (BCVA) with a Snellen chart at a distance of 20 feet, slit lamp biomicroscopy of the anterior segment and fundus, and spectral domain optical coherence tomogra- phy (SD-OCT). The data recorded included complains as scotoma, blurred vision and metamorphopsia. The intravitreal injection of dobesilate was performed in accordance with the guidelines for intravitreal injec- tions [18]. Before injection administration, the eye was washed with povidone-iodine (5%) and the eyelashes and lid region were then wiped, also with povidone-iodine (5%). Then, each patient received 18.75 mg of dobesilate in a single intravitreal injection of 150 μl of a solution of diethylamonium 2,5-dihydroxybenzesulfonate (etamsy- late; dycinone®, Sanofi-Aventis, Paris, France). Antibi- otic eye drops were then applied. Patients returned to the outpatient clinic for routine postinjection follow-up at day one and day three after injection; a slit lamp exami- nation and pressure measurements were performed to rule out intraocular inflammation or elevated intraocular pressure (IOP). BCVA, slit lamp biomicroscopy of the anterior segment and fundus, and SDCOT were con- ducted again at 1 month postinjection. The ocular symp- toms, such as scotoma, metamorphopsia and blurred vi- sion were examined by questioning each patient before and after treatment. Scotoma and metamorphopsia on the Amsler grid were scored according to Verma et al. [19]. Accordingly, scotoma and metamorphopsia were graded numerically from 0 to IV, where a grade 0 indicates ab- sence of scotoma and metamorhopsia, and a grade IV indicates very severe scotoma and metamorphopsia. Blurred vision was scored from 0 to 4, where a score of 0 indicates no vision disturbance and a score of 4 indicates very severe blurred vision. 2.4. Endpoints The primary endpoint was the improvement of visual acuity at 1 month compared with baseline. The second endpoint was the effect of treatment in the normalization of retinal structure at the same time after treatment. 2.5. Statistics The paired t-test was used to statistically evaluate changes in visual acuity at baseline and one month after treatment. A p value < 0.05 was considered to be statis- tically significant. Graphic representation of the data is expressed as mean ± standard error of the mean (SEM). 3. RESULTS 3.1. Visual Outcomes In the current study, 36 eyes of 36 patients of both sexes were enrolled. All patients tolerated well the injection. The mean BCVA at baseline was 0.44 ± 0.03. At one Copyright © 2013 SciRes. OPEN ACCESS 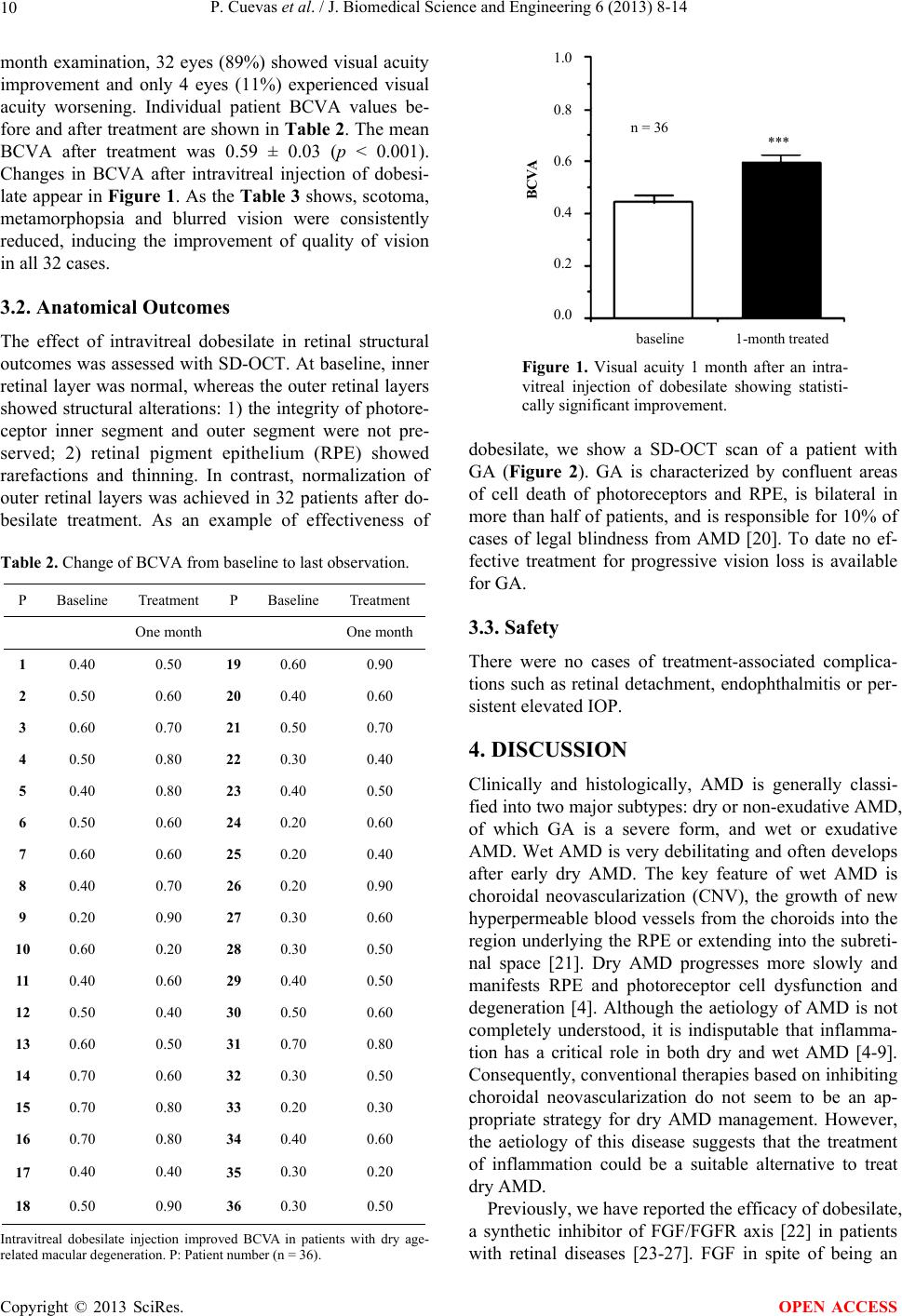 P. Cuevas et al. / J. Biomedical Science and Engineering 6 (2013) 8-14 10 month examination, 32 eyes (89%) showed visual acuity improvement and only 4 eyes (11%) experienced visual acuity worsening. Individual patient BCVA values be- fore and after treatment are shown in Table 2. The mean BCVA after treatment was 0.59 ± 0.03 (p < 0.001). Changes in BCVA after intravitreal injection of dobesi- late appear in Figure 1. As the Table 3 shows, scotoma, metamorphopsia and blurred vision were consistently reduced, inducing the improvement of quality of vision in all 32 cases. 3.2. Anatomical Outcomes The effect of intravitreal dobesilate in retinal structural outcomes was assessed with SD-OCT. At baseline, inner retinal layer was normal, whereas the outer retinal layers showed structural alterations: 1) the integrity of photore- ceptor inner segment and outer segment were not pre- served; 2) retinal pigment epithelium (RPE) showed rarefactions and thinning. In contrast, normalization of outer retinal layers was achieved in 32 patients after do- besilate treatment. As an example of effectiveness of Table 2. Change of BCVA from baseline to last observation. P Baseline Treatment P Baseline Treatment One month One month 1 0.40 0.50 19 0.60 0.90 2 0.50 0.60 20 0.40 0.60 3 0.60 0.70 21 0.50 0.70 4 0.50 0.80 22 0.30 0.40 5 0.40 0.80 23 0.40 0.50 6 0.50 0.60 24 0.20 0.60 7 0.60 0.60 25 0.20 0.40 8 0.40 0.70 26 0.20 0.90 9 0.20 0.90 27 0.30 0.60 10 0.60 0.20 28 0.30 0.50 11 0.40 0.60 29 0.40 0.50 12 0.50 0.40 30 0.50 0.60 13 0.60 0.50 31 0.70 0.80 14 0.70 0.60 32 0.30 0.50 15 0.70 0.80 33 0.20 0.30 16 0.70 0.80 34 0.40 0.60 17 0.40 0.40 35 0.30 0.20 18 0.50 0.90 36 0.30 0.50 Intravitreal dobesilate injection improved BCVA in patients with dry age- related macular degeneration. P: Patient number (n = 36). n = 36 *** 1.0 0.8 0.6 0.4 0.2 0.0 BCVA aseline 1-month treated Figure 1. Visual acuity 1 month after an intra- vitreal injection of dobesilate showing statisti- cally significant improvement. dobesilate, we show a SD-OCT scan of a patient with GA (Figure 2). GA is characterized by confluent areas of cell death of photoreceptors and RPE, is bilateral in more than half of patients, and is responsible for 10% of cases of legal blindness from AMD [20]. To date no ef- fective treatment for progressive vision loss is available for GA. 3.3. Safety There were no cases of treatment-associated complica- tions such as retinal detachment, endophthalmitis or per- sistent elevated IOP. 4. DISCUSSION Clinically and histologically, AMD is generally classi- fied into two major subtypes: dry or non-exudative AMD, of which GA is a severe form, and wet or exudative AMD. Wet AMD is very debilitating and often develops after early dry AMD. The key feature of wet AMD is choroidal neovascularization (CNV), the growth of new hyperpermeable blood vessels from the choroids into the region underlying the RPE or extending into the subreti- nal space [21]. Dry AMD progresses more slowly and manifests RPE and photoreceptor cell dysfunction and degeneration [4]. Although the aetiology of AMD is not completely understood, it is indisputable that inflamma- tion has a critical role in both dry and wet AMD [4-9]. Consequently, conventional therapies based on inhibiting choroidal neovascularization do not seem to be an ap- propriate strategy for dry AMD management. However, the aetiology of this disease suggests that the treatment of inflammation could be a suitable alternative to treat dry AMD. Previously, we have reported the efficacy of dobesilate, a synthetic inhibitor of FGF/FGFR axis [22] in patients with retinal diseases [23-27]. FGF in spite of being an Copyright © 2013 SciRes. OPEN ACCESS  P. Cuevas et al. / J. Biomedical Science and Engineering 6 (2013) 8-14 11 Table 3. Change in ocular symptoms score from baseline to last observation. P B T P B T P B T P BT S 1 II I 10 II II 19 III 0 28 II 0 M II I I 0 II 0 III I BV 1 0 1 1 1 0 31 S 2 III II 11 I III 20 II I 29 II I M I I I II II I III 0 BV 1 0 1 2 2 1 20 S 3 I 0 12 I 0 21 I 0 30 II I M I 0 II 0 I I I0 BV 0 0 1 0 1 0 10 S 4 0 0 13 I I 22 II I 31 00 M I 0 I III II 0 00 BV 1 0 0 1 1 0 10 S 5 I 0 14 II II 23 I 0 32 00 M 0 0 I 0 I I 00 BV 1 0 1 1 1 0 10 S 6 I 0 15 I II 24 II I 33 00 M 0 0 I II II I I0 BV 0 0 1 1 1 0 10 S 7 I 0 16 II I 25 III I 34 III II M 0 0 I I II 0 III II BV 0 0 0 1 3 0 22 S 8 II I 17 I 0 26 II 0 35 I0 M II 0 I 0 II 0 I0 BV 1 0 1 0 1 0 11 S 9 III 0 18 I I 27 III I 36 II III M II 0 II II III I II II BV 1 0 1 1 3 0 22 Dobesilate injection in the vitreous gel improved ocular symptoms associ- ated to dry age-related macular degeneration. S: Scotoma; M: Metamor- phopsia; BV: Blurred Vision; P: Patient number (n = 36); B: Baseline; T: after one month of Treatment. angiogenesis promoter [28] is involved also in inflam- mation [10-16] and seems to play key roles in neurode- generative diseases such as Alzheimer’s and Parkinson’s diseases as well as in AMD [17]. Dobesilate features suggest that its intravitreal appli- cation could be of potential clinical benefit in dry AMD management. In this report, we describe the rapid nor- malization of retinal structure, running parallel to a con- (a) (b) → T (c) Figure 2. Fundus photographs of the left eye of an enrolled patient at baseline with peripheral areas of pigment accumula- tion, autofluorescence deposits and patches of geographic at- rophy of the retinal pigment epithelium (a). Optical coherence tomography of the central macula before treatment with dobe- silate (b), showing loss in some areas of both outer retinal lay- ers and pigment epithelial cells, and one month after treatment (c), with improvement of vision from 0.20 to 0.60. Note the anatomical recovery of outer retinal layers and retinal pigment epithelium after a single intravitreal injection of dobesilate. siderable improvement of visual acuity in patients with dry AMD after a single intravitreal administration of dobesilate. This study adds some more valuable data to the recently reported promising good results to the use of dobesilate in both dry and wet AMD and also in other retinal diseases [23-27]. It may result somehow surprising that an inhibitor of FGF shows the good safety profile of dobesilate, given the broad spectrum of physiological activities in which FGF is involved [29-32]. In effect, FGF has been de- tected in most adult tissues tightly bound to the sulphated glycosaminoglycans (GAG) of the extracellular matrix Copyright © 2013 SciRes. OPEN ACCESS  P. Cuevas et al. / J. Biomedical Science and Engineering 6 (2013) 8-14 12 (ECM), at times at very high levels [22]. Thus, under normal physiological conditions, FGF does not act as a signalling molecule in solution, but as a solid phase growth factor. As far as FGF remains part of the ECM, it cannot be inhibited by dobesilate, which has an affinity constant for FGF approximately 3000 times lower than that of the sulphated GAG of the ECM; furthermore, it reaches extraordinarily high concentrations [22]. Never- theless, the physiological FGF signalling system gets sometimes subverted, causing very serious physiological disturbances, when high concentrations of free FGF are accumulated, either through uncontrolled synthesis or mobilization by heparanases and other specialized pro- teins which could be upregulated in inflammatory condi- tions [33-35]. In contrast to ECM bounded FGF, dobesi- late efficiently inhibits free FGF [22]. Consequently, it inhibits mainly pathological FGF, leaving the relatively untouched physiological FGF pool. Several limitations are inherent in the current study. First, the design was prospective, the sample size was small and there was no control group. Second, this one time follow-up of patients does not describe visual acuity evolution over time and the results may be transient. However, this study shows for the first time the efficacy and safety of a new therapy for dry age-related macular degeneration that has been considered as an orphan dis- ease. Furthermore, treatment with dobesilate increased visual acuity above the expected baseline decrease dur- ing the natural evolution of untreated dry AMD. In sum, dobesilate with a long history of use, and abundant pre- clinical data supporting its biological effects and its po- tential efficacy, is promising as a FGF targeted therapy for AMD. A large population cohort study is needed to establish the effectiveness of intravitreal dobesilate in treating dry AMD patients. 5. AUTHOR CONTRIBUTIONS Conceived and designed the study: PC, GGG, LO. Per- formed the study LO, CA. Analyzed the data: LO, CA, JA, GGG, and PC. PC, GGG, wrote the paper. All au- thors read and approved the final manuscript. 6. ACKNOWLEDGEMENTS We are indebted to the patients who participated in this study. REFERENCES [1] Ambati, J., Ambati, B.K., Yoo, S.H., Ianchulev, S. and Adamis, A.P. (2003) Age-related macular degeneration: Etiology, pathogenesis, and therapeutic strategies. Survey of Ophthalmology, 48, 257-293. http://dx.doi.org/10.1016/S0039-6257(03)00030-4 [2] van Leeuwen, R., Klaver, C.C., Vingerling, J.R., Hofman, A. and de Jong, P.T. (2003) Epidemiology of age-related maculopathy: A review. European Journal of Epidemi- ology, 18, 845-854. http://dx.doi.org/10.1023/A:1025643303914 [3] Klein, R., Klein, B.E., Knudtson, M.D., Meuer, S.M., Swift, M. and Gangnon, R.E. (2007) Fifteen-year cumu- lative incidence of age-related macular degeneration: The beaver dam eye study. Ophthalmology, 114, 253-262. http://dx.doi.org/10.1016/j.ophtha.2006.10.040 [4] Rodrigues, E.B. (2007) Inflammation in dry age-related macular degeneration. Ophthalmologica, 221, 143-152. http://dx.doi.org/10.1159/000099293 [5] Nowak, J.Z. (2006) Age-related macular degeneration (AMD): Pathogenesis and therapy. Pharmacological Re- ports, 58, 353-363. [6] Patel, M. and Chan, C.C. (2008) Immunopathological aspects of age-related macular degeneration. Seminars in Immunopathology, 30, 97-110. http://dx.doi.org/10.1159/000099293 [7] Hollyfield, J.G., Bonilha, V.L., Rayborn, M.E., Yang, X., Shadrach, K.G., Lu, L., Ufret, R.L, Salomon, R.G. and Perez, V.L. (2008) Oxidative damage-induced inflamma- tion initiates age-related macular degeneration. Nature Medicine, 14, 194-198. http://dx.doi.org/10.1038/nm1709 [8] Buschini, E., Piras, A., Nuzzi, R. and Vercelli, A. (2011) Age related macular degeneration and drusen: Neuroin- flammation in the retina. Progress in Neurobiology, 95, 14-25. http://dx.doi.org/10.1016/j.pneurobio.2011.05.011 [9] Telander, D.G. (2011) Inflammation and age-related macular degeneration (AMD). Seminars in Ophthalmol- ogy, 26, 192-197. http://dx.doi.org/10.1016/j.pneurobio.2011.05.011 [10] Byrd, V.M., Ballard, D.W., Miller, G.G. and Thomas, J.W. (1999) Fibroblast growth factor-1 (FGF-1) enhances IL-2 production and nuclear translocation of NF-kappaB in FGF receptor-bearing Jurkat T cells. Journal of Im- munology, 162, 5853-5859. [11] Meij, J.T., Sheikh, F., Jiménez, S.K., Nickerson, P.W., Kardami, E. and Cattini, P.A. (2002) Exacerbation of myocardial injury in transgenic mice overexpressing FGF- 2 is T cell dependent. American Journal of Physiology— Heart and Circulatory Physiology, 282, H547-H555. [12] Rossini, M., Cheunsuchon, B., Donnert, E., Ma, L.J., Thomas, J.W., Neilson, E.G. and Fogo, A.B. (2005) Im- munolocalization of fibroblast growth factor-1 (FGF-1), its receptor (FGFR-1), and fibroblast-specific protein-1 (FSP-1) in inflammatory renal disease. Kidney Interna- tional, 68, 2621-2628. http://dx.doi.org/10.1111/j.1523-1755.2005.00734.x [13] Zittermann, S.I. and Issekutz, A.C. (2006) Basic fibro- blast growth factor (bFGF, FGF-2) potentiates leukocyte recruitment to inflammation by enhancing endothelial adhesion molecule expression. American Journal of Pa- thology, 168, 835-846. http://dx.doi.org/10.2353/ajpath.2006.050479 [14] Andrés, G., Leali, D., Mitola, S., Coltrini, D., Camozzi, M., Corsini, M., Belleri, M., Hirsch, E., Schwendener, R.A., Christofori, G., Alcami, A. and Presta, M. (2009) A pro-inflammatory signatura mediates FGF2-induced an- Copyright © 2013 SciRes. OPEN ACCESS  P. Cuevas et al. / J. Biomedical Science and Engineering 6 (2013) 8-14 Copyright © 2013 SciRes. 13 OPEN ACCESS giogenesis. Journal of Cellular and Molecular Medicine, 13, 2083-2108. http://dx.doi.org/10.1111/j.1582-4934.2008.00415.x [15] Strowig, T., Henao-Mejía, J., Elinav, E. and Flawell, R. (2012) Inflammosomes in health and disease. Nature, 481, 278-296. http://dx.doi.org/10.1038/nature10759 [16] Stewart, M.W. (2012) The expanding role of vascular endothelial growth factor inhibitors in ophthalmology. Mayo Clinic Proceedings, 87, 77-88. http://dx.doi.org/10.1016/j.mayocp.2011.10.001 [17] Lee, M., Kang, Y., Suk, K., Schwab, C., Yu, S. and McGeer, P.L. (2011) Acidic fibroblast growth factor (FGF) potentiates glial-mediated neurotoxicity by acti- vating FGFR2 IIIb protein. Journal of Biological Che- mistry, 286, 41230-41245. http://dx.doi.org/10.1074/jbc.M111.270470 [18] Aiello, L.P., Brucker, A.J., Chang, S., Cunningham Jr, E.T., D’Amico, D.J., Flynn Jr., H.W., Grillote, L.R., Hutcherson, S., Liebmann, J.M., O’Brien, T.P., Scout, I.U., Spaide, R.F., Ta, C. and Trese, M.T. (2004) Evolving guidelines for intravitreous injections. Retina, 24, S3- S19. http://dx.doi.org/10.1097/00006982-200410001-00002 [19] Verma, L., Sinha, R., Venkatesh, P. and Tewari, H.K. (2004) Comparative evaluation of diode laser versus ar- gon laser photocoagulation in patients with central serous retinopathy: A pilot, randomized controlled trial [ISRC- TN84128484]. BMC Ophthalmology, 4, 15. http://dx.doi.org/10.1186/1471-2415-4-15 [20] Sarks, J.P., Sarks, S.H. and Killingsworth, M.C. (1988) Evolution of geographic atrophy of the retinal pigment epithelium. Eye (Lond), 2, 552-577. http://dx.doi.org/10.1038/eye.1988.106 [21] Chappelow, A.V. and Kaiser, P.K. (2008) Neovascular age-related macular degeneration: Potential therapies. Drugs, 68, 1029-1036. http://dx.doi.org/10.1038/eye.1988.106 [22] Fernández, I.S., Cuevas, P., Angulo, J., López-Navajas, P., Canales-Mayordomo, A., Lozano, R.M., Valverde, S., Jiménez-Barbero, J., Romero, A. and Giménez-Gallego, G. (2010) Gentisic acid, a compound associated with plant defence and a metabolite of aspirin, heads a new class of in vivo FGF inhibitor. The Journal of Biological Chemis- try, 285, 1714-1729. http://dx.doi.org/10.1074/jbc.M109.064618 [23] Cuevas, P., Outeiriño, L.A., Azanza, C. and Giménez- Gallego, G. (2012) Intravitreal dobesilate in the treatment of choroidal neovascularization associated with age-re- lated macular degeneration. Report of two cases. British Medical Journal (BMJ) Case Reports. [24] Cuevas, P., Outeiriño, L.A., Azanza, C., Angulo, J. and Giménez-Gallego, G. (2012) Short-term efficacy of in- travitreal dobesilate in central serous chorioretinopathy. European Journal of Medical Research, 17, 22. http://dx.doi.org/10.1186/2047-783X-17-22 [25] Cuevas, P., Outeiriño, L.A., Angulo, J. and Giménez- Gallego, G. (2012) Chronic cystoid macular oedema treated with intravitreal dobesilate. British Medical Journal (BMJ) Case Reports. [26] Cuevas, P., Outeiriño, L.A., Angulo, J. and Giménez- Gallego, G. (2012) Treatment of dry age-related macular degeneration with dobesilate. British Medical Journal (BMJ) Case Reports. [27] Cuevas, P., Outeiriño, L.A., Angulo, J. and Giménez- Gallego, G. (2012) Treatment of Stargardt disease with dobesilate. British Medical Journal (BMJ) Case Reports. [28] Thomas, K.A. and Giménez-Gallego, G. (1986) Fibro- blast growth factors: Broad spectrum mitogens with po- tent angiogenic activity. Trends in Biochemical Sciences, 11, 81-84. http://dx.doi.org/10.1016/0968-0004(86)90271-9 [29] Cuevas, P., Carceller, F., Ortega, S., Zazo, M., Nieto, I. and Giménez-Gallego, G. (1991) Hypotensive activity of fibroblast growth factor. Science, 254, 1208-1210. http://dx.doi.org/10.1126/science.1957172 [30] Galan, J.M., Cuevas, B., Dujovny, N., Giménez-Gallego, G. and Cuevas, P. (1996) Sleep promoting effects of in- travenously administered acidic fibroblast growth factor. Neurological Research, 18, 567-569. [31] Cuevas, P., Angulo, J. and Giménez-Gallego, G. (2011) Topical treatment of contact dermatitis by pine proces- sionary caterpillar. British Medical Journal (BMJ) Case Reports. http://casereports.bmj.com/content/2011/bcr.06.2011.435 1.full.pdf [32] Hanai, K., Oomura, Y., Kai, Y., Nishikawa, K., Shimizu, N., Morita, H. and Plata-Salamán, C.R. (1989) Central action of acidic fibroblast growth factor in feeding regu- lation. American Journal of Physiology, 256, R217-R223. [33] Bame, K.J. (2001) Heparanases: Endoglycosidases that degrade heparan sulfate proteoglycans. Glycobiology, 11, 91R-98R. http://dx.doi.org/10.1093/glycob/11.6.91R [34] Yuan, K., Hong, T.M., Chen, J.J., Tsai, W.H. and Lin, M.T. (2004) Syndecan-1 up-regulated by ephrinB2/EphB4 plays dual roles in inflammatory angiogenesis. Blood, 104, 1025-1033. http://dx.doi.org/10.1182/blood-2003-09-3334 [35] Chen, G., Wang, D., Vikramadithyan, R., Yagyu, H., Saxena, U., Pillarisetti, S. and Goldberg, I.J. (2004) In- flammatory cytokines and fatty acids regulate endothelial cell heparanase expression. Biochemistry, 43, 4971-4977. http://dx.doi.org/10.1021/bi0356552  P. Cuevas et al. / J. Biomedical Science and Engineering 6 (2013) 8-14 14 ABBREVIATIONS BCVA: Best corrected visual acuity SD-OCT: Spectral domain optical coherence tomography FGF: Fibroblast growth factor FGFR: Fibroblast growth factor receptors Copyright © 2013 SciRes. OPEN ACCESS
|