 American Journal of Analytical Chemistry, 2013, 4, 1-7 http://dx.doi.org/10.4236/ajac.2013.410A3001 Published Online October 2013 (http://www.scirp.org/journal/ajac) Hygroscopic Behavior of Lyophilized Powder of Grugru Palm (Acrocomia aculeata) Dalany Menezes Oliveira1*, Edmar Clemente2, Marcos Rodrigues A morim Afonso3, José Maria Correia da Costa3 1Center of Agricultural Sciences, State University of Maringá, Maringá, Brazil 2Department of Chemistry, State University of Maringá, Maringá, Brazil 3Department of Food Technology, Federal University of Ceará, Fortaleza, Brazil Email: *dalany5@yahoo.com.br, eclemente@uem.br, mafonso@ufc.br, correiacostaufc@gmail.com Received June 23, 2013; revised July 24, 2013; accepted August 19, 2013 Copyright © 2013 Dalany Menezes Oliveira et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The objective of this study was to determine adsorption isotherms and hygroscopic behavior of lyophilized powder from grugru palm. The powders of grugru palm were obtained by lyophilization process without maltodextrin (T1) and with 8% matodextrin (T2). The experimental data were obtained through the static gravimetric method at temperatures (25˚C, 30˚C, 35˚C and 40˚C), with different saturated solutions of salts. The models of GAB, BET, Henderson and Os- win were fitted to experimental data. The values obtained for hygroscopicity were 7.68% and 6.86% and the degrees of caking were 0.33% and 0.09% for T1 and T2, respectively. Mathematical models of adsorption isotherms for grugru palm powders can be classified as Type III. The GAB and Oswin models represented better the behavior of isotherms for T1 and T2. Grugru palm powder showed an increase in the humidity of the monolayer Xm along with increasing temperature. The grugru palm powder demonstrated to be a non-hygroscopic product, non-caking features. Keywords: Acrocomia aculeate; Adsorption Isotherms; Hygroscopicity; Degree of Caking 1. Introduction Grugru palm (Acrocomia aculeata (Jacq.) Lodd. ex-Mart.) is native to the grasslands, savannas and open forests of Tropical America and occurs in dense populations in some localities [1]. It presents a fresh pulp, which is consumed fresh or used in the production of flour that can be utilized in dif- ferent foods; it has a sweet taste and is mucilaginous. This fruit can be eaten fresh or cooked, and used in soft drinks and ice creams [2]. Ramos et al. [3] demonstrated the potential of grugru palm pulp as a nourishing food, able to contribute to the enrichment of the regional diet in supplemental feeding programs, and a natural source of β-carotene, vitamin A and minerals as copper, potassium and zinc. Dehydration of fruits and food is used as a method of preservation and storage, providing a new product on the market, which has motivated the investments in agricul- tural production and processing [4,5]. According to Toneli et al. [6], foods in storage period are exposed to various relative humidities and to differ- ent conditions of temperature; so it is necessary to adjust the moisture in order to reach equilibrium with the envi- ronment. These authors observed physical changes (cak- ing) in inulin powder when there is humidity variation. Mathlouthi and Rogé [7] stated that to explain the be- havior of different insoluble food products, several ma- thematical models were proposed for sorption isotherms. Studies by Kaymak-Ertekin and Gedik [8] for sorption isotherms in apples and potatoes showed an increase in temperature, resulting in decrease of equilibrium for moisture content. The understanding of moisture sorption isotherms in food has great importance in food science and technol- ogy for many uses, such as design and optimization of industrial processes, e.g., drying, assessing the problems of packaging, modeling of moisture changes that occur during drying, forecasting the stability of shelf life and predicting mixtures of ingredients [9]. Obtaining grugru palm powder is still handmade, and studies are necessary to show the best techniques for ob- taining and storing this product. Given the above, the objective of this work was to evaluate the hygroscopic *Corresponding author. C opyright © 2013 SciRes. AJAC  D. M. OLIVEIRA ET AL. 2 behavior and the application of mathematical models in predicting the adsorption isotherms of lyophilized pow- ders from grugru palm. 2. Material and Methods 2.1. Raw Material Grugru palm fruits were harvested in Araripe Plateau in the Cariri Region, Ceará State, Brazil, and taken to the Laboratory of Food Quality Control and Drying (UFCE), which were selected and sanitized and subsequently peeled for pulp separation. The pulp was stored at −20˚C until the lyophilization process. Two lyophilized powders of grugru palm were used: one treatment without addition of drying adjuvant and other with maltodextrin of dextrose equivalent (DE) 20. Control treatment (T1)—powder without maltodextrin Treatment with maltodextrin (T2)—powder with addition of maltodextrin 8%. 2.2. Lyophilization The pulp was unfrozen and triturated; after this process the pulp of T1 and T2 were spread on stainless steel trays with diameter of 15 cm. The trays were placed in ul- tra-freezer (CL 90 - 40 V, Terroni) at −40˚C for 24 hours; after this period the pulp was taken to a bench lyophilizer (LS 3000, Terroni) for 25 hours. Right after the lyophili- zation, the dried product was taken to a rotating-knives mill (MA 048, Marconi) in order to get a powder with homogeneous granularity. 2.3. Modeling of Adsorption Isotherms In determining the moisture adsorption isotherms, a static gravimetric method described by Wolf et al. [10] was applied using saturated solutions of salts according to Greenspan [11], at room temperature of 25˚C ± 2˚C. The solutions of salts were prepared and put in containers of tempered glass closed with silicone. Measurements of adsorption isotherms were per- formed in triplicate. For each cell about 0.20 g of each treatment were weighed in pre-librated aluminum cruci- bles. Afterward, the crucibles were taken to cells, which contained the saturated solutions of salts. The process was monitored by weighing of samples on analytical balance every 24 hours to obtain equilibrium. After balance the water activity of the samples was de- termined at temperatures of 25˚C, 30˚C, 35˚C and 40˚C, using aw meter, model AQUALab 4TEV. Then they were taken to a lab chamber with air circulation at 105˚C for determination of moisture content. The equilibrium moisture (Xeq, Equation (1)) was cal- culated as the difference between the mass of balanced sample and the dried mass: eq s eq s mm Xm (1) where: Xeq = equilibrium moisture (db); meq = mass of balanced sample (g); ms = mass of dried sample (g). For adjusting of the experimental data from adsorption isotherms of grugru palm powder, mathematical models of GAB (Equation (2)), BET (Equation (3)), Henderson (Equation (4)) and Oswin (Equation (5)) were used, rep- resented respectively by the equations in Table 1. The calculations of parameters of each model were performed using the software Statistic version 7.0 [12]. The quality of adjusting different models was evalua- ted through the best values of the determination coeffi- cient (R2) and the relative mean deviation (E%, Equation (6)), defined by Iglesias and Chirife [17]: 1 100 %e n i e p eq Xeq EnXeq (6) where: E% = relative mean error; Xeqe = experimental Table 1. Mathematical models used to adjust the experimental data of adsorption isotherms. Models Equations GAB (Guggenheim-Andersen-de Boer) [10] 11 mw eq ww XCKa X w aKaCKa (2) BET [11] 21 1 11 111 n ww mw n eq www nana XCa XaCa Ca (3) Henderson [12] 1 ln 1w e a q a Xb (4) Oswin [13] 1 b w eq w a Xa a (5) *aw = water activity; Xm = moisture content in the molecular monolayer (g H2O·g−1 dry basis); Xeq = equilibrium moisture content (g H2O·g−1 dry basis); C = constant related to heat of sorption of the molecular layer; a, b, K = adjusting parameters. Copyright © 2013 SciRes. AJAC 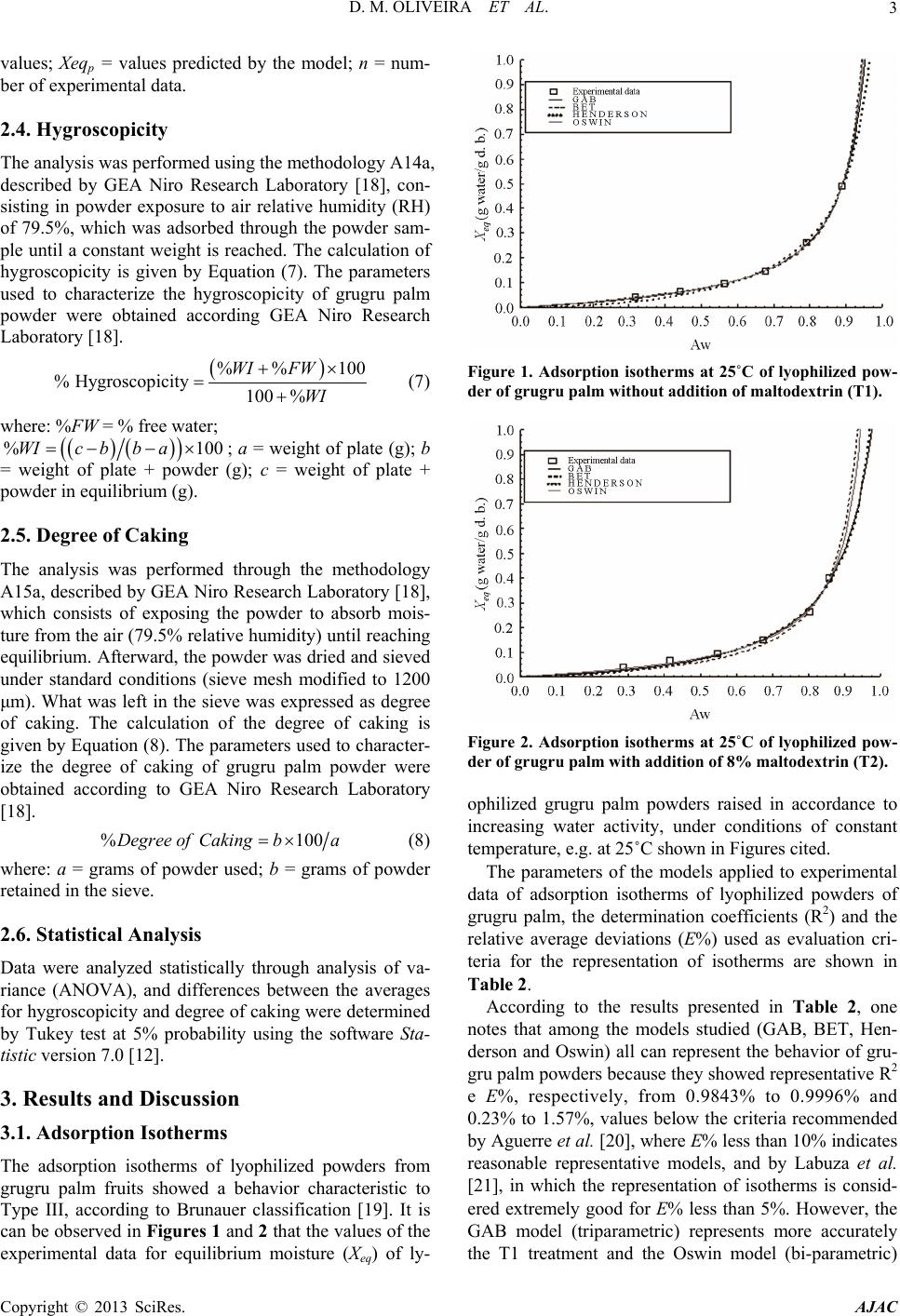 D. M. OLIVEIRA ET AL. 3 values; Xeqp = values predicted by the model; n = num- ber of experimental data. 2.4. Hygroscopicity The analysis was performed using the methodology A14a, described by GEA Niro Research Laboratory [18], con- sisting in powder exposure to air relative humidity (RH) of 79.5%, which was adsorbed through the powder sam- ple until a constant weight is reached. The calculation of hygroscopicity is given by Equation (7). The parameters used to characterize the hygroscopicity of grugru palm powder were obtained according GEA Niro Research Laboratory [18]. %% 10 % Hygroscopicity100 % WI FW WI 0 (7) where: %FW = % free water; % 100WI b ac b ; a = weight of plate (g); b = weight of plate + powder (g); c = weight of plate + powder in equilibrium (g). 2.5. Degree of Caking The analysis was performed through the methodology A15a, described by GEA Niro Research Laboratory [18], which consists of exposing the powder to absorb mois- ture from the air (79.5% relative humidity) until reaching equilibrium. Afterward, the powder was dried and sieved under standard conditions (sieve mesh modified to 1200 μm). What was left in the sieve was expressed as degree of caking. The calculation of the degree of caking is given by Equation (8). The parameters used to character- ize the degree of caking of grugru palm powder were obtained according to GEA Niro Research Laboratory [18]. %Degree ofCakingba100 (8) where: a = grams of powder used; b = grams of powder retained in the sieve. 2.6. Statistical Analysis Data were analyzed statistically through analysis of va- riance (ANOVA), and differences between the averages for hygroscopicity and degree of caking were determined by Tukey test at 5% probability using the software Sta- tistic version 7.0 [12]. 3. Results and Discussion 3.1. Adsorption Isotherms The adsorption isotherms of lyophilized powders from grugru palm fruits showed a behavior characteristic to Type III, according to Brunauer classification [19]. It is can be observed in Figures 1 and 2 that the values of the experimental data for equilibrium moisture (Xeq) of ly- Figure 1. Adsorption isotherms at 25˚C of lyophilized pow- der of grugru palm without addition of maltodextrin (T1). Figure 2. Adsorption isotherms at 25˚C of lyophilized pow- der of grugru palm with addition of 8% maltodextrin (T2). ophilized grugru palm powders raised in accordance to increasing water activity, under conditions of constant temperature, e.g. at 25˚C shown in Figures cited. The parameters of the models applied to experimental data of adsorption isotherms of lyophilized powders of grugru palm, the determination coefficients (R2) and the relative average deviations (E%) used as evaluation cri- teria for the representation of isotherms are shown in Table 2. According to the results presented in Table 2, one notes that among the models studied (GAB, BET, Hen- derson and Oswin) all can represent the behavior of gru- gru palm powders because they showed representative R2 e E%, respectively, from 0.9843% to 0.9996% and 0.23% to 1.57%, values below the criteria recommended by Aguerre et al. [20], where E% less than 10% indicates reasonable representative models, and by Labuza et al. [21], in which the representation of isotherms is consid- ered extremely good for E% less than 5%. However, the GAB model (triparametric) represents more accurately the T1 treatment and the Oswin model (bi-parametric) Copyright © 2013 SciRes. AJAC 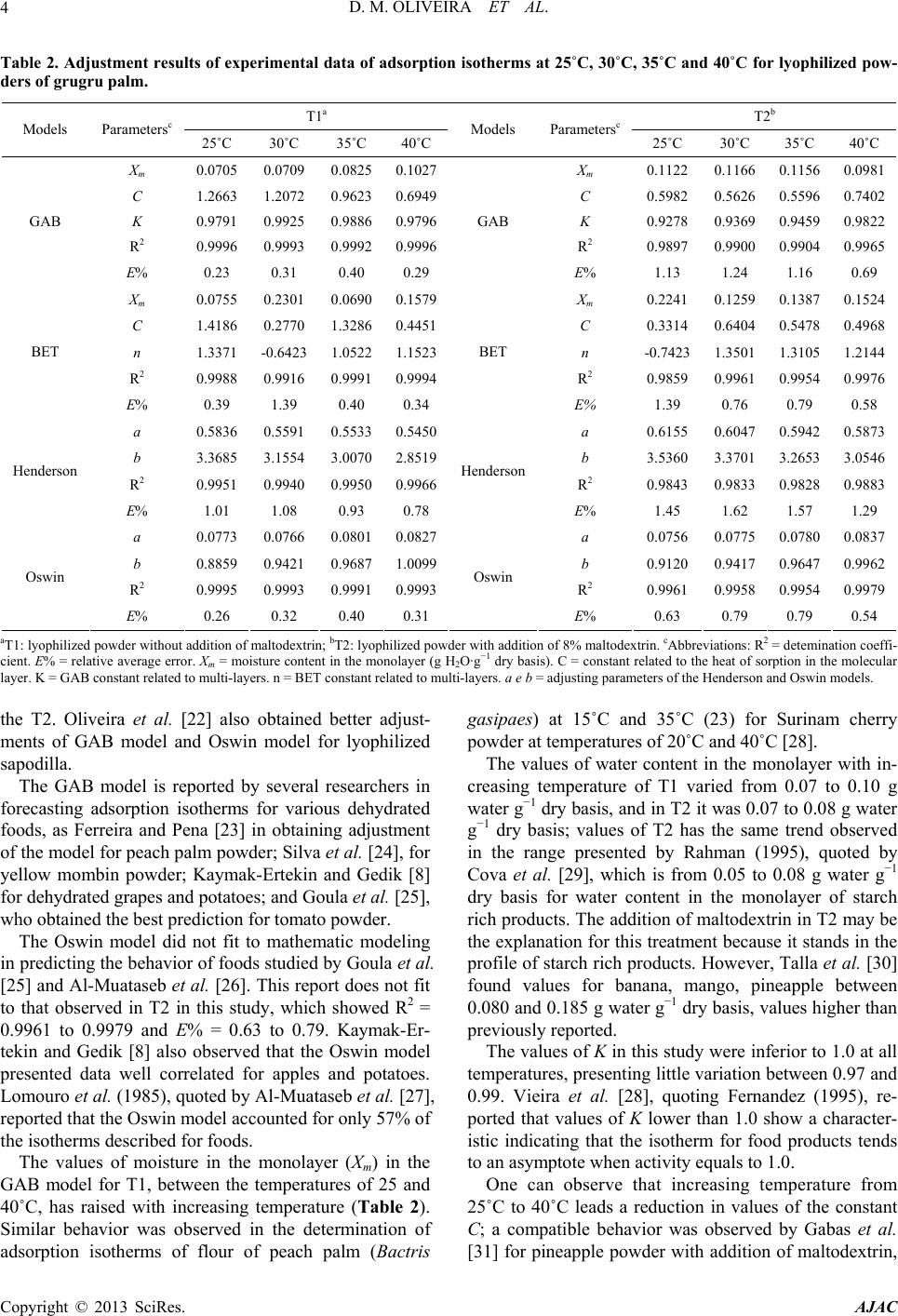 D. M. OLIVEIRA ET AL. 4 Table 2. Adjustment results of experimental data of adsorption isotherms at 25˚C, 30˚C, 35˚C and 40˚C for lyophilized pow- ders of grugru palm. T1a T2b Models Parametersc 25˚C 30˚C 35˚C 40˚C Models Parametersc 25˚C 30˚C 35˚C 40˚C Xm 0.07050.0709 0.08250.1027Xm 0.1122 0.1166 0.11560.0981 C 1.2663 1.2072 0.96230.6949C 0.5982 0.5626 0.55960.7402 K 0.9791 0.9925 0.98860.9796K 0.9278 0.9369 0.94590.9822 R2 0.9996 0.9993 0.99920.9996R2 0.9897 0.9900 0.99040.9965 GAB E% 0.23 0.31 0.40 0.29 GAB E% 1.13 1.24 1.16 0.69 Xm 0.07550.2301 0.06900.1579Xm 0.2241 0.1259 0.13870.1524 C 1.4186 0.2770 1.32860.4451C 0.3314 0.6404 0.54780.4968 n 1.3371-0.6423 1.0522 1.1523n -0.7423 1.3501 1.3105 1.2144 R2 0.9988 0.9916 0.99910.9994R2 0.9859 0.9961 0.99540.9976 BET E% 0.39 1.39 0.40 0.34 BET E% 1.39 0.76 0.79 0.58 a 0.58360.5591 0.55330.5450a 0.6155 0.6047 0.59420.5873 b 3.36853.1554 3.00702.8519b 3.5360 3.3701 3.26533.0546 R2 0.9951 0.9940 0.99500.9966R2 0.9843 0.9833 0.98280.9883 Henderson E% 1.01 1.08 0.93 0.78 Henderson E% 1.45 1.62 1.57 1.29 a 0.07730.0766 0.08010.0827a 0.0756 0.0775 0.07800.0837 b 0.88590.9421 0.96871.0099b 0.9120 0.9417 0.96470.9962 R2 0.9995 0.9993 0.99910.9993R2 0.9961 0.9958 0.99540.9979 Oswin E% 0.26 0.32 0.40 0.31 Oswin E% 0.63 0.79 0.79 0.54 aT1: lyophilized powder without addition of maltodextrin; bT2: lyophilized powder with addition of 8% maltodextrin. cAbbreviations: R2 = detemination coeffi- cient. E% = relative average error. Xm = moisture content in the monolayer (g H2O·g−1 dry basis). C = constant related to the heat of sorption in the molecular layer. K = GAB constant related to multi-layers. n = BET constant related to multi-layers. a e b = adjusting parameters of the Henderson and Oswin models. the T2. Oliveira et al. [22] also obtained better adjust- ments of GAB model and Oswin model for lyophilized sapodilla. The GAB model is reported by several researchers in forecasting adsorption isotherms for various dehydrated foods, as Ferreira and Pena [23] in obtaining adjustment of the model for peach palm powder; Silva et al. [24], for yellow mombin powder; Kaymak-Ertekin and Gedik [8] for dehydrated grapes and potatoes; and Goula et al. [25], who obtained the best prediction for tomato powder. The Oswin model did not fit to mathematic modeling in predicting the behavior of foods studied by Goula et al. [25] and Al-Muataseb et al. [26]. This report does not fit to that observed in T2 in this study, which showed R2 = 0.9961 to 0.9979 and E% = 0.63 to 0.79. Kaymak-Er- tekin and Gedik [8] also observed that the Oswin model presented data well correlated for apples and potatoes. Lomouro et al. (1985), quoted by Al-Muataseb et al. [27], reported that the Oswin model accounted for only 57% of the isotherms described for foods. The values of moisture in the monolayer (Xm) in the GAB model for T1, between the temperatures of 25 and 40˚C, has raised with increasing temperature (Table 2). Similar behavior was observed in the determination of adsorption isotherms of flour of peach palm (Bactris gasipaes) at 15˚C and 35˚C (23) for Surinam cherry powder at temperatures of 20˚C and 40˚C [28]. The values of water content in the monolayer with in- creasing temperature of T1 varied from 0.07 to 0.10 g water g−1 dry basis, and in T2 it was 0.07 to 0.08 g water g−1 dry basis; values of T2 has the same trend observed in the range presented by Rahman (1995), quoted by Cova et al. [29], which is from 0.05 to 0.08 g water g−1 dry basis for water content in the monolayer of starch rich products. The addition of maltodextrin in T2 may be the explanation for this treatment because it stands in the profile of starch rich products. However, Talla et al. [30] found values for banana, mango, pineapple between 0.080 and 0.185 g water g−1 dry basis, values higher than previously reported. The values of K in this study were inferior to 1.0 at all temperatures, presenting little variation between 0.97 and 0.99. Vieira et al. [28], quoting Fernandez (1995), re- ported that values of K lower than 1.0 show a character- istic indicating that the isotherm for food products tends to an asymptote when activity equals to 1.0. One can observe that increasing temperature from 25˚C to 40˚C leads a reduction in values of the constant C; a compatible behavior was observed by Gabas et al. [31] for pineapple powder with addition of maltodextrin, Copyright © 2013 SciRes. AJAC  D. M. OLIVEIRA ET AL. 5 which demonstrates temperature is dependent to the con- stant C. These same authors reported that strong adsor- bate-adsorbent interactions are favored by lower tem- peratures, reflecting on C increase and showing that this variation along with temperature may be the result of a mathematic compensation between C and K. In Figures 3 and 4, there is a graphical representation of the adsorption isotherms of moisture of grugru palm powder, for all temperatures studied, adjusted by GAB and Oswin model for T1 and T2, respectively. There is little influence of temperature on equilibrium moisture content of grugru palm powder for water activ- ity below 0.55 in T1, being observed from this point that for the same aw there is an increase in the equilibrium moisture content along with rising temperatures (Figure 3). In T2, this effect was observed from water activity above 0.50 (Figure 4). In studies carried out with Suri- nam cherry powder, it was observed an influence of temperature on the equilibrium moisture content from aw above 0.30 [28]; this shows that grugru palm powder presents a hygroscopicity below that observed in Suri- nam cherry powder. As may be observed, the curves of Figures 3 and 4 do not intersect themselves with the increasing temperature, a fact also reported by Kaymak-Ertekin and Gedik [8] for apples with high content of pectin and sugar, and for po- tatoes with high content of starch. This similar behavior of grugru palm powder to data of the previously cited authors is due to its sweet fruit with approximately 35% carbohydrates [32], being 13.3% glucose [33]. 3.2. Hygroscopicity and Degree of Caking Table 3 shows the values of hygroscopicity and degree of caking for T1 and T2. Treatment T1 presented greater caking in relation to T2. This fact may be related to the Figure 3. Best model (GAB) for adsorption isotherms at different temperatures of lyophilized grugru palm powder without maltodextrin (T1). addition of drying adjuvant, maltodextrin, in which its use entails a reduction in the powder hygroscopicity; this fact is also evidenced in adsorption isotherms of this work. Powder caking is an undesirable phenomenon, which initially consists of transformation of powder into solid and sticky material, resulting in decreased functionality and fluidity, so quality loss, and the main cause of ag- glomeration is the presence of water induced by plastici- zation of the surface of particles [34]. In accordance with GEA Niro Research Laboratory [18], in which is characterized the hygroscopic behavior and the degree of caking, respectively, the grugru palm powder is classified as a non-hygroscopic and non-cak- ing product, Oliveira et al. [35] showed similar response about this classification (hygroscopicity 5.17% and 6.39%; degree of caking 0.03% and 3.11%) for powder grugru palm obtained by oven dryer. In studies carried out with sucrose, Mathlouth and Rogé [7] reported the formation of agglomerates is re- lated to the solid bridges created during the process, which turns out sucrose more hygroscopic. The same can be observed in T1, where the powder formed the greatest caking and consequently making the grugru palm powder more hygroscopic. Costa et al. [36] stated that different variations in the hygroscopic behavior of food powders are also attributed to their size, as finer particles have higher contact surface and therefore higher number of active sites. This report may explain the non-hygroscopicity behavior of grugru palm powder when compared to that of fruits found in the literature, which showed high hygroscopicity, as the particle size of grugru palm powder is 1200 μm and the particle size of most fruit powders is in the range from 500 to 600 μm. Figure 4. Best model (Oswin) for adsorption isotherm at different temperatures of lyophilized grugru palm powder with 8% maltodextrin (T2). Copyright © 2013 SciRes. AJAC  D. M. OLIVEIRA ET AL. 6 Table 3. Hygroscopicity and degree of caking of lyophilized powders from grugru palm. Treatmenta Hygroscopicity (%) Degree of caking (%) T1 7.68 a* 0.33 a T2 6.86 a 0.09 b aT1 = lyophilized powder without maltodextrin; T2 = lyophilized powder with 8% maltodextrin. *Equal lower-case letters in the same column do not differ by Tukey test at 5% probability. 4. Conclusions Mathematical models of adsorption isotherms for grugru palm powders can be classified as Type III, according to Brunauer classification. All models studied adjust themselves to lyophilized powder of grugru palm, and GAB and Oswin models represent best the behavior of the adsorption isotherms for T1 and T2, respectively. Grugru palm powder showed an increase in the hu- midity of the monolayer X m along with increasing tem- perature. The values of C were reduced along with in- creasing temperature. The constant K suffered small var- iations in function to temperature. Lyophilized powders of grugru palm were characte- rized as non-hygroscopic and non-caking product. 5. Acknowledgements To Laboratory of Food Quality Control and Drying (UFCE) for the research supporting, to CNPq through INCT, and to Araucaria Foundation for the scholarship granting. REFERENCES [1] C. R. Clement, E. Lleras Pérez and J. Van Leeuwen, “O Potencial das Palmeiras Tropicais no Brasil: Acertos e Fracassos das Últimas d’Écadas,” Agrociencias, Vol. 9, No. 1-2, 2005, pp. 67-71. [2] Ministério da Saúde, “Alimentos Regionais Brasileiros,” Ministério da Saúde, Brasília, 2002. [3] M. I. L. Ramos, M. M. Ramos Filho, P. A. Hiane, J. A. Braga Neto and E. M. A. Siqueira, “Qualidade Nutri- cional da Polpa de Bocaiúva Acrocomia aculeata (Jacq.) Lodd,” Ciência e Tecnologia de Alimentos, Vol. 28, 2008, pp. 90-94. [4] E. C. Soares, G. S. F. Oliveira, G. A. Maia, J. C. S. Monteiro, A. Silva Jr. and M. S. S. Filho, “Desidratação da Polpa de Acerola (Malpighia emarginata D.C.) Pelo Processo Foam-Mat,” Ciência e Tecnologia de Alimentos, Vol. 21, No. 2, 2001, pp. 164-170. http://dx.doi.org/10.1590/S0101-20612001000200008 [5] P. M. A. Gomes, R. M. F. Figueirêdo and A. J. M. Queiroz, “Armazenamento da Polpa de Acerola em Pó a Temperatura Ambiente,” Ciência e Tecnologia de Ali- mentos, Vol. 24, No. 3, 2004, pp. 384-389. http://dx.doi.org/10.1590/S0101-20612004000300014 [6] J. T. C. L.Toneli, K. J. Park, F. E. X. Murr and A. A. Negreiros, “Efeito da Umidade Sobre a Microestrutura da Inulina em Pó,” Ciência e Tecnologia de Alimentos, Vol. 28, No. 1, 2008, pp. 122-131. http://dx.doi.org/10.1590/S0101-20612008000100018 [7] M. Mathlouthi and M. B. Rogé, “Water Vapour Sorption Isotherms and the Caking of Food Powders,” Food Chemistry, Vol. 82, No. 1, 2003, pp. 61-71. http://dx.doi.org/10.1016/S0308-8146(02)00534-4 [8] F. Kaymak-Ertekin and A. Gedik, “Sorption Isotherms Andisosteric Heat of Sorption for Grapes, Apricots, Ap- ples and Potatoes,” LWT-Food Science and Technology, Vol. 37, No. 4, 2004, pp. 429-438. http://dx.doi.org/10.1016/j.lwt.2003.10.012 [9] A. Jamali, M. Kouhila, L. A. Mohamed, J. T. Jaouhari and N. Abdenouri, “Sorption Isotherms of Chenopodium Ambrosioides, Leaves at Three Temperatures,” Journal of Food Engineering, Vol. 72, No. 1, 2006, pp. 77-84. http://dx.doi.org/10.1016/j.jfoodeng.2004.11.021 [10] W. Wolf, W. E. L. Spiess and G. Jung, “Standarization of Isotherm Measurements,” In: D. Simatos and J. L. Multon, Eds., Properties of Water in Foods, Martinus Nijhoff, Leiden, 1985, pp. 661-679. http://dx.doi.org/10.1007/978-94-009-5103-7_40 [11] L. Greenspan, “Humidity Fixed Points of Binary Satured Aqueuos Solutions,” Journal of Research of the National Bureau of Standards-A, Physics and Chemistry, Vol. 81, No. 1, 1977, pp. 89-96. [12] Statsoft, “Statistica for Window, Computer Programa Manual,” 7th Edition, Statsoft Inc., Tulsa, 2007. [13] C. Van Den Berg and S. Bruin, “Water Activity and Its Estimation in Food Systems: Theoretical Aspects,” In: L. B. Rockland and G. E. Stewart, Eds., Theoretical Aspects, Academic Press, New York, 1981, pp. 45-58. [14] S. Brunauer, P. H. Emmett and E. Teller, “Adsorption of Gases in Multimolecular Layer,” Journal of the American Chemistry Society, Vol. 60, No. 2, 1938, pp. 309-319. http://dx.doi.org/10.1021/ja01269a023 [15] S. M. Henderson, “A Basic Concept of Equilibrium Moisture,” Agricultural Engineering, Vol. 33, 1952, pp. 29-32. [16] C. R. Oswin, “The Kinetics of Packing Life. III. The Iso- therm,” Journal of the Society of Chemical Industry, Vol. 65, No. 12, 1946, pp. 419-423. http://dx.doi.org/10.1002/jctb.5000651216 [17] H. A. Iglesias and J. Chirife, “A Model for Describing the Water Sorption Behaviour of Foods,” Journal of Food Science, Vol. 41, No. 5, 1976, pp. 984-992. http://dx.doi.org/10.1111/j.1365-2621.1976.tb14373.x [18] GEA Niro Research Laboratory, “Analytical Methods Dry Milk Products. GEA Niro Analytical Methods, Me- thods 14 a and 15 a,” Soeborg, 2003. [19] S. S. H. Rizvi, “Thermodynamic Properties of Foods in Dehydration,” In: M. A. Rao and S. S. H. Rizvi, Eds., Engineering Properties of Foods, Marcel Dekker, New York, 1995, pp. 239-313. [20] R. J. Aguerre, C. Suarez and P. E. Viollaz, “New BET Copyright © 2013 SciRes. AJAC  D. M. OLIVEIRA ET AL. Copyright © 2013 SciRes. AJAC 7 Type Multilayer Sorption Isotherms Part II: Modelling Water Sorption in Foods,” LWT-Food Science and Tech- nology, Vol. 22, No. 4, 1989, pp. 192-195. [21] T. P. Labuza, A. Kaanane and J. Y. Chen, “Effects of Temperature on the Moisture Sorption Isotherms and Water Activity Shift of Two Dehydrated Foods,” Journal of Food Science, Vol. 50, No. 3, 1985, pp. 385-392. [22] V. S. Oliveira, M. R. A. Afonso and J. M. C. Costa, “Caracterização Físico-Química e Comportamento Higro- scópico de Sapoti Liofilizado,” Revista Ciência Agro- nômica, Vol. 42, No. 2, 2011, pp. 342-348. http://dx.doi.org/10.1590/S1806-66902011000200012 [23] C. D. Ferreira and R. S. Pena, “Comportamento Higro- scópico da Farinha de Pupunha (Bactris gasipaes),” Ciên- cia e Tecnologia de Alimentos, Vol. 23, No. 2, 2003, pp. 251-255. http://dx.doi.org/10.1590/S0101-20612003000200025 [24] Y. C. Silva, M. E. R. M. C. Mata and M. E. M. Duarte, “Atividade de Água em Pó Microencapsulado com Amido Modificado: Estudo de Dois Modelos Mate- máticos,” Simpósio Brasileiro de Pós Colheita de Frutos Tropicais, João Pessoa, 2005. [25] A. M. Goula, T. D. Karapantsios, D. S. Achilias and K. G. Adamopoulos, “Water Sorption Isotherms and Glass Transition Temperature of Spray Dried Tomato Pulp,” Journal of Food Engineering, Vol. 85, No. 1, 2008, pp. 73-83. http://dx.doi.org/10.1016/j.jfoodeng.2007.07.015 [26] A. H. Al-Muhtaseb, W. A. M. McMinn and T. R. A. Magee, “Water Sorption Isotherms of Starch Powders Part 1: Mathematical Description of Experimental Data,” Journal of Food Engineering, Vol. 61, No. 3, 2004, pp. 297-307. http://dx.doi.org/10.1016/S0260-8774(03)00133-X [27] A. H. Al-Muhtaseb, W. A. M. McMinn and T. R. A. Ma- gee, “Moisture Sorption Isotherm Characteristics of Food Products: A Review,” Food and Bioproducts Pro- cessing e Ciencias da Terra, Vol. 7, No. 1, bohydrate , Vol. 80, No. 2, 2002, pp. 118-128. [28] A. H. Vieira, R. M. F. Figueirêdo and A. J. M. Queiroz, “Isotermas de Adsorção de Umidade da Pitanga em Pó,” Revista de Biologia 2007, pp. 11-20. [29] A. Cova, A. J. Sandoval, V. Balsamo and A. J. Müller, “The Effect of Hydrophobic Modifications on the Ad- sorption Isotherms of Cassava Starch,” Car Polymers, Vol. 81, No. 3, 2010, pp. 660-667. http://dx.doi.org/10.1016/j.carbpol.2010.03.028 [30] A. Talla, Y. Jannot, G. E. Nkeing and J. R. Puigalli, “Ex- perimental Determination and Modeling of Sorption Iso- therms of Tropical Fruits: Banana, Mando and Pineap- ple,” Drying Technology: An Inte 23, No. 7, 2005, pp. 1477-1498. rnational Journal, Vol. http://dx.doi.org/10.1081/DRT-200063530 [31] A. L. Gabas, V. R. N. Telis, P. J. A. Sobral and J. Te- lis-Romero, “Effect of Maltodextrin and Arabic Gum in Water Vapor Sorption Thermodynamic Properties of Va- cuum Dried Pineapple Pulp Powder,” Journal o Engineering, Vol. 82, No. 2, 2007, pp. 246-252. f Food http://dx.doi.org/10.1016/j.jfoodeng.2007.02.029 [32] M. R. Silva, D. B. C. L. Lacerda, G. G. Santos and D. M. O. Martins, “Caracterização Química de Frutos Nativos do Cerrado,” 1790-1793. Ciência Rural, Vol. 38, No. 6, 2008, pp. http://dx.doi.org/10.1590/S0103-84782008000600051 [33] A. L. S. Oliveira, M. A. Torres, S. J. Freire, T. B. Pereira, T. F. Santos, V. O. Silva and L. C. Azevêdo, “Carac- terização Físico-Química da Macaúba (Acrocomia acu- leata Jacq. Lodd.) Cultivada No Sertão Pernambucano,” Anais do, IV Congresso de Pesquisa e Inovação da Rede Norte e Nordeste de Educação Tecnológica, Belém, Para, ce & Technology, Vol. 6, No. 5, 1995, pp. 2009. [34] J. M. Aguilera, J. M. Del Valle and M. Karel, “Caking Phenomena in Amorphous Food Powder,” Trends in Food Scien 149-155. http://dx.doi.org/10.1016/S0924-2244(00)89023-8 [35] D. M. Oliveira, E. Clemente, J. M. C. Costa, “Hygro- scopic Behavior and Degree of Caking of Grugru Palm (Acrocomia aculeata) Powder,” Journal of Food Science sta Ciência Agronômica, Vol. 34, No. 1, 2003, pp. 39-43. and Technology, Vol. on line, 2012, pp. 1-7. [36] J. M. C. Costa, M. F. D. Medeiros and A. L. M. L. Mata, “Isotermas de Adsorção de Pós de Beterraba (Beta vulgaris L.), Abóbora (Cucurbita moschata) e Cenoura (Daucus carota) Obtidos Pelo Processo de Secagem em Leito de Jorro: Estudo Comparativo,” Revi
|