Z. G. HOU ET AL.
598
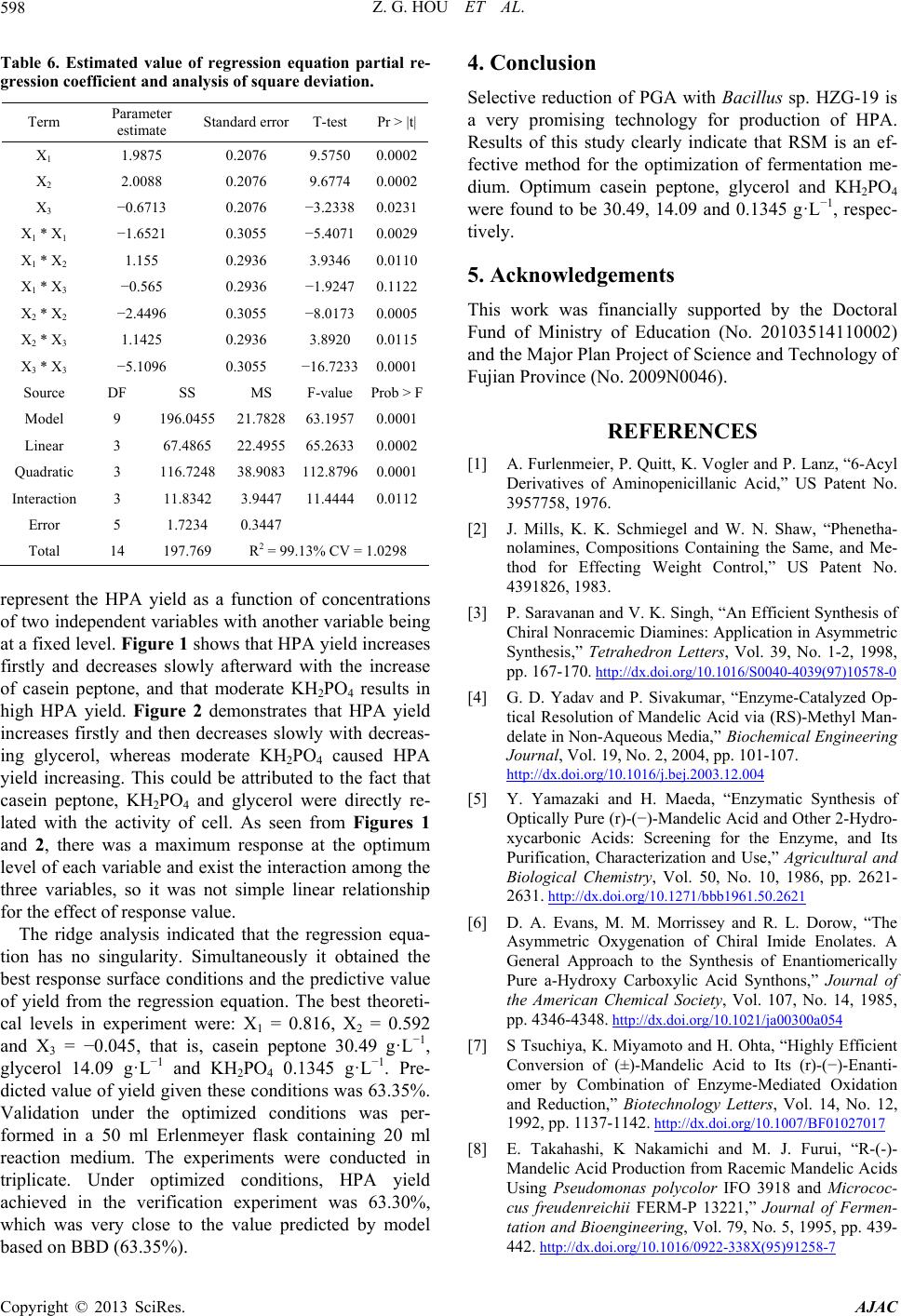
Table 6. Estimated value of regression equation partial re-
ression coefficient and analysis of squareg deviation.
|t|Term Parameter
estimate Standard error T-test Pr >
X1 1.9875 0.2076 9.5750 0.0002
X2 2.0088 0.2076 9.6774 0.0002
X3 − −
X1
− −
−
−
DF SS P
196.55 21.8
Quadr 112.
I
197. 13% CV = 1.0298
0.6713 0.2076 3.2338 0.0231
1 * X−1.6521 0.3055 −5.4071 0.0029
X1 * X2 1.155 0.2936 3.9346 0.0110
X1 * X3 0.565 0.2936 1.9247 0.1122
X2 * X2 2.4496 0.3055 −8.0173 0.0005
X2 * X3 1.1425
−
0.2936 3.8920 0.0115
X3 * X3 5.1096 0.3055 16.7233 0.0001
Source MSF-valuerob > F
Model 9 0478263.19570.0001
Linear 3 67.4865
116.
22.4955 65.26330.0002
atic 3 7248 38.9083 87960.0001
nteraction 3 11.8342 3.9447 11.44440.0112
Error 5 1.7234 0.3447
2
Total 14 769 R = 99.
rep the fu
f two independent variables with another variable bei
neously it obtained the
be
logy for production of HPA.
dy clearly indicate that RSM is an ef-
ork was financially supported by the Doctoral
Fund of Ministry of Education (No. 20103514110002)
Science and Technology of
er and P. Lanz, “6-Acyl
Derivatives of Aminopenicillanic Acid,” US Patent No.
3957758, 1976
[2] J. Mills, K. K Shaw, “Phenetha-
doi.org/10.1016/S0040-4039(97)10578-0
resentHPA yield as anction of concentrations
ng [
o
at a fixed level. Figure 1 shows that HPA yield increases
firstly and decreases slowly afterward with the increase
of casein peptone, and that moderate KH2PO4 results in
high HPA yield. Figure 2 demonstrates that HPA yield
increases firstly and then decreases slowly with decreas-
ing glycerol, whereas moderate KH2PO4 caused HPA
yield increasing. This could be attributed to the fact that
casein peptone, KH2PO4 and glycerol were directly re-
lated with the activity of cell. As seen from Figures 1
and 2, there was a maximum response at the optimum
level of each variable and exist the interaction among the
three variables, so it was not simple linear relationship
for the effect of response value.
The ridge analysis indicated that the regression equa-
tion has no singularity. Simulta
st response surface conditions and the predictive value
of yield from the regression equation. The best theoreti-
cal levels in experiment were: X1 = 0.816, X2 = 0.592
and X3 = −0.045, that is, casein peptone 30.49 g·L−1,
glycerol 14.09 g·L−1 and KH2PO4 0.1345 g·L−1. Pre-
dicted value of yield given these conditions was 63.35%.
Validation under the optimized conditions was per-
formed in a 50 ml Erlenmeyer flask containing 20 ml
reaction medium. The experiments were conducted in
triplicate. Under optimized conditions, HPA yield
achieved in the verification experiment was 63.30%,
which was very close to the value predicted by model
based on BBD (63.35%).
Selective reduction of PGA with Bacillus sp. HZG-19 is
a very promising techno
4. Conclusion
Results of this stu
fective method for the optimization of fermentation me-
dium. Optimum casein peptone, glycerol and KH2PO4
were found to be 30.49, 14.09 and 0.1345 g·L−1, respec-
tively.
5. Acknowledgements
This w
and the Major Plan Project of
Fujian Province (No. 2009N0046).
REFERENCES
[1] A. Furlenmeier, P. Quitt, K. Vogl
.
. Schmiegel and W. N.
nolamines, Compositions Containing the Same, and Me-
thod for Effecting Weight Control,” US Patent No.
4391826, 1983.
3] P. Saravanan and V. K. Singh, “An Efficient Synthesis of
Chiral Nonracemic Diamines: Application in Asymmetric
Synthesis,” Tetrahedron Letters, Vol. 39, No. 1-2, 1998,
pp. 167-170. http://dx.
[4] G. D. Yadav and P. Sivakumar, “Enzyme-Catalyzed Op-
tical Resolution of Mandelic Acid via (RS)-Methyl Man-
delate in Non-Aqueous Media,” Biochemical Engineering
Journal, Vol. 19, No. 2, 2004, pp. 101-107.
http://dx.doi.org/10.1016/j.bej.2003.12.004
[5] Y. Yamazaki and H. Maeda, “Enzymatic Synthesis of
Optically Pure (r)-(−)-Mandelic Acid and Other 2-Hydro-
xycarbonic Acids: Screening for the Enzyme, and Its
Purification, Characterization and Use,”
Agricultural and
Biological Chemistry, Vol. 50, No. 10, 1986, pp. 2621-
2631. http://dx.doi.org/10.1271/bbb1961.50.2621
[6] D. A. Evans, M. M. Morrissey and R. L. Dorow, “The
Asymmetric Oxygenation of Chiral Imide Enolates. A
General Approach to the Synthesis of Enantiomerically
Pure a-Hydroxy Carboxylic Acid Synthons,” Journal of
the American Chemical Society, Vol. 107, No. 14, 1985,
pp. 4346-4348. http://dx.doi.org/10.1021/ja00300a054
[7] S Tsuchiya, K. Miyamoto and H. Ohta, “Highly Efficient
Conversion of (±)-Mandelic Acid to Its (r)-(−)-Enanti-
omer by Combination of Enzyme-Mediated Oxidation
and Reduction,” Biotechnology Letters, Vol. 14, No. 12,
1992, pp. 1137-1142. http://dx.doi.org/10.1007/BF01027017
[8] E. Takahashi, K Nakamichi and M. J. Furui, “R-(-)-
Mandelic Acid Production from Racemic Mandelic Acids
Using Pseudomonas polycolor IFO 3918 and Micrococ-
cus freudenreichii FERM-P 13221,” Journal of Fermen-
tation and Bioengineering, Vol. 79, No. 5, 1995, pp. 439-
442. http://dx.doi.org/10.1016/0922-338X(95)91258-7
Copyright © 2013 SciRes. AJAC