Paper Menu >>
Journal Menu >>
 Advances in Ma terials Physics and Che mistry, 2012, 2, 267-269 doi:10.4236/ampc.2012.24B068 Published Online December 2012 (http ://www.SciRP.org/journal/ampc) Copyright © 2012 SciRes. AMPC Activation of Quart z Gr ain Surface with Chloride Ions D. N. Bondaletov, V. A. Fedorova OJSC “Gusevsky glas sworks named aft er F.E. Dzerzhinsky Received 2012 ABSTRACT Alteration o f technolo gical and opti cal states of glass a ctivated with chloride ions, entered t o the surface of qu artz sand and quartz grain by way of sodium chloride was investigated in the article. Concentration optimum of activating agent was determined. Keywords: Activating Agent of Surface; Flo r Ion; Quartz Sand; Grain; Optical Transmission The present paper is concerned with certain methods of im- proving optical properties of special-purpose glasses: soda-lime glasses used for making solar batteries and very-high-purity quart z glasses. The ra w materials used for making pho tovoltaic glass are known for strict requirements to be made on their iron content (0.012%). Quartz glass, by virtue of its structure and when containing impurities and admixtures, is furthermore capable of undergoing some considerable changes in its proper- ties and poss i bly structure.[1,2] As the assessment criterion the variation of iron content, as well as of the optical transmission of the glass, which had been molten using sand and quartz grains activated with chloride ions, has been taken. The research works carried out featured the introduction of chloride ions directly onto the quartz raw material at the batch preparation stage. A 0.1 mass % NaCl solution has been used for this purpose. The objectives pursued by introducing chloride ions by way of a solution are as follows: -to obtain an uniform per-quartz-grain distribution of the ac- tive admixt ure. - to remove the iron impurities during the process of melting a multicomponent glass or quartz. 1. Soda-lime Glass Sand chlorination has been carried out at th e stage of the batch preparation in using the raw material with a 0,015% iron con- tent. A 0.1% NaCl water solution has been prepared preliminarily. Sand has been further poured with the NaCl water solution at 4% batch moisture content and stirred thoroughly. The sand has been dried at 40°C and 120°С. Quartz grains having a porous and crumbling surface get coated with a uniform chloride-ion cont aining layer. The theoretical chemical interreactions to take place at the quartz grain surface are as follows : Fe2O3 + 3NaCl + 3H2O → FeCl3↑ +3Na (OH) + Fe (OH)3, (1) NaOH + SiO2 → Na2SiO3, (2) Electron microscopic studies of the activated quartz grain sur- face and the resulting coating layer content by scanning elec- tron microscopy method and using an electron probe X-ray spectroscopic microanalyzer have been carri ed out. Micro photograph s of the sample su rface have been 0 made at various magnifications. In Fig ure 1a microphotograph of the sand grains is shown without chloride ions applied. In that microphotograph the crystal-form grains are visible having cl ear ly defined b orders and cr acks. A qualitative elemental analysis has shown the quartz grains without NaCl treatment to be represented by the following principal elemental composition: Si, O, C, Al, Fe (s. Figure 3(а)). The qualitative elemental composition has changed after the chloride-ion treatment due to appearance of Cl and Na on the grain surface (s. Figure 3(b)). Glasses have been molten at 1450°C using the batch without chlorine and with 0.1; 0.2; 0.5% NaCl admi xt ures. In all obtained glasses the ferric oxide residual has been de- termined and the integral optical transmission has been meas- ured in the range between 300 nm and 2500 nm for the purpose of determining the sunlight transmission. The total ferric o xide percentage in the initi al glass has been determined at 0.0148% and it has decreased by 18% down to 0.0122% for the glass that has been activated with 0.1% NaCl. On further increasing the introduction of the activator, the proces s of the ferric o xide co ntent d ecrease has d ecelerat ed and its percentage has been determined at 0.0120% and 0.0117% when having introduced 0.2% and 0,5% of chloride ions, cor- respondingly. Figure 1. Microphotograph of quartz grains.  D. N. BONDALETOV, V. A. FEDOROVA Copyright © 2012 SciRes. AMPC 268 а – material dried at 40˚С; b – ma terial dried at 120˚С Figure 2. Microphotographs of quartz grains activated with 0.1% NaCl. (a) (b) а – surface without chloride ion activation ; б - surface activated with chloride i ons Figure 3. Qualitative elemental composition of quartz sand grain surface. A reduction in the content of iron impurities due to formation and volatilization of FeCl3 according to the above-stated reac- tion has thus been observed in all the glasses under study. The iron impurities partly remain in glass what is apparently attri- buted to ti me and temperature factors. Optical transmission of glasses, which has been measured in the ran ge bet ween 30 0 nm and 2500 nm, has shown that a 0.1% chloride ion admixture results in an effective increase of the optical transmission of glass from 89 % up to 91 .8% and in ca se the admixture percentage is increased up to 0.5% the optical 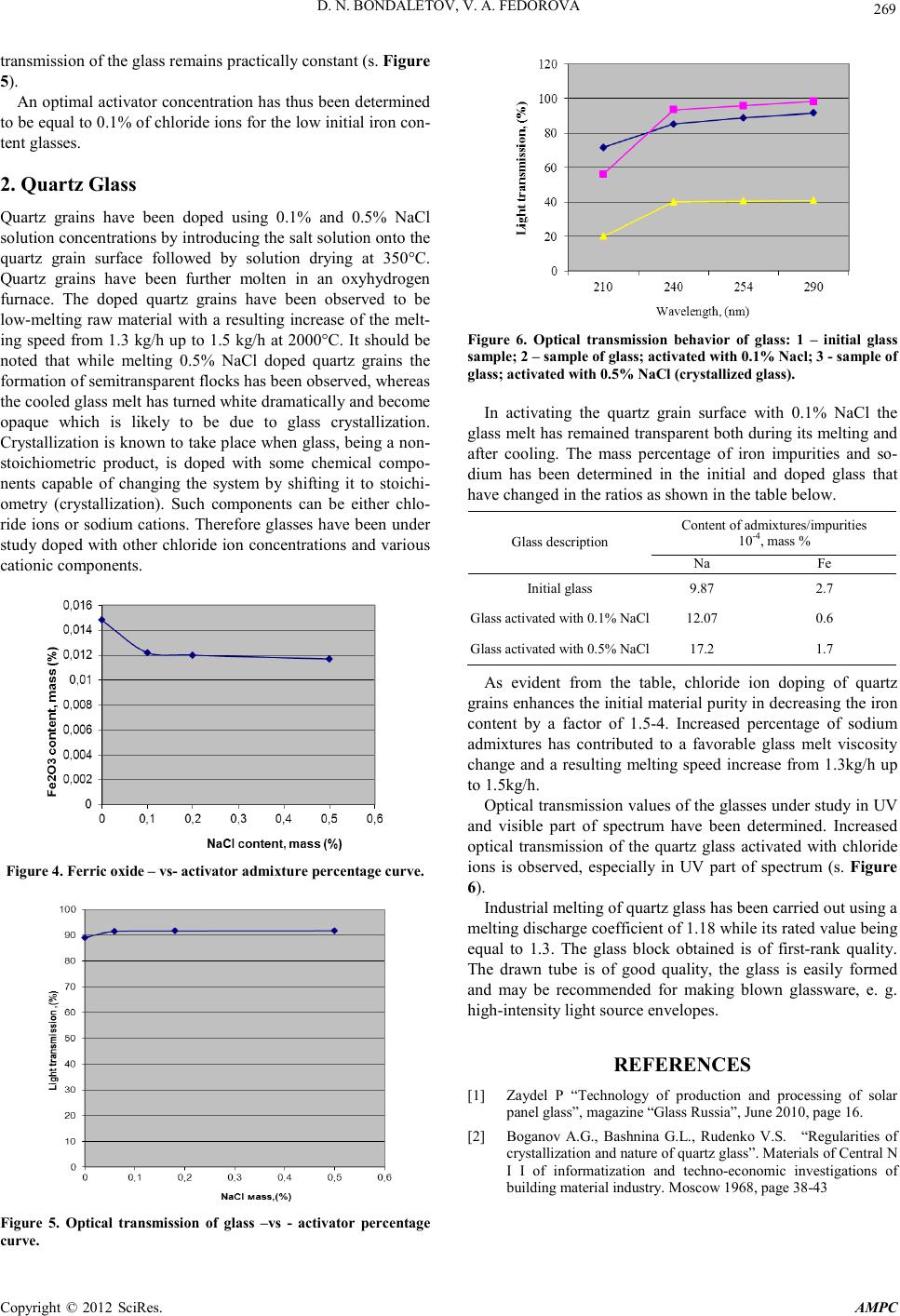 D. N. BONDALETOV, V. A. FEDOROVA Copyright © 2012 SciRes. AMPC 269 transmission of the glass remains practically constant (s. Figure 5). An optimal activator concentration has thus been determined to be equal to 0.1% of chloride ions for the low initial iron con- tent glas ses . 2. Quartz Glass Quartz grains have been doped using 0.1% and 0.5% NaCl solution concentrations by introducing the salt solution onto the quartz grain surface followed by solution drying at 350°C. Quartz grains have been further molten in an oxyhydrogen furnace. The doped quartz grains have been observed to be low-melting raw material with a resulting increase of the melt- ing speed from 1.3 kg/h up to 1.5 kg/h at 2000°C. It should be noted that while melting 0.5% NaCl doped quartz grains the formation of semitransparent flocks has been observed, whereas the cool ed gl ass melt has t urn ed white dramati call y and beco me opaque which is likely to be due to glass crystallization. Crystallization is known to take place when glass, bein g a non- stoichiometric product, is doped with some chemical compo- nents capable of changing the system by shifting it to stoichi- ometry (crystallization). Such components can be either chlo- ride ions or sodium cations. Therefore glasses have been under study doped with other chloride ion concentrations and various cationic components. Figure 4. Ferric oxide – vs- ac tivator admixture percentage curve. Figure 5. Optical transmission of glass –vs - activator percentage curve. Figure 6. Optical transmission behavior of glass: 1 – initial glass sample; 2 – s ample of gla s s; activated with 0.1% Nacl; 3 - sample of glass; activated with 0.5% NaCl (crystallized glass). In activating the quartz grain surface with 0.1% NaCl the glass melt has remained transparent both during its melting and after cooling. The mass percentage of iron impurities and so- dium has been determined in the initial and doped glass that have chan ged in the ratios as shown in the table below. Glas s description Content of admixtures/impurities 10-4, mass % Na Fe Initial glass 9.8 7 2.7 Glass activated with 0.1% NaCl 12.07 0.6 Glass activated with 0.5% NaCl 17.2 1.7 As evident from the table, chloride ion doping of quartz grains en hances th e initial materi al purit y in decreasing th e iron content by a factor of 1.5-4. Increased percentage of sodium admixtures has contributed to a favorable glass melt viscosity change and a resulting melting speed increase from 1.3kg/h up to 1.5kg/h . Optical transmission values of the glasses under study in UV and visible part of spectrum have been determined. Increased optical transmission of the quartz glass activated with chloride ions is observed, especially in UV part of spectrum (s. Figure 6). Industrial melting of quartz glass has been carried out using a meltin g disch arge co efficien t of 1.18 while its rated value being equal to 1.3. The glass block obtained is of first-rank quality. The drawn tube is of good quality, the glass is easily formed and may be recommended for making blown glassware, e. g. high-intensity light source envelopes. REFERENCES [1] Zaydel P “Technology of production and processing of solar panel glass ”, magazin e “G l ass Russi a”, June 2010, p age 16. [2] Boganov A.G., Bashnina G.L., Rudenko V.S. “Regularities of crystallization and nature of quartz glass”. Materials of Central N I I of informatization and techno-economic investigations of building material industry. Moscow 1968, page 38-43 |

