 American Journal of Plant Sciences, 2013, 4, 1976-1982 http://dx.doi.org/10.4236/ajps.2013.410245 Published Online October 2013 (http://www.scirp.org/journal/ajps) Effect of Ammonium- and Potassium-Loaded Zeolite on Kale (Brassica alboglabra) Growth and Soil Property Junxi Li1,2*, Chido Wee1, Bokyoon Sohn1 1Department of Agricultural Chemistry, Sunchon National University, Suncheon, South Korea; 2School of Biology and Biotechnol- ogy Sciences, Murdoch University, Perth, Australia. Email: *junxi1981@gmail.com Received August 1st, 2013; revised September 5th, 2013; accepted September 23rd, 2013 Copyright © 2013 Junxi Li et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT We investigated the feasibility of using ammonium- and potassium-loaded zeolite (NK-Z) as carriers for fertilizer and for slow release of nitrogen (N) and potassium (K). Plant growth response and soil analysis were performed. The results indicated an increase in the total harvest weight of kale (Brassica alboglabra Bailey). Furthermore, higher levels of N and K were detected in soil applied with NK-Z than in soil applied with compound fertilizers. The leaf fresh weight of kale in the fertilizer treatments including NK-Z were 2118.4 and 2111.3 g·plant−1, while the leaf fresh weights of kale in the treatment without NK-Z was 2018.0 g·plant−1. The N and K contents in the soils were maintained in the high level in the NK-Z treatment compared that in control treatment. The results indicate that NK-Z has a great potential as the slow-release fertilizer reducing pollution by preventing leaching to the ground water. Keywords: Zeolite; Slow-Release Fertilizer; Soil Property; Kale; Growth Response 1. Introduction Zeolites have been used in commercial applications be- cause of their unique adsorption, ion-exchange, molecu- lar sieve, and catalytic properties. In agricultural field, natural zeolite can be provided in large quantities with uniform characteristics and unique properties (cation ex- change capacity and pH) for application and commercial processing [1]. The negative charge created when Al3+ replaces Si4+ in structural tetrahedra is counterbalanced by cations (e.g., Na+, K+, 4 NH , and Ca2+, Mg2+). These charge sites are located in large structural channels and cavities throughout the structure and will be referred to as zeolitic exchange sites. Due to this special structure, natural zeolite can adsorb many kinds of ions with vary- ing degrees of ability. Natural zeolite is highly selective for 4 and K+ relative to Na+ or divalent cations such as Ca2+ and Mg2+ due to the location and density of nega- tive charge in the structure and the dimensions of interior channels (0.40 - 0.72 nm diameter) [2]. NH Natural zeolites in soil help to retain nutrients and im- prove long-term soil quality by enhancing its absorption ability. It influences soil retention of the most important plant nutrients such as N and K, Ca, Mg, and many kinds of micro-elements. Zeolite can retain these nutrients in the root zone to be used by plants as needed. The appli- cation of natural zeolite also enhances plant growth and reduces nutrient loss. Many laboratory and field expe- riments carried in recent decades have shown that sur- factant-modified zeolite and nutrient-loaded zeolite can be used as a kind of slow-release fertilizer to supply long- lasting nutrients for crop growth [3,4]. The application of zeolite can enhance the plant growth and development by reducing the loss of nutrients. The main use of natural zeolites in agriculture is for ammonium (4) exchange, storage, slow release and decrease losses of nitrogen through nitrification. NH Nitrogen leaching from irrigated cropland significantly contributes to increased nitrate (3) levels in ground and surface water [5]. To overcome the problems associ- ated with nitrogen leaching during fertilizer application, different approaches were taken to control N release. Ge- nerally, slow-release fertilizers (SRF) are usually expen- sive, and the release of N is slow at the time of high N need. However, in a 4-week long experiment, Perrin et al. [6] reported that soil amendment with ammonium-loaded clinoptilolite (A-CP) significantly reduced leaching and provided sufficient N for normal corn growth. However, corn begins to take up exponentially higher levels of N NO *Corresponding author. Copyright © 2013 SciRes. AJPS 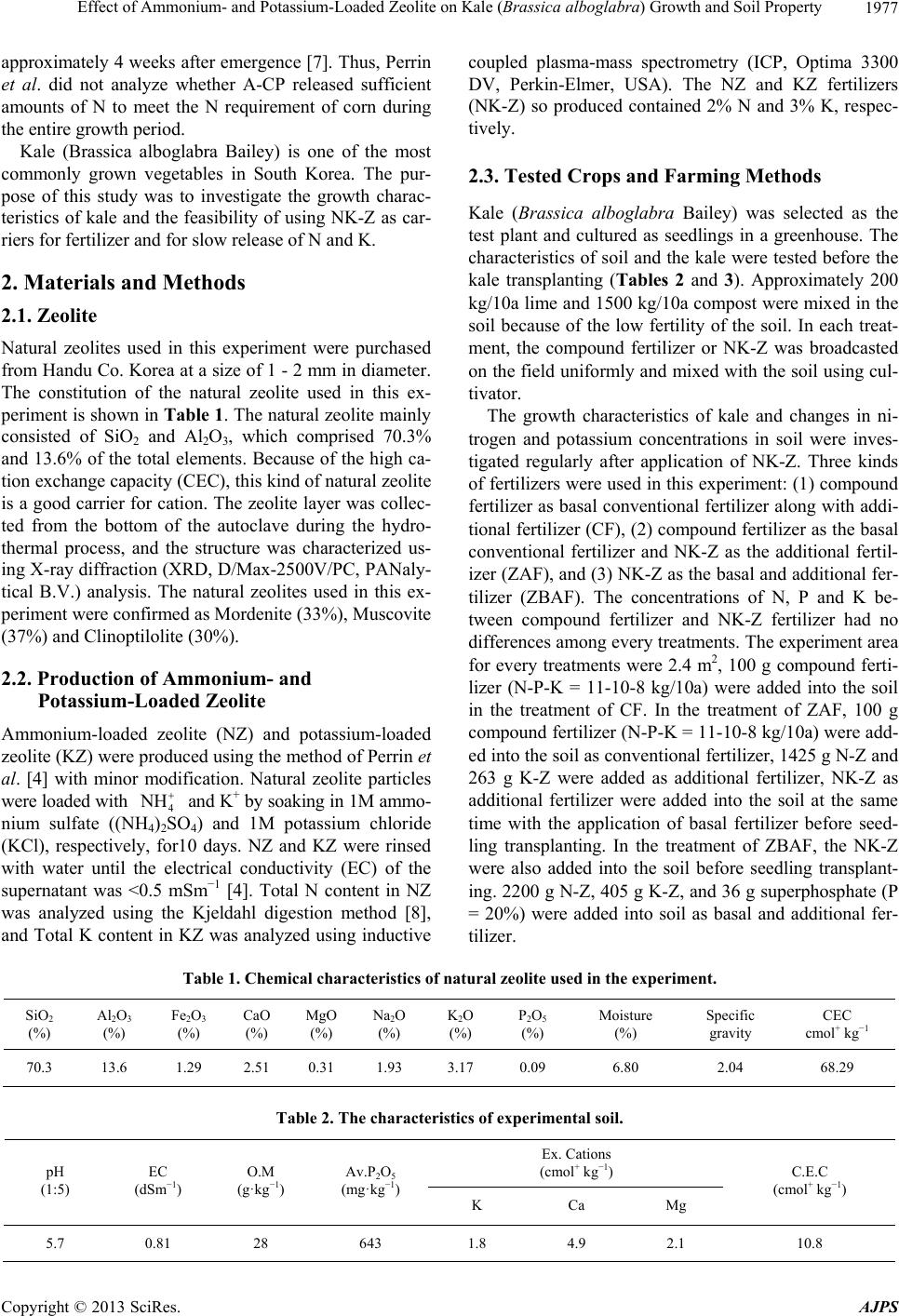 Effect of Ammonium- and Potassium-Loaded Zeolite on Kale (Brassica alboglabra) Growth and Soil Property 1977 approximately 4 weeks after emergence [7]. Thus, Perrin et al. did not analyze whether A-CP released sufficient amounts of N to meet the N requirement of corn during the entire growth period. Kale (Brassica alboglabra Bailey) is one of the most commonly grown vegetables in South Korea. The pur- pose of this study was to investigate the growth charac- teristics of kale and the feasibility of using NK-Z as car- riers for fertilizer and for slow release of N and K. 2. Materials and Methods 2.1. Zeolite Natural zeolites used in this experiment were purchased from Handu Co. Korea at a size of 1 - 2 mm in diameter. The constitution of the natural zeolite used in this ex- periment is shown in Table 1. The natural zeolite mainly consisted of SiO2 and Al2O3, which comprised 70.3% and 13.6% of the total elements. Because of the high ca- tion exchange capacity (CEC), this kind of natural zeolite is a good carrier for cation. The zeolite layer was collec- ted from the bottom of the autoclave during the hydro- thermal process, and the structure was characterized us- ing X-ray diffraction (XRD, D/Max-2500V/PC, PANaly- tical B.V.) analysis. The natural zeolites used in this ex- periment were confirmed as Mordenite (33%), Muscovite (37%) and Clinoptilolite (30%). 2.2. Production of Ammonium- and Potassium-Loaded Zeolite Ammonium-loaded zeolite (NZ) and potassium-loaded zeolite (KZ) were produced using the method of Perrin et al. [4] with minor modification. Natural zeolite particles were loaded with 4 and K+ by soaking in 1M ammo- nium sulfate ((NH4)2SO4) and 1M potassium chloride (KCl), respectively, for10 days. NZ and KZ were rinsed with water until the electrical conductivity (EC) of the supernatant was <0.5 mSm−1 [4]. Total N content in NZ was analyzed using the Kjeldahl digestion method [8], and Total K content in KZ was analyzed using inductive NH coupled plasma-mass spectrometry (ICP, Optima 3300 DV, Perkin-Elmer, USA). The NZ and KZ fertilizers (NK-Z) so produced contained 2% N and 3% K, respec- tively. 2.3. Tested Crops and Farming Methods Kale (Brassica alboglabra Bailey) was selected as the test plant and cultured as seedlings in a greenhouse. The characteristics of soil and the kale were tested before the kale transplanting (Tables 2 and 3). Approximately 200 kg/10a lime and 1500 kg/10a compost were mixed in the soil because of the low fertility of the soil. In each treat- ment, the compound fertilizer or NK-Z was broadcasted on the field uniformly and mixed with the soil using cul- tivator. The growth characteristics of kale and changes in ni- trogen and potassium concentrations in soil were inves- tigated regularly after application of NK-Z. Three kinds of fertilizers were used in this experiment: (1) compound fertilizer as basal conventional fertilizer along with addi- tional fertilizer (CF), (2) compound fertilizer as the basal conventional fertilizer and NK-Z as the additional fertil- izer (ZAF), and (3) NK-Z as the basal and additional fer- tilizer (ZBAF). The concentrations of N, P and K be- tween compound fertilizer and NK-Z fertilizer had no differences among every treatments. The experiment area for every treatments were 2.4 m2, 100 g compound ferti- lizer (N-P-K = 11-10-8 kg/10a) were added into the soil in the treatment of CF. In the treatment of ZAF, 100 g compound fertilizer (N-P-K = 11-10-8 kg/10a) were add- ed into the soil as conventional fertilizer, 1425 g N-Z and 263 g K-Z were added as additional fertilizer, NK-Z as additional fertilizer were added into the soil at the same time with the application of basal fertilizer before seed- ling transplanting. In the treatment of ZBAF, the NK-Z were also added into the soil before seedling transplant- ing. 2200 g N-Z, 405 g K-Z, and 36 g superphosphate (P = 20%) were added into soil as basal and additional fer- tilizer. Table 1. Chemical characteristics of natural zeolite used in the experiment. SiO2 (%) Al2O3 (%) Fe2O3 (%) CaO (%) MgO (%) Na2O (%) K2O (%) P2O5 (%) Moisture (%) Specific gravity CEC cmol+ kg−1 70.3 13.6 1.29 2.51 0.31 1.93 3.17 0.09 6.80 2.04 68.29 Table 2. The characteristics of experimental soil. Ex. Cations (cmol+ kg−1) pH (1:5) EC (dSm−1) O.M (g·kg−1) Av.P2O5 (mg·kg−1) K Ca Mg C.E.C (cmol+ kg−1) 5.7 0.81 28 643 1.8 4.9 2.1 10.8 Copyright © 2013 SciRes. AJPS  Effect of Ammonium- and Potassium-Loaded Zeolite on Kale (Brassica alboglabra) Growth and Soil Property 1978 Table 3. The growth characteristics of kale before trans- planting. Species No. of leaves (ea) Leaf area (cm2) Shoot fresh weight (g) Brassica alboglabra Bailey 5.0 ± 0.0 112.25 ± 1.96 8.02 ± 0.26 2.4. Test Methods and Statistical Analysis Soil in the different treatment fields were sampled 0, 3, 6, and 18 weeks after transplanting, Weight 5 g dry soil into a plastic triangular flask, add 50 ml distilled water and shake for 30 minutes at the speed of 120 rpm. The clear solution was accepted after filtering using what man No.2 filter paper. The nitrogen content was analyzed by the Kjeldahl digestion method [8] and potassium was analyzed by Inductive Coupled Plasma (ICP, Optima 3300 DV, Perkin-Elmer, USA) using the method of NI- AST [9]. EC and pH were analyzed using Istek Conduc- tivity meter and Mettler Toledo 340 pH meter, respec- tively. Phosphorus and Soil CEC were also analyzed us- ing the method of NIAST [9]. Chlorophyll content of fully expanded kale leaves was measured using an in situ SPAD-502 chlorophyll meter (Minolta Co. Ltd., Japan). Actual chlorophyll content Y (mg/100 cm2) was calculated by substituting the SPAD reading for X in the standard formula Y = 0.0996X − 0.152 [10]. Leaf length and leaf width were measured us- ing vernier caliper, shoot weight were measured using platform balance (A&D HF-2000 g). For chemical analy- sis, plant samples were finely ground after drying at 65˚C for 48 h. A 0.5 g sample was placed in a 100 ml flask with 10 ml of concentrated H2SO4. Next, 0.5 ml H2O2 was added to the sample every 10 min for 90 min (total: 4.5 ml). After cooling, the solution was filtered through a Whatman No. 6 filter into 100 ml flasks. Inorganic con- stituents contents were determined using Inductive Cou- pled Plasma (ICP, Optima 3300DV, Perkin-Elmer, USA). The data from different treatment combinations were ana- lyzed using SPSS for windows. 3. Results and Discussion 3.1. Crop Growth Characteristics Significant increases in the yields of wheat (13% - 15%), eggplant (19% - 55%), apples (13% - 38%), and carrots (63%) were reported when 4 - 8 tons of zeolite were added per acre [11]. In our study, leaf number, leaf length, leaf width, shoot weight, and chlorophyll content were estimated three weeks after transplanting (Table 4). The leaf length of kale in ZAF and ZBAF were 12.61% and 9.28% longer, respectively, than in CF. However, the leaf number did not differ significantly among the groups. The shoot weight in ZAF and ZBAF was 3.74% and 3.61% higher, respectively, than in CF. Thus, the results indicate that NK-Z enhances plant growth during the ear- ly stage. The leaf fresh weight per plant was estimated at 6 in- tervals of harvesting. NK-Z improved the harvest of kale significantly; the yield in ZAF and ZBAF was 4.97% and 4.65% higher, respectively, as compared with CF (Fig- ure 1). It has been reported that leaf area, dry weight, and root fresh weight was higher in radishes fertilized with A-CP than in those fertilized with ammonium sul- fate [12]. In this experiment, we showed that plant growth and kale harvest was higher both in ZAF and ZBAF as compared to that in CF. 3.2. Inorganic Matter Contents in Kale Leaf There was rare experiment adjustment the effective of zeolite on crop inorganic matter contents. In this paper, we tested the inorganic matter contents between every group in the kale leaf to investigate the effective of nutri- tion loaded zeolite on improving crop inorganic matter contents 18 weeks after transplanting (Table 5). In the group of ZAF, all of the tested inorganic constituents Table 4. Growth responses of kale 3 weeks after trans- planting. Treatments No. of leaves (ea) Shoot weight (g/plant) Leaf length (cm) Leaf width (cm) Chlorophyll contents (mg/100 cm2) CF* 9.58141.24 19.83 18.50 4.67 ZAF* 9.41146.52 22.33* 19.67 4.89 ZBAF* 9.69146.34 21.67 19.33 4.92 LSD 0.05*0.379 25.9711.972 2.568 0.386 *CF, Compound fertilizer as basal conventional fertilizer along with addi- tional fertilizer; *ZAF, Compound fertilizer as basal conventional fertilizer, NK-Z as additional fertilizer; *ZBAF, NK-Z as basal and additional fertilizer. *Each value was compared with least significant difference (LSD). 0 500 1000 1500 2000 2500 CFZAF ZBAF Treatment group Leaf fresh wight(g/plant) 1 st harvesting2nd harvesting3rd harvesting 4th harvesting5th harvesting6th harvesting Figure 1. The fresh weight of kale leaf via 6 times of har- vesting. Copyright © 2013 SciRes. AJPS 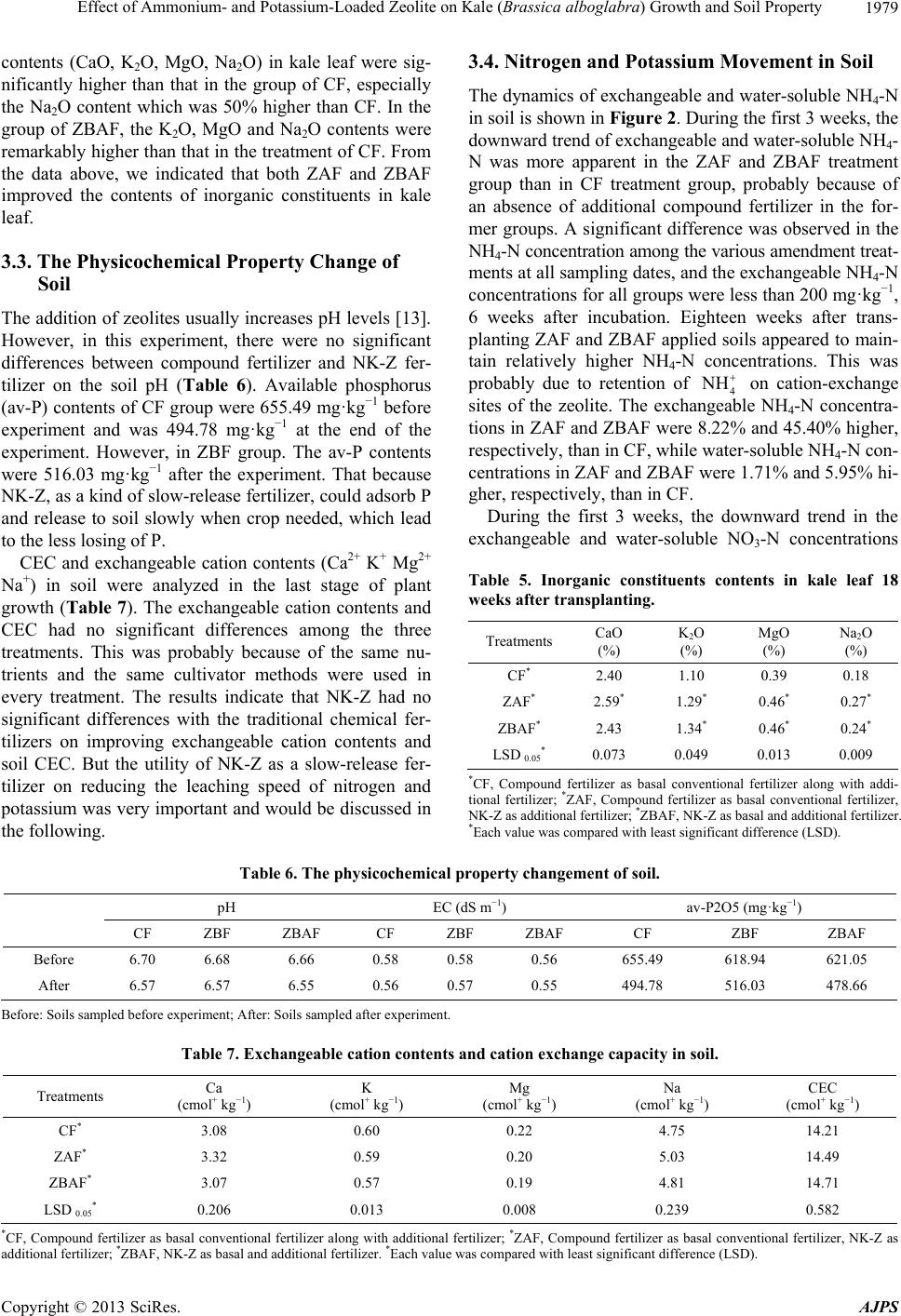 Effect of Ammonium- and Potassium-Loaded Zeolite on Kale (Brassica alboglabra) Growth and Soil Property 1979 contents (CaO, K2O, MgO, Na2O) in kale leaf were sig- nificantly higher than that in the group of CF, especially the Na2O content which was 50% higher than CF. In the group of ZBAF, the K2O, MgO and Na2O contents were remarkably higher than that in the treatment of CF. From the data above, we indicated that both ZAF and ZBAF improved the contents of inorganic constituents in kale leaf. 3.3. The Physicochemical Property Change of Soil The addition of zeolites usually increases pH levels [13]. However, in this experiment, there were no significant differences between compound fertilizer and NK-Z fer- tilizer on the soil pH (Table 6). Available phosphorus (av-P) contents of CF group were 655.49 mg·kg−1 before experiment and was 494.78 mg·kg−1 at the end of the experiment. However, in ZBF group. The av-P contents were 516.03 mg·kg−1 after the experiment. That because NK-Z, as a kind of slow-release fertilizer, could adsorb P and release to soil slowly when crop needed, which lead to the less losing of P. CEC and exchangeable cation contents (Ca2+ K+ Mg2+ Na+) in soil were analyzed in the last stage of plant growth (Table 7). The exchangeable cation contents and CEC had no significant differences among the three treatments. This was probably because of the same nu- trients and the same cultivator methods were used in every treatment. The results indicate that NK-Z had no significant differences with the traditional chemical fer- tilizers on improving exchangeable cation contents and soil CEC. But the utility of NK-Z as a slow-release fer- tilizer on reducing the leaching speed of nitrogen and potassium was very important and would be discussed in the following. 3.4. Nitrogen and Potassium Movement in Soil The dynamics of exchangeable and water-soluble NH4-N in soil is shown in Figure 2. During the first 3 weeks, the downward trend of exchangeable and water-soluble NH4- N was more apparent in the ZAF and ZBAF treatment group than in CF treatment group, probably because of an absence of additional compound fertilizer in the for- mer groups. A significant difference was observed in the NH4-N concentration among the various amendment treat- ments at all sampling dates, and the exchangeable NH4-N concentrations for all groups were less than 200 mg·kg−1, 6 weeks after incubation. Eighteen weeks after trans- planting ZAF and ZBAF applied soils appeared to main- tain relatively higher NH4-N concentrations. This was probably due to retention of 4 on cation-exchange sites of the zeolite. The exchangeable NH4-N concentra- tions in ZAF and ZBAF were 8.22% and 45.40% higher, respectively, than in CF, while water-soluble NH4-N con- centrations in ZAF and ZBAF were 1.71% and 5.95% hi- gher, respectively, than in CF. NH During the first 3 weeks, the downward trend in the exchangeable and water-soluble NO3-N concentrations Table 5. Inorganic constituents contents in kale leaf 18 weeks after transplanting. Treatments CaO (%) K2O (%) MgO (%) Na2O (%) CF* 2.40 1.10 0.39 0.18 ZAF* 2.59* 1.29* 0.46* 0.27* ZBAF* 2.43 1.34* 0.46* 0.24* LSD 0.05* 0.073 0.049 0.013 0.009 *CF, Compound fertilizer as basal conventional fertilizer along with addi- tional fertilizer; *ZAF, Compound fertilizer as basal conventional fertilizer, NK-Z as additional fertilizer; *ZBAF, NK-Z as basal and additional fertilizer. *Each value was compared with least significant difference (LSD). Table 6. The physicochemical property changement of soil. pH EC (dS m−1) av-P2O5 (mg·kg−1) CF ZBF ZBAF CF ZBF ZBAF CF ZBF ZBAF Before 6.70 6.68 6.66 0.58 0.58 0.56 655.49 618.94 621.05 After 6.57 6.57 6.55 0.56 0.57 0.55 494.78 516.03 478.66 Before: Soils sampled before experiment; After: Soils sampled after experiment. Table 7. Exchangeable cation contents and cation exchange capacity in soil. Treatments Ca (cmol+ kg−1) K (cmol+ kg−1) Mg (cmol+ kg−1) Na (cmol+ kg−1) CEC (cmol+ kg−1) CF* 3.08 0.60 0.22 4.75 14.21 ZAF* 3.32 0.59 0.20 5.03 14.49 ZBAF* 3.07 0.57 0.19 4.81 14.71 LSD 0.05* 0.206 0.013 0.008 0.239 0.582 *CF, Compound fertilizer as basal conventional fertilizer along with additional fertilizer; *ZAF, Compound fertilizer as basal conventional fertilizer, NK-Z as additional fertilizer; *ZBAF, NK-Z as basal and additional fertilizer. *Each value was compared with least significant difference (LSD). Copyright © 2013 SciRes. AJPS 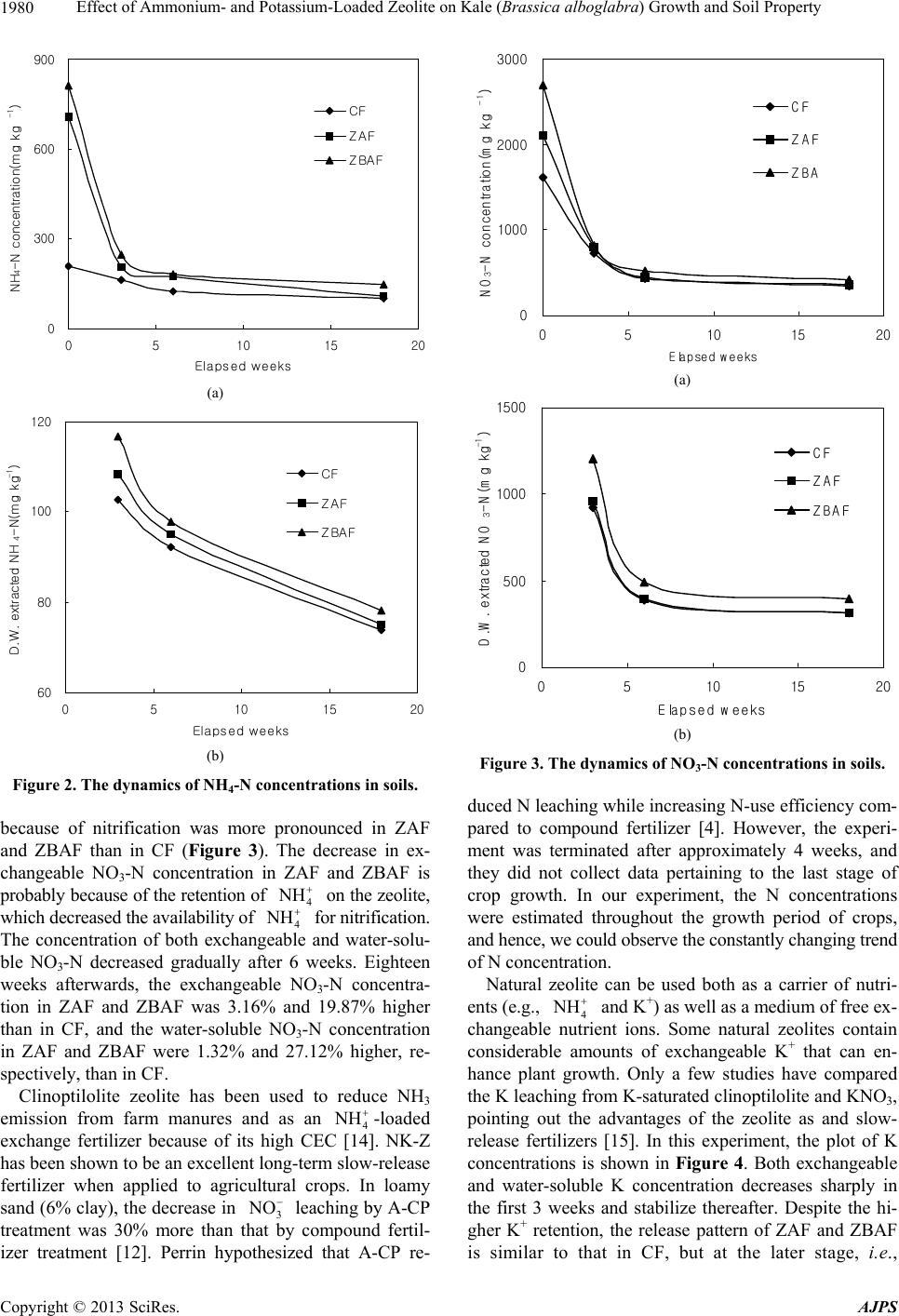 Effect of Ammonium- and Potassium-Loaded Zeolite on Kale (Brassica alboglabra) Growth and Soil Property 1980 0 300 600 900 0510 15 20 Elapsed weeks NH4-N concentration(mg kg-1) CF ZAF ZBAF (a) 60 80 100 120 051015 20 Elapsed weeks D.W. extracted NH4-N(mg kg -1) CF ZAF ZBAF (b) Figure 2. The dynamics of NH4-N concentrations in soils. because of nitrification was more pronounced in ZAF and ZBAF than in CF (Figure 3). The decrease in ex- changeable NO3-N concentration in ZAF and ZBAF is probably because of the retention of 4 H on the zeolite, which decreased the availability of 4 H for nitrification. The concentration of both exchangeable and water-solu- ble NO3-N decreased gradually after 6 weeks. Eighteen weeks afterwards, the exchangeable NO3-N concentra- tion in ZAF and ZBAF was 3.16% and 19.87% higher than in CF, and the water-soluble NO3-N concentration in ZAF and ZBAF were 1.32% and 27.12% higher, re- spectively, than in CF. Clinoptilolite zeolite has been used to reduce NH3 emission from farm manures and as an 4 H -loaded exchange fertilizer because of its high CEC [14]. NK-Z has been shown to be an excellent long-term slow-release fertilizer when applied to agricultural crops. In loamy sand (6% clay), the decrease in 3 leaching by A-CP treatment was 30% more than that by compound fertil- izer treatment [12]. Perrin hypothesized that A-CP re- NO 0 1000 2000 3000 05101520 Elapsed weeks NO3-N concentration(mg kg-1) CF ZAF ZBA (a) 0 500 1000 1500 0510 1520 Elapsed weeks D .W. extracted N O3-N(mg kg -1) CF ZAF ZBAF (b) Figure 3. The dynamics of NO3-N concentrations in soils. duced N leaching while increasing N-use efficiency com- pared to compound fertilizer [4]. However, the experi- ment was terminated after approximately 4 weeks, and they did not collect data pertaining to the last stage of crop growth. In our experiment, the N concentrations were estimated throughout the growth period of crops, and hence, we could observe the constantly changing trend of N concentration. Natural zeolite can be used both as a carrier of nutri- ents (e.g., 4 H and K+) as well as a medium of free ex- changeable nutrient ions. Some natural zeolites contain considerable amounts of exchangeable K+ that can en- hance plant growth. Only a few studies have compared the K leaching from K-saturated clinoptilolite and KNO3, pointing out the advantages of the zeolite as and slow- release fertilizers [15]. In this experiment, the plot of K concentrations is shown in Figure 4. Both exchangeable and water-soluble K concentration decreases sharply in the first 3 weeks and stabilize thereafter. Despite the hi- gher K+ retention, the release pattern of ZAF and ZBAF is similar to that in CF, but at the later stage, i.e., Copyright © 2013 SciRes. AJPS 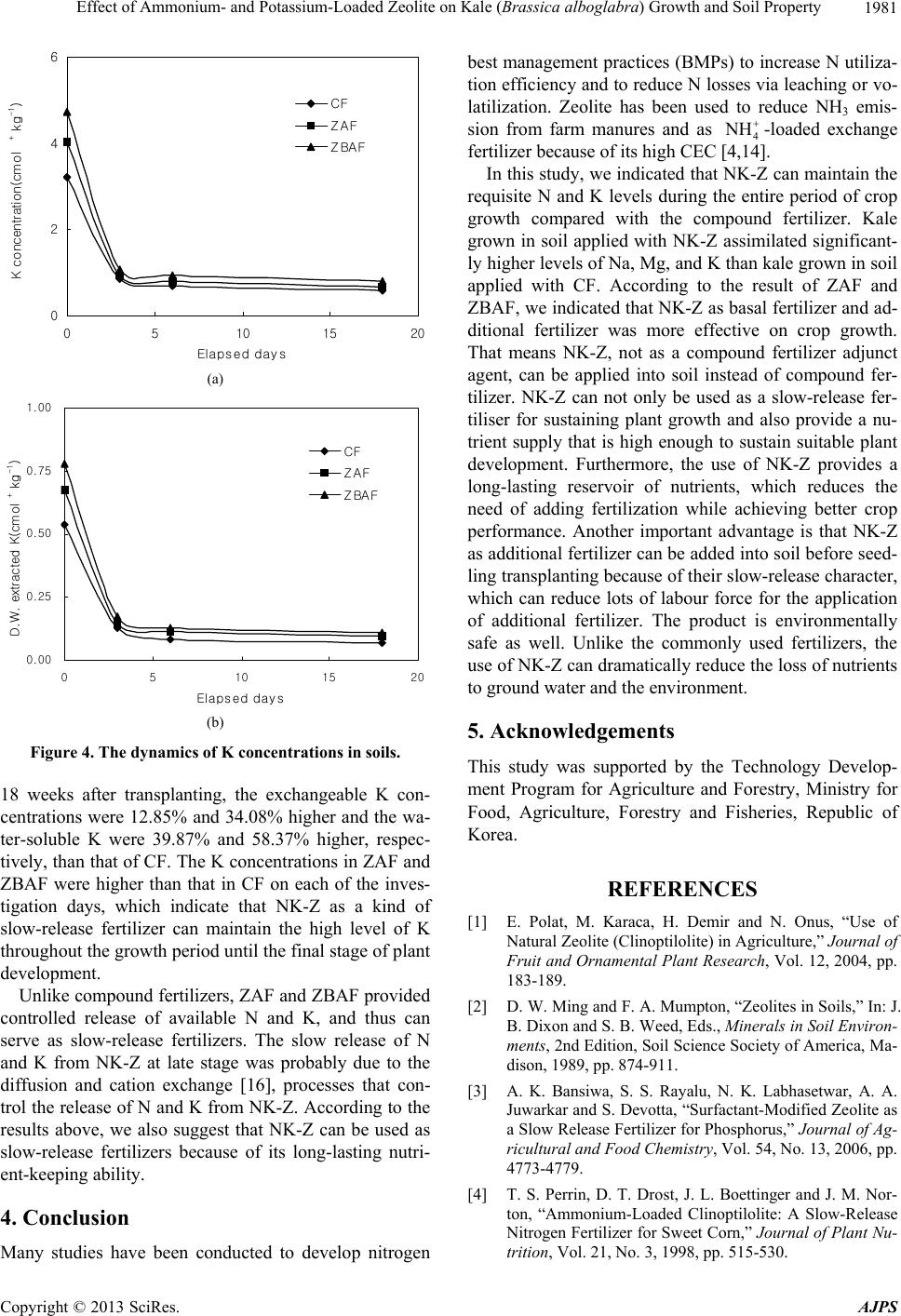 Effect of Ammonium- and Potassium-Loaded Zeolite on Kale (Brassica alboglabra) Growth and Soil Property 1981 0 2 4 6 0510 1520 Elapsed days K concentration(cmol+ kg-1) CF ZAF ZBAF (a) 0. 00 0. 25 0. 50 0. 75 1. 00 0510 1520 Elapsed days D.W. extracted K(cmol + kg-1) CF ZAF ZBAF (b) Figure 4. The dynamics of K concentrations in soils. 18 weeks after transplanting, the exchangeable K con- centrations were 12.85% and 34.08% higher and the wa- ter-soluble K were 39.87% and 58.37% higher, respec- tively, than that of CF. The K concentrations in ZAF and ZBAF were higher than that in CF on each of the inves- tigation days, which indicate that NK-Z as a kind of slow-release fertilizer can maintain the high level of K throughout the growth period until the final stage of plant development. Unlike compound fertilizers, ZAF and ZBAF provided controlled release of available N and K, and thus can serve as slow-release fertilizers. The slow release of N and K from NK-Z at late stage was probably due to the diffusion and cation exchange [16], processes that con- trol the release of N and K from NK-Z. According to the results above, we also suggest that NK-Z can be used as slow-release fertilizers because of its long-lasting nutri- ent-keeping ability. 4. Conclusion Many studies have been conducted to develop nitrogen best management practices (BMPs) to increase N utiliza- tion efficiency and to reduce N losses via leaching or vo- latilization. Zeolite has been used to reduce NH3 emis- sion from farm manures and as 4 H-loaded exchange fertilizer because of its high CEC [4,14]. In this study, we indicated that NK-Z can maintain the requisite N and K levels during the entire period of crop growth compared with the compound fertilizer. Kale grown in soil applied with NK-Z assimilated significant- ly higher levels of Na, Mg, and K than kale grown in soil applied with CF. According to the result of ZAF and ZBAF, we indicated that NK-Z as basal fertilizer and ad- ditional fertilizer was more effective on crop growth. That means NK-Z, not as a compound fertilizer adjunct agent, can be applied into soil instead of compound fer- tilizer. NK-Z can not only be used as a slow-release fer- tiliser for sustaining plant growth and also provide a nu- trient supply that is high enough to sustain suitable plant development. Furthermore, the use of NK-Z provides a long-lasting reservoir of nutrients, which reduces the need of adding fertilization while achieving better crop performance. Another important advantage is that NK-Z as additional fertilizer can be added into soil before seed- ling transplanting because of their slow-release character, which can reduce lots of labour force for the application of additional fertilizer. The product is environmentally safe as well. Unlike the commonly used fertilizers, the use of NK-Z can dramatically reduce the loss of nutrients to ground water and the environment. 5. Acknowledgements This study was supported by the Technology Develop- ment Program for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea. REFERENCES [1] E. Polat, M. Karaca, H. Demir and N. Onus, “Use of Natural Zeolite (Clinoptilolite) in Agriculture,” Journal of Fruit and Ornamental Plant Research, Vol. 12, 2004, pp. 183-189. [2] D. W. Ming and F. A. Mumpton, “Zeolites in Soils,” In: J. B. Dixon and S. B. Weed, Eds., Minerals in Soil Environ- ments, 2nd Edition, Soil Science Society of America, Ma- dison, 1989, pp. 874-911. [3] A. K. Bansiwa, S. S. Rayalu, N. K. Labhasetwar, A. A. Juwarkar and S. Devotta, “Surfactant-Modified Zeolite as a Slow Release Fertilizer for Phosphorus,” Journal of Ag- ricultural and Food Chemistry, Vol. 54, No. 13, 2006, pp. 4773-4779. [4] T. S. Perrin, D. T. Drost, J. L. Boettinger and J. M. Nor- ton, “Ammonium-Loaded Clinoptilolite: A Slow-Release Nitrogen Fertilizer for Sweet Corn,” Journal of Plant Nu- trition, Vol. 21, No. 3, 1998, pp. 515-530. Copyright © 2013 SciRes. AJPS  Effect of Ammonium- and Potassium-Loaded Zeolite on Kale (Brassica alboglabra) Growth and Soil Property Copyright © 2013 SciRes. AJPS 1982 http://dx.doi.org/10.1080/01904169809365421 [5] E. Lichtenberg and L. S. Shapiro, “Agriculture and Ni- trate Concentrations in Maryland Community Water Sys- tem Wells,” Journal of Environmental Quality, Vol. 26, No. 1, 1997, pp. 145-153. [6] T. S. Perrin, D. T. Drost, J. L. Boettinger and J. M. Nor- ton, “Ammonium-Loaded Clinoptilolite: A Slow-Release Nitrogen Fertilizer for Sweet Corn,” Journal of Plant Nu- trition, Vol. 21, No. 3, 2008, pp. 515-530. [7] S. W. Ritchie, J. J. Hanway and G. O. Benson, “How a Corn Plant Develops,” Iowa State University of Science and Technology Cooperative Extension Service, Special Report, 1997. [8] J. M. Bremner and C. S. Mulvaney, “Nitrogen-Total,” In: A. L. Page, R. H. Miller and D. R. Keeney, Eds., Part 2. Methods of Soil Analysis, Agronomy 9, American Society of Agronomy, 1982, pp. 403-430. [9] NIAST, “Methods of Soil Chemical Analysis, National Institute of Agricultural Science and Technology,” RDA, Suwon, 2000. [10] Y. Watanabe, F. Uchiyama and K. Yoshida, “Compositio- nal Changes in Spinach (Spinacia oleracea L.) Grown in the Summer and in the Fall,” Journal of Japanese Society of Horticulture, Science, Vol. 62, 1994, pp. 889-895. [11] K. Torri, “Utilization of Natureal Zeolite in Japan,” In: L. B. Sand and F. A. Mumpton, Eds., Natural zeolites: Oc- currence, Properties, Use, Pergamon, Elmsford, 1978, pp. 441-450. [12] M. D. Lewis, F. D. Moore and K. L. Goldsberry, “Am- monium-Exchanged Clinoptilolite and Granulated Clino- ptilolite with Urea as Nitrogen Fertilizers,” In: W. G. Pond and F. A. Mumpton, Eds., Zeo-Agriculture: Use of Natural Zeolites in Agriculture and Aquaculture. West- view Press, Boulder, 1984, pp. 105-111. [13] D. W. Ming and J. L. Boettinger, “Zeolites in Soil Envi- ronments,” Reviews in Mineralogy and Geochemistry, Vol. 45, No. 1, 2001, pp. 323-345. http://dx.doi.org/10.2138/rmg.2001.45.11 [14] M. Amon, M. Dobeic, R. W. Sneath, V. R. Philips, T. H. Mis-selbrook and B. E. Pain, “A Farm-Scale Study on the Use of Clinoptilolite Zeolite and De-Odorase for Reduc- ing Odor and Ammonia Emissions from Broiler Houses,” Bioresearches Technology, Vol. 61, 1997, pp. 229-237. [15] D. R. Hershey, J. L. Paul and R. M. Carlson, “Evaluation of Potassium-Enriched Clinoptilolite as a Potassium Source for Potting Media,” Horticultural Science, Vol. 15, 1980, pp. 87-89. [16] D. W. Ming, D. J. Barta, D. C. Golden, C. Galindo and D. L. Henninger, “Zeoponic Plant-Growth Substrates for Space Applications,” In: D. W. Ming and F. A. Mumpton, Eds., Natural Zeolites: Occurrences, Properties, Use, Interna- tional Committee on Natural Zeolites, Brockport, 1995, pp. 505-513.
|