 Modern Research in Catalysis, 2013, 2, 119-126 http://dx.doi.org/10.4236/mrc.2013.24017 Published Online October 2013 (http://www.scirp.org/journal/mrc) N-Hexane Isomerization on Ni-Pt/Catalysts Supported on Mordenite Geovana S. V. Martins1, Everton R. F. dos Santos1, Meiry G. F. Rodrigues1, Gina Pecchi2, Carlos M. N. Yoshioka3, Dilson Cardoso3 1Chemical Engineering Department, Federal University of Campina Grande, Catalysis Laboratory (LABNOV), Campina Grande, Brazil 2Facultad de Ciencias Químicas, UniversidadConcepción, Concepción, Chile 3Chemical Engineering Department, Federal University of São Carlos, Catalysis Laboratory (LabCat), São Carlos, Brazil Email: meiry@deq.ufcg.edu.br Received August 7, 2013; revised September 5, 2013; accepted September 17, 2013 Copyright © 2013 Geovana S. V. Martins et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The aim of this work was to evaluate the catalytic properties for n-hexane isomerization of bifunctional monometallic (Ni or Pt) and bimetallic catalysts (Pt-Ni), using HMOR zeolite as support. The method used for metal dispersion in the zeolite was competitive ion exchange using ammine complexes [Ni(NH3)6]Cl2 and [Pt(NH3)4]Cl2 as precursors. The catalysts were characterized by X-Ray diffraction, X-Ray energy dispersion spectroscopy, temperature-programmed re- duction and transmission electron microscopy. The n-hexane isomerization reaction using the catalysts was carried out to evaluate the catalyst activity. The reaction was carried out in a fixed bed reactor operating at 250˚C, 1 atm, H2/C6 = 9 molar ratio. The profiles obtained from TPR suggest that, for bimetallic catalysts, the presence of platinum facilitates the reduction of Ni2+ cations. The bimetallic catalysts presented a higher activity in the isomerization of n-hexane when compared to the monometallic ones, as well better stability as the Pt content in the solid increases. Keywords: N-Hexane; Isomerization; Mordenite; Nickel; Platinum 1. Introduction Several factors determine gasoline quality. One of the key specifications of gasoline is the octane number, which corresponds to the fuel knocking (self-igniting) property in internal combustion engine. High octane numbers cor- relate to a low knocking intensity that is related to good engine performance [1]. Usually branched paraffins have higher octane num- bers than corresponding linear paraffins. For example, linear hexane has an octane number equal to 25, while 2,2-dimethylbutane, an hexane isomer, has an octane number equal to 92. For that reason, isomerization of linear paraffins, a process in which straight-chain hydro- carbon molecules rearrange to form branched hydrocar- bons, is used to improve gasoline quality [2]. Commonly, users of paraffin isomerization technology had the choice between robust zeolite based systems [3-8]. While zeolite catalysts are characterized by their outstanding tolerance of feedstock poison such as sulphur and water—this is particularly true for Sud-ChemieHysopar catalyst that operates commercially at sulphur levels ex- ceeding 100 ppm—the chlorinated catalysts suffer from extreme sensitivity to all kinds of feed contaminants [9]. The cases of Mordenite zeolite are employed in rela- tively high temperature (250˚C) necessary to form the carbocationic isomerization reactions C5/C6 that the case of long paraffins such as n-heptane ends cracking occur- ring faster than favoring the formation of coke and avoids getting high fractions of branched isomers [10,11]. These catalysts are bifunctional, i.e. they consist of a metal supported on a zeolite, and since the reaction, and mechanism requires the dehydrogenation of the initial alkane to form an intermediate alkene. This alkene can then proceed through a carbocationic intermediate either to yield the isomerized products or to undergo cracking through a B-scission to give unwanted gaseous products [12,13]. For this kind of bifunctional catalysts, Guisnetet al. [14] estimated an optimum in the number of acid sites per available platinum atom 6. If this ratio is exceeded, the cracking reaction will be favored. Moreover, those catalysts without a proper balance between the metallic C opyright © 2013 SciRes. MRC  G. S. V. MARTINS ET AL. 120 and acid functions are expected to follow an alternative mechanistic pathway involving bimolecular intermedi- ates [12]. The second ingredient of bifunctional catalyst is the hydrogenation/dehydrogenation site, being Pt and Pd very active in comparison with other transition metals. According to Jordão et al. [16], they research with bi- metallic catalysts Pt-Ni and Pt-Cu/HUSY in the isomeri- zation of n-hexane, seeking a possible alternative for the decrease of the cost of the catalyst, doing a substitution from platinum to metals of low cost (Ni or Co). It was verified that the bimetallic catalysts presented a great activity when compared with the monometallic ones, even that the pure platinum. In this same perspective, it was analyzed that the proportions of the catalysts varied the percentage of platinum and nickel for the isomeriza- tion of n-hexane reaction. Recent studies on Pt-Ni systems supported on H-USY zeolite and Beta zeolite [15-18] showed that these pro- vided catalytic activities were higher than those of mono- metallic Pt catalysts. Many studies [19-23] have been conducted using the Pt/Mordenite catalyst in the reaction of n-hexane isom- erization, but the literature is sparse regarding the Ni-Pt/ Mordenite catalyst. Mordenite has an intrinsic activity for isomerization and the initial rate is rather lowered by the presence of Pt and hydrogen. Thus, the most important role of the metal component is to stabilize the catalytic activity and to of- fer higher selectivity for isomerization [24]. Mordenite consists of parallel 12-membered ring (MR) channels (0.67 × 0.70 nm) with 8 MR side pockets (0.34 × 0.48 nm) [25]. Due to the small size of the 8 MR, for most guest species, mordenite structure is generally con- sidered as one-dimensional, which can induce diffusion limitations in catalysis applications [26]. The objective of this work was to investigate the effect of nickel on Pt/Mordenite catalyst in the reaction of n-hexane isomerization. The catalysts were prepared by competitive ion exchange and were characterized by X- ray diffraction, X-Ray energy dispersion spectroscopy, temperature-programmed reduction and transmission electron microscopy. 2. Experimental 2.1. Catalysts Preparation The starting material used to prepare all the catalysts was commercial zeolite Mordenite (NH4MOR, Si/Al ratio = 10), supplied by Zeolyst International. Monometallic Catalysts: The platinum-containing ca- talysts were obtained by subjecting NH4MOR zeolite to a competitive ion exchange [19] involving the cations of the metal complex [Pt(NH3)4]2+ and 4 ions. For this, a solution of 0.05 mol·L−1 [Pt(NH3)4]2+ containing 4 NH NH (to give an 4 NH /[Pt(NH3)6]2+ ratio of 10) was used. To perform the exchange, the solution was added slowly (0.2 mL·min−1, while stirring at room temperature) to a suspension of NH4MOR that contained the volume of water required to give a final concentration of 0.005 mol·L−1 [Pt(NH3)4]2+. After a period of 70 h, the solid was separated by filtration, washed with deionized water and dried at 110˚C for 2 h. For precursors containing only Ni supported on NH4MOR, we used the same methodology described be- fore to obtain Pt containing catalysts. However, we used a solution containing 4 NH ions and [Ni(NH3)6]2+ at a 4 NH /[Ni(NH3)6]2+ molar ratio of 20 was used. Bimetallic Catalysts: To obtain precursors of bime- tallic catalysts containing Pt-Ni, two solutions were ini- tially prepared: one containing the [Ni(NH3)6]Cl2 com- plex and the other containing the [Pt(NH3)4]Cl2 complex, which were simultaneously added to a NH4MOR zeolite suspension in water using the same methodology used to obtain the monometallic catalysts described above. After stirring for 1 hour, the solid was filtered and washed with deionized water, and dried at 110˚C for 2 hours [27]. Calcination: After the precursors were prepared, they were submitted to calcinations. This was done to remove the ligands coordinated to the metal and decompose the NH4+ cations presents in the NH4MOR zeolite, thus, forming Bronsted acid sites (HMOR). The samples were heated at 10˚C·min−1 rate from room temperature to 200˚C, under N2 flow (100 cm3·min−1·gcat−1) and the sample remained at this temperature for 1 h. Then, the samples were subjected to syntheticair flow (100 cm3·min−1·gcat−1) and the temperature was increased up to 500˚C at 2˚C·min−1. With the samples remaining under the air flow, the 500˚C temperature was kept constant for 2 h, in order to complete the calcinations process. X-Ray Energy Dispersion Spectroscopy (EDX): Elemental analysis was determined through energy dis- persive X-Ray spectrophotometry, in a Shimadzu EDX- 700 instrument. X-Ray Diffraction (XRD): The powder method has been used, whereby the samples were sieved in an ABNT no 200 (0.074 mm) sieve and then placed in an aluminum sample door for the X-Ray diffraction, using a Shimadzu XRD 6000 equipment. Operational details of the tech- nique have been set as follows: Copper Kα radiation at 40 KV/30 mA, with a goniometer velocity of 2˚/min and a step of 0.02˚ in the range of 2θ scanning from 2˚ to 45˚. The average diameter of the sample crystallites was de- termined by the Scherrer equation. Analysis by Temperature Programmed Reduction (TPR): Calcinated samples were characterized by TPR Copyright © 2013 SciRes. MRC 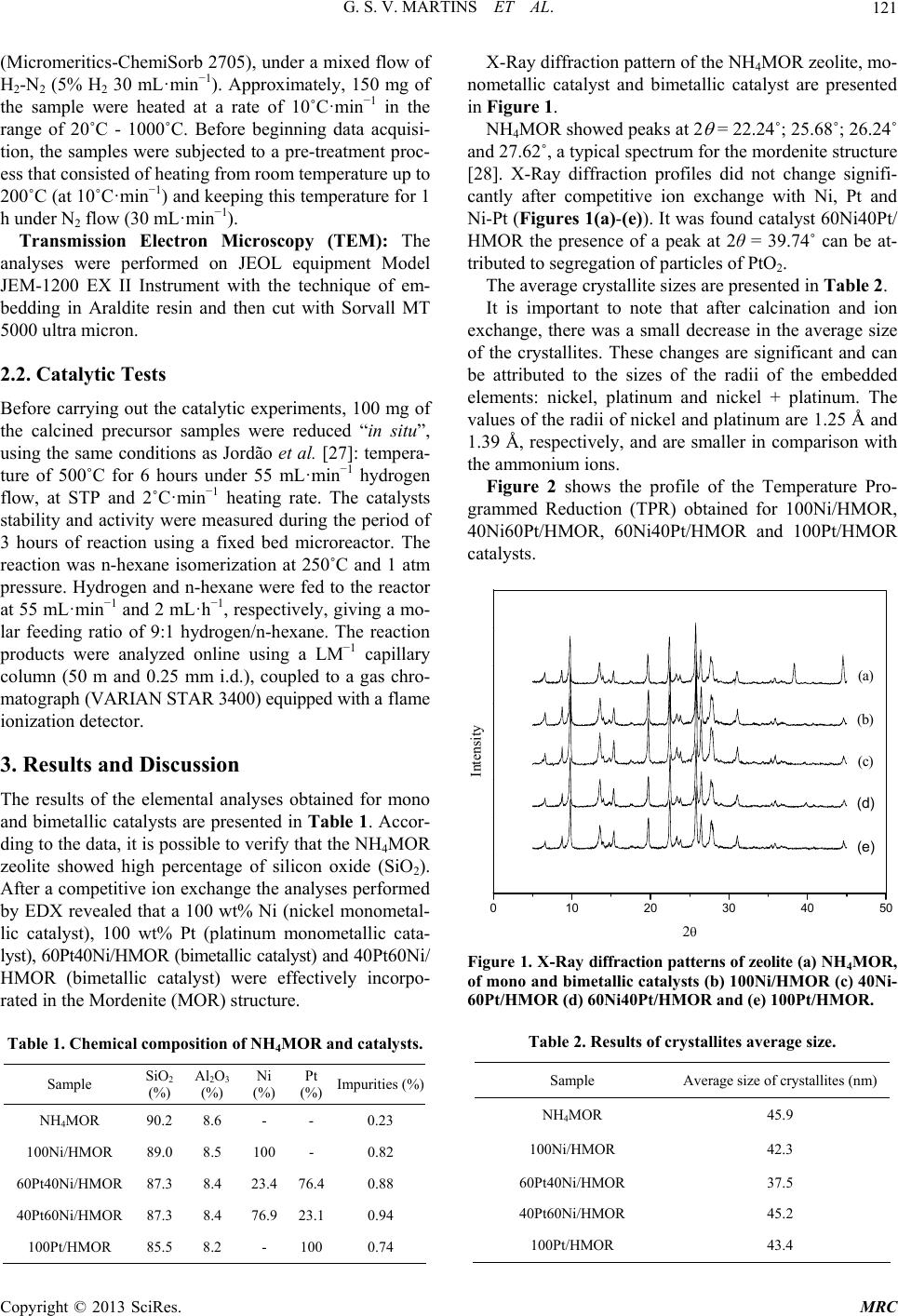 G. S. V. MARTINS ET AL. 121 (Micromeritics-ChemiSorb 2705), under a mixed flow of H2-N2 (5% H2 30 mL·min−1). Approximately, 150 mg of the sample were heated at a rate of 10˚C·min−1 in the range of 20˚C - 1000˚C. Before beginning data acquisi- tion, the samples were subjected to a pre-treatment proc- ess that consisted of heating from room temperature up to 200˚C (at 10˚C·min−1) and keeping this temperature for 1 h under N2 flow (30 mL·min−1). Transmission Electron Microscopy (TEM): The analyses were performed on JEOL equipment Model JEM-1200 EX II Instrument with the technique of em- bedding in Araldite resin and then cut with Sorvall MT 5000 ultra micron. 2.2. Catalytic Tests Before carrying out the catalytic experiments, 100 mg of the calcined precursor samples were reduced “in situ”, using the same conditions as Jordão et al. [27]: tempera- ture of 500˚C for 6 hours under 55 mL·min−1 hydrogen flow, at STP and 2˚C·min−1 heating rate. The catalysts stability and activity were measured during the period of 3 hours of reaction using a fixed bed microreactor. The reaction was n-hexane isomerization at 250˚C and 1 atm pressure. Hydrogen and n-hexane were fed to the reactor at 55 mL·min−1 and 2 mL·h−1, respectively, giving a mo- lar feeding ratio of 9:1 hydrogen/n-hexane. The reaction products were analyzed online using a LM−1 capillary column (50 m and 0.25 mm i.d.), coupled to a gas chro- matograph (VARIAN STAR 3400) equipped with a flame ionization detector. 3. Results and Discussion The results of the elemental analyses obtained for mono and bimetallic catalysts are presented in Table 1. Accor- ding to the data, it is possible to verify that the NH4MOR zeolite showed high percentage of silicon oxide (SiO2). After a competitive ion exchange the analyses performed by EDX revealed that a 100 wt% Ni (nickel monometal- lic catalyst), 100 wt% Pt (platinum monometallic cata- lyst), 60Pt40Ni/HMOR (bimetallic catalyst) and 40Pt60Ni/ HMOR (bimetallic catalyst) were effectively incorpo- rated in the Mordenite (MOR) structure. Table 1. Chemical composition of NH4MOR and catalysts. Sample SiO2 (%) Al2O3 (%) Ni (%) Pt (%) Impurities (%) NH4MOR 90.2 8.6 - - 0.23 100Ni/HMOR 89.0 8.5 100 - 0.82 60Pt40Ni/HMOR 87.3 8.4 23.4 76.4 0.88 40Pt60Ni/HMOR 87.3 8.4 76.9 23.1 0.94 100Pt/HMOR 85.5 8.2 - 100 0.74 X-Ray diffraction pattern of the NH4MOR zeolite, mo- nometallic catalyst and bimetallic catalyst are presented in Figure 1. NH4MOR showed peaks at 2 = 22.24˚; 25.68˚; 26.24˚ and 27.62˚, a typical spectrum for the mordenite structure [28]. X-Ray diffraction profiles did not change signifi- cantly after competitive ion exchange with Ni, Pt and Ni-Pt (Figures 1(a)-(e)). It was found catalyst 60Ni40Pt/ HMOR the presence of a peak at 2θ = 39.74˚ can be at- tributed to segregation of particles of PtO2. The average crystallite sizes are presented in Table 2. It is important to note that after calcination and ion exchange, there was a small decrease in the average size of the crystallites. These changes are significant and can be attributed to the sizes of the radii of the embedded elements: nickel, platinum and nickel + platinum. The values of the radii of nickel and platinum are 1.25 Å and 1.39 Å, respectively, and are smaller in comparison with the ammonium ions. Figure 2 shows the profile of the Temperature Pro- grammed Reduction (TPR) obtained for 100Ni/HMOR, 40Ni60Pt/HMOR, 60Ni40Pt/HMOR and 100Pt/HMOR catalysts. 0 1020304050 (b) (e) (d) (c) (a) Intensit 2 Figure 1. X-Ray diffraction patterns of zeolite (a) NH4MOR, of mono and bimetallic catalysts (b) 100Ni/HMOR (c) 40Ni- 60Pt/HMOR (d) 60Ni40Pt/HMOR and (e) 100Pt/HMOR. Table 2. Results of crystallites average size. Sample Average size of crystallites (nm) NH4MOR 45.9 100Ni/HMOR 42.3 60Pt40Ni/HMOR 37.5 40Pt60Ni/HMOR 45.2 100Pt/HMOR 43.4 Copyright © 2013 SciRes. MRC 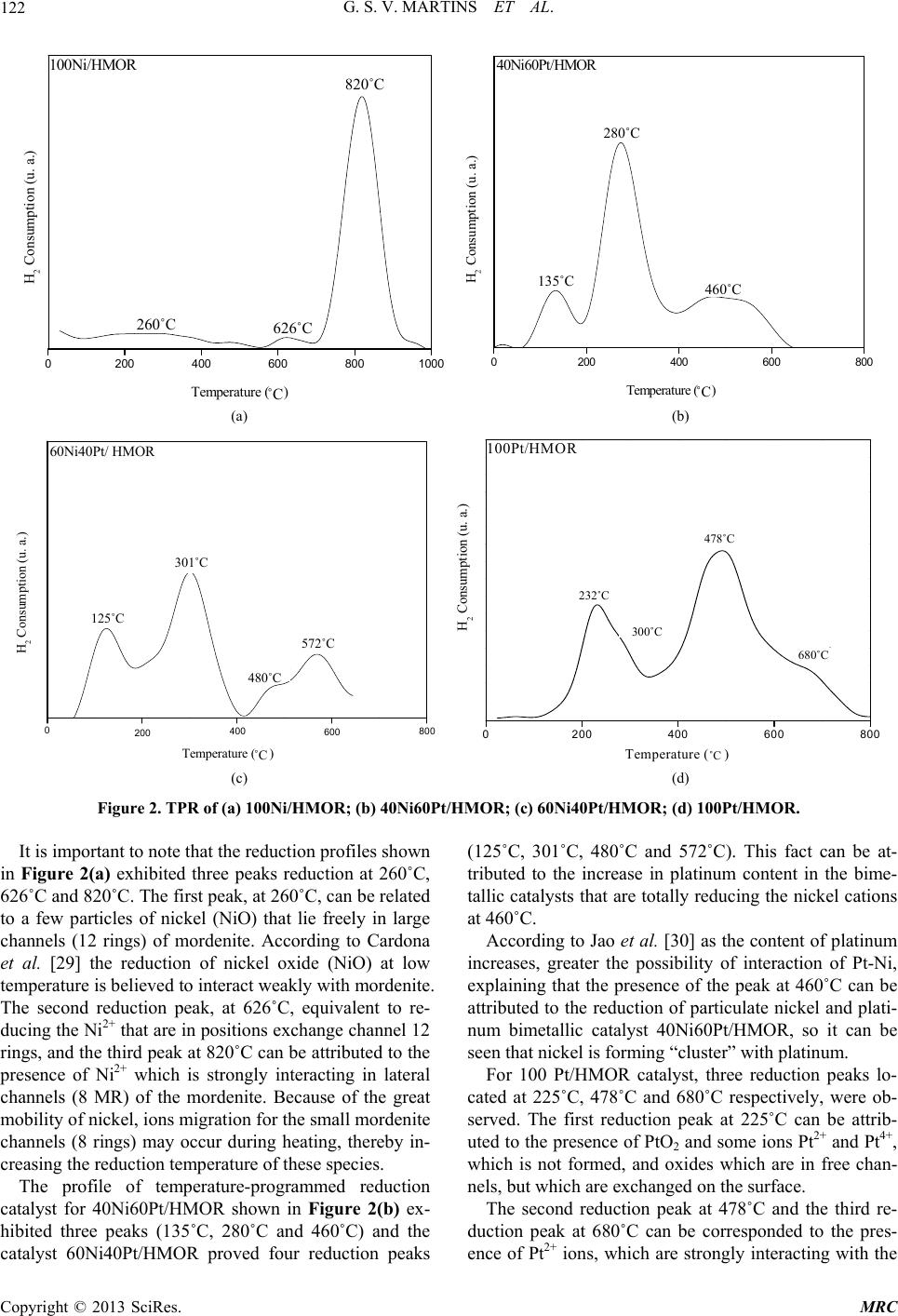 G. S. V. MARTINS ET AL. Copyright © 2013 SciRes. MRC 122 0200 400 600 8001000 癈 820 癈 626 H2 Consumption (u. a.) 癈 Temperature () 100Ni / HMOR 癈 260 260˚C 626˚C ˚C 820˚C 0200 400 600 800 癈 460 癈 280 癈 135 癈 Temperature () H2 Consumption (u. a.) 40Ni60Pt/ HMOR ˚C 460˚C 135˚C 280˚C (a) (b) 0200 400 600 800 癈 680 癈 478 癈 300 癈 232 癈 Temperature () H2 Consumption (u. a.) 100Pt/HMOR 癈 572 癈 480 癈 301 癈 125 癈 Temperature () H2 Consumption (u. a.) 800 600 400 200 0 60Ni40Pt/ HMOR ˚C 572˚C 480˚C 301˚C 125˚C ˚C 300˚C 232˚C 478˚C 680˚C (c) (d) Figure 2. TPR of (00Pt/HMOR. is impor in - a) 100Ni/HMOR; (b) 40Ni60Pt/HMOR; (c) 60Ni40Pt/HMOR; (d) 1 tant to note that the reduction profiles shown (125˚C, 301˚C, 480˚C and 572˚C). This fact can be atIt Figure 2(a) exhibited three peaks reduction at 260˚C, 626˚C and 820˚C. The first peak, at 260˚C, can be related to a few particles of nickel (NiO) that lie freely in large channels (12 rings) of mordenite. According to Cardona et al. [29] the reduction of nickel oxide (NiO) at low temperature is believed to interact weakly with mordenite. The second reduction peak, at 626˚C, equivalent to re- ducing the Ni2+ that are in positions exchange channel 12 rings, and the third peak at 820˚C can be attributed to the presence of Ni2+ which is strongly interacting in lateral channels (8 MR) of the mordenite. Because of the great mobility of nickel, ions migration for the small mordenite channels (8 rings) may occur during heating, thereby in- creasing the reduction temperature of these species. The profile of temperature-programmed reduction catalyst for 40Ni60Pt/HMOR shown in Figure 2(b) ex- hibited three peaks (135˚C, 280˚C and 460˚C) and the catalyst 60Ni40Pt/HMOR proved four reduction peaks tributed to the increase in platinum content in the bime- tallic catalysts that are totally reducing the nickel cations at 460˚C. According to Jao et al. [30] as the content of platinum increases, greater the possibility of interaction of Pt-Ni, explaining that the presence of the peak at 460˚C can be attributed to the reduction of particulate nickel and plati- num bimetallic catalyst 40Ni60Pt/HMOR, so it can be seen that nickel is forming “cluster” with platinum. For 100 Pt/HMOR catalyst, three reduction peaks lo- cated at 225˚C, 478˚C and 680˚C respectively, were ob- served. The first reduction peak at 225˚C can be attrib- ut 2+ ed to the presence of PtO2 and some ions Pt and Pt4+, which is not formed, and oxides which are in free chan- nels, but which are exchanged on the surface. The second reduction peak at 478˚C and the third re- duction peak at 680˚C can be corresponded to the pres- ence of Pt2+ ions, which are strongly interacting with the  G. S. V. MARTINS ET AL. 123 channels (8 MR) of the mordenite, according Jimenez et al be noted that the addition of platinum alters si creasing reduction te e Ni2+ cations present in the zeo- lit well distributed along the catalyst grain. The catalyst 100 Ni/ H ce the temperature at which the reaction is pe platinum assists in the reduction of ni tic . [31]. Comparing the reduction profiles of monometallic and bimetallic catalysts, we observed a shift of peak reduc- tion of cations to lower temperatures. It may gnificantly reducing the profiles, indicating that bi- metallic catalysts, for the presence of platinum is facili- tating the reduction of cations Ni2+, de mperature of cations. Yoshioka et al. [15] observed that this behavior occurs because initially the platinum is reduced causing metal sites that dissociate hydrogen molecules into atomic hy- drogen, which reduce th e mordenite. Representative TEM images of the catalysts after re- duction are shown in Figure 3. The metal particles found in Figures 3(a)-(d) present diameters in the range of 8 - 18 nm that are very Figure 4 shows the activities in the isomerization of n-hexane during 3 hours of Pt-Ni catalysts supported on HMOR zeolite with different Pt/Ni ratios and a total metal content of 180 μmol/gcat. Figure 4 shows the results of the activities for the mono-and bimetallic catalysts. MOR showed lower performance than the other cata- lysts (40Ni60Pt/HMOR, 60Ni40Pt/HMOR and 100Pt/ HMOR). This is due to the difficulty that the nickel particles have to redu rformed (250˚C) as was observed in the reduction pro- files shown in Figure 2(a). It is noticed that few nickel particles are reduced, and this implies that there are few metal sites formed. There is an initial increase in activity with increasing platinum content as ckel cations in the bimetallic catalysts, in such a way that these catalysts have higher activities. This fact can be explained with the dispersion of metals in the zeolite. The formation of metallic platinum is influenced by the presence of nickel, resulting in a smaller average par- le size. That is, Ni-Pt/HMOR bimetallic catalysts have metal particles with smaller diameter when compared to monometallic Ni/HMOR or Pt/HMOR. Therefore, the 15 nm 100Ni (a) 40Ni60Pt (b) 12.5 nm 8 nm (a) (b) 60Ni40Pt (c) 14 nm 12.5 nm 100Pt (d) 18 nm (c) (d) Figure 3. Micrographs of samples: (a) 100Ni/HMOR; (b) i/HMOR; (c) 40Pt60Ni/HMOR; and (d) 100Pt/HOR. 60Pt40N M Copyright © 2013 SciRes. MRC  G. S. V. MARTINS ET AL. 124 bimetallic catalysts have higher fu ening of 6.5 to 7 Å (12 ring mem- be on. metal dispersion for the nction, thus presenting a greater activity in the isom- erization of n-hexane. The structure of mordenite shows a system formed by channels with large op rs) connected by parallel channels of small dimensions from 2.7 to 5.7 Å (8 rings members). In view of the di- mensions of organic molecules is important to note that the diffusion of n-hexane, whose diameter has dimen- sions of 4.3 Å and its isomers larger number octane (3- methyl-pentane, 2,2-dimethyl-butane and 2,3-dimethyl- butane) have diameters of 5 Å, 6.2 Å and 5.6 Å diffusion occurs only in the large channels of mordenite 12 mem- bers. However, because of its one-dimensional porous system, mordenite is susceptible to deactivation due to blocking of its channels by deposition of coke formed during the course of the reaction. Figure 5 shows the selectivity of mono-branched pro- ducts as a function global conversi 050100 150 200 0 10 20 30 40 50 Activity/(m mol g-1cat-1h-1) TOS h 100Pt/HMOR 40Ni60Pt/HMOR 60Ni40Pt/HMOR 100Ni/HMOR Figure 4. The activity of catalysts with 180 μmol/gcat dur- ing 3-h reaction. 100 0510 15 20 25 30 60 65 70 75 80 85 90 95 100Ni/HMOR 40Ni60Pt/HMOR 60Ni40Pt/HMOR 100Pt/HMOR Selectivity(%) Conversion (%) Figure 5. Selectivity for mono-branched products as a func- tion of global conversion. es obtained for the mono-and - When comparing the valu bimetallic catalysts it is clear that the selectivity was al ways above 65%, except for the catalyst only containing nickel (100Ni/HMOR) which proved to be less selective isomerization thus confirming its weak dehydrogenating power capacity and low adsorption of molecules of n- hexane. However, it appears that increasing the platinum content bimetallic catalysts for the conversion of the re- actant increases and the selectivity to isomerization. Stu- dies by Jordão [32] showed that the isomerization selec- tivity is low (~60%) and this fact gives great ability of nickel to promote the formation of cracking products stemmed from the hydrogenolysis reactions of this metal. Figures 6 and 7 illustrate the relationship between the mono- and di-branched isomers as a function of conver- sion for mono e bimetallic catalysts. Figure 6 shows that the catalysts exhibited a high 1,8 2,0 0510 15 20 25 30 0,0 0,2 0,4 0,6 0,8 1,0 1,2 1,4 1,6 ______________________ ______ ______ ______ _____ 2mC5/3mC5 (molar) 100Ni/HMOR 40Ni60Pt/HMOR 60Ni40Pt/HMOR 100Pt/HMOR Conversion (%) Figure 6. Ratio between the mono-branched isomers of n- hexane as a function of the conversion for mono and bi- metallic catalysts. 0510 15 20 25 30 0,0 0,5 1,0 1,5 2,0 3,0 2,5 2,2-dmC4/2,3-dmC4 (molar) Conversion % _________________________________________________ 100Ni/HMOR 40Ni60Pt/HMOR 60Ni40Pt/HMOR 100Pt/HMOR Figure 7. Ratio between the di-branched isomers of n-hex- ane as a function of the conversion for mono and bimetallic catalysts. Copyright © 2013 SciRes. MRC  G. S. V. MARTINS ET AL. 125 value of the ratio of mono-branched isomers (2-mC5/ 3-mC5) and this ratio was almost constant for all catalysts, the balance being above (1.5) only 60Ni40Pt/HMOR catalyst showed less than the equilibrium value which can be attributed to segregation of particles of this cata- lyst PtO2 observed by XRD analysis. These results indicate that there is a higher selectivity to the formation of 2-mC5 the formation of 3-mC5, which makes them the most promising catalysts for isomeriza- tion, since the 2-methyl-pentane has a higher octane number (RON = 75) than 3-mC5 (RON = 73). It is noted in Figure 7 that the molar ratio of branched bi-products (2,2-dmC4/2,3-dmC4) for all the catalysts was considerably below the equilibrium value (2, 5). Th behavior can be explained by the higher stability o t -dmC requires two successive branches th 0) is 2,3-dimethyl- bu elho Nacional de Desenvolvimento Ci- entifico e Tecnológico (CNPq, Brazil) and Petrobras for rt to this research. is fhe tertiary carbocation, compared to the secondary, promot- ing the formation of the product 2,3-dmC4. The forma- tion of 2,34 protonation of cyclopropane in the acidic sites. This branch corresponds to the limiting step of the bifunc- tional mechanism of 2,3-dmC4 succeed in that methyl pentane, simultaneously with the formation of 2,2-dmC4. Thus, this is advantageous because the isomer which has a higher octane number (RON = 10 rough tane, while 2,2-dimethyl-butane presented RON equal to 92, which is somewhat lower when compared to 2,3- dimethyl-butane. 4. Conclusions The profiles obtained from TPR suggest that, for bime- tallic catalysts, the presence of platinum facilitates the reduction of Ni2+ cations. The mono and bimetallic catalysts were more selective for the formation of isomers with high octane index (2- mC5 dmC4 and 2.3) which are products of interest in the petroleum industry. The bimetallic catalysts presented a higher activity in the isomerization of n-hexane when compared to the mo- nometallic ones, as well better stability as the Pt content in the solid increases. 5. Acknowledgements The authors would like to make special acknowledge- ments to the Cons their financial suppo REFERENCES [1] A. D. Estrada-Villagrana and C. L. Paz-Zavala, “Applica- tion of Chemical Equilibrium for Hydrocarbon Isomeri- zation Analysis,” Fuel, Vol. 86, No. 9, 2007, pp. 1325- 1330. http://dx.doi.org/10.1016/j.fuel.2006.08.016 [2] J. H. Gary and G. E. Handwerk, “Petroleum Refining: n-Hexane over Platinum Mazzite and Catalysts Kinetics and Mechanism,” ral, Vol. 152, No. 2, 1997, pp. Technology and Economics,” 3th Edition, M. Dekker, New York, 1994. [3] J. F. Allain, P. Magnoux, P. Schulz and M. Guisnet, “Hy- droisomerization of Platinum Mordenite Applied Catalysis A: Gene 221-235. http://dx.doi.org/10.1016/S0926-860X(96)00346-8 [4] J.-K. Lee and H.-K. Rhee, “Characteristics of Pt/H-Beta and Pt/H-Mordenite Catalysts for the Isomerization of n-Hexane,” Catalysis Today, Vol. 38, No. 2, 1997, pp. 235-242. http://dx.doi.org/10.1016/S0920-5861(97)00072-2 [5] A. van de Run. Stobbelaar, J. van straat, J. A. Kamp, P. J Grondelle, S. Krijnen and R. A. van Santen, “Kinetics of Hydro-Isomerization of n-Hexane over Platinum Con- taining Zeolites,” Journal of Catalysis, Vol. 171, No. 1, 1997, pp. 77-84. http://dx.doi.org/10.1016/S0920-5861(97)00072-2 [6] A. Holló, J. Hancsók and D. Kalló, “Kinetics of Hydroi- somerization of C5-C7 Alkanes and Their Mixtures over Platinum Containing Mordenite,” Applied Catalysis A: General, Vol. 229, No. 1-2, 2002, pp. 93-102. http://dx.doi.org/10.1016/S0926-860X(02)00018-2 [7] S. Calero, M. Schenk, D. Dubbeldam, T. L. M. Maesen and B. Smit, “The Selectivity of n-Hexane Hydroconver- sion on MOR-, MAZ-, and FAU-Type Zeolites,” Jo of Catalysis, Vol. 228, No. 1, 2004, pp. 212-129. urnal http://dx.doi.org/10.1016/j.jcat.2004.08.019 [8] S. Yuvaraj, T.-H. Chang and C.-T. Yeh, “Segregation of Platinum from Mordenite Channels on Calcination and Reduction Pretreatments,” Journal of Catalysis, Vo No. 2, 2004, pp. 466-473. l. 221, http://dx.doi.org/10.1016/j.jcat.2003.09.008 [9] H. Weyda and E. K. Köhler, “Modern Refining Con- cepts—An Update on Naphtha-Isomerization to Modern Gasoline Manufacture,” Catalysis Today, Vol. 81, No. 1, 2003, pp. 51-55. http://dx.doi.org/10.1016/S0920-5861(03)00101-9 [10] R. Roldán, F. J. Romero, C. Jiménez-Sanchidrián and J. M. Marinas, “Influence of Acidity and Pore Geometry on the Product Distribution in the Hydroisomerization of Light Paraffins on Zeolites,” Applied Catalysis A: eral, Vol. 288, No. 1-2, 2005, pp. 104-115. Gen- http://dx.doi.org/10.1016/j.apcata.2005.04.029 [11] A. Patrigeon, E. Benazzi, C. Travers and J. Y. Bernhard, “Influence of the Zeolite Structure and Acidity on the Hydroisomerization of n-Heptane,” Catalysis Toda 65, No. 2-4, 2001, pp. 149-155. y, Vol. http://dx.doi.org/10.1016/S0920-5861(00)00558-7 [12] E. Blomsma, J. A. Martens and P. A. Jacobs, “Reaction Mechanisms of Isomerization and Cracking of Heptane on Pd/H-Beta Zeolite,” Journal of Catalys No. 1, 1995, pp. 141-147. is, Vol. 155, http://dx.doi.org/10.1016/S0920-5861(00)00558-7 [13] E. Blomsma, J. A. Martens and P. A. Jacobs, “Mecha- nisms of Heptane Isomerization on Bifunctional Pd/H- Beta Zeolites,” Journal of Catalysis, Vol. 159, 1996, pp. 323-331. No. 2, Copyright © 2013 SciRes. MRC  G. S. V. MARTINS ET AL. Copyright © 2013 SciRes. MRC 126 http://dx.doi.org/10.1006/jcat.1996.0094 [14] M. Guisnet, F. Alvarez, G. Giannetto and G. Perot, “Hy- droisomerization and Hydrocracking of n-Heptane on Pth Zeolites. Effect of the Porosity and of the Distribution of Metallic and Acid Sites,” Catalysis Today, Vo 1987, pp. 415-433. l. 1, No. 4, http://dx.doi.org/10.1016/0920-5861(87)80007-X [15] C. M. N. Yoshioka, T. Garetto and D. Cardoso, “n-Hex- ane Isomerization on Ni-Pt Catalysts/Supported on HUSY Zeolite: The Influence from a Metal Content Catalysis Today, Vol. 107-108, 2005, pp. 693-698. ,” http://dx.doi.org/10.1016/j.cattod.2005.07.056 [16] M. H. Jordão, V. Simões and D. Cardoso, “Zeolite Sup- ported Pt-Ni Catalysts in n-Hexane Isomerization,” Ap- plied Catalysis A: General, Vol. 319, 2007, pp. 1-6. http://dx.doi.org/10.1016/j.apcata.2006.09.039 [17] F. V. Barsi and D. Cardoso, “Bimetallic Pt-Ni Catalysts Supported on USY Zeolite for n-Hexane Isomerization,” Brazilian Journal of Chemical Engineering, Vol. 26, No. 2, 2009, pp. 353-360. http://dx.doi.org/10.1590/S0104-66322009000200012 016/j.cattod.2011.02.031 [18] P. M. Lima, T. Garetto, C. L. Cavalcante Jr. and D. Car- doso, “Isomerization of n-Hexane on Pt-Ni Catalysts Sup- ported on Nanocrystalline H-BEA Zeolite,” Catalysis Today, Vol. 172, No. 1, 2011, pp. 195-202. http://dx.doi.org/10.1 F. Ro- 006 [19] B. V. Sousa, K. D. Brito, J. J. N. Alves and M. G. drigues, C. M. N. Yoshioka and D. Cardoso, “n-Hexane Isomerization on Pt/HMOR: Effect of Platinum Content,” Reaction Kinetics, Mechanisms and Catalysis, Vol. 102, No. 2, 2011, pp. 473-485. N. Viswanadham, L. Dixit, J. K. Gupta and M. O. Garg, [20] “Effect of Acidity and Porosity Changes of Dealuminated Mordenites on n-Hexane Isomerization,” Journal of Mo- lecular Catalysis A: Chemical, Vol. 258, No. 1-2, 2 pp. 15-21. , http://dx.doi.org/10.1016/j.molcata.2006.04.067 [21] M. Guisnet and V. Fouche, “Isomerization of n-Hexane on Platinum Dealuminated Mordenite Catalysts III. In- fluence of Hydrocarbon Impurities,” Applied Catalysis A General, Vol. 71, No. 2, 1991, pp. 307-317. : http://dx.doi.org/10.1016/0166-9834(91)85088-D [22] I. Eswaramoorthi, A. G. Bhavani and N. Lingappan, “Ac- tivity, Selectivity and Stability of Ni-Pt Loaded Zeolite-β and Mordenite Catalysts for Hydroisomer Heptane,” Applied Catalysis A: General, Vol. 2 isation of n- 53, No. 2, 2003, pp. 469-486. http://dx.doi.org/10.1016/S0926-860X(03)00538-6 [23] S. V. Konnov, I. I. Ivanova, O. A. Ponomavera and V. I. Zaikovskii, “Hydroisomerization of n-Alkanes over Pt- Modified Micro/Mesoporous Materials Obtain denite Recrystallization,” Microporous and Meso ed by Mor- porous 016/S0920-5861(03)00097-X Materials, Vol. 164, No. 1, 2012, pp. 222-231. [24] Y. Ono, “A Survey of the Mechanism in Catalytic Isom- erization of Alkanes,” Catalysis Today, Vol. 81, No. 1, 2003, pp. 3-16. http://dx.doi.org/10.1 lo- [25] W. M. Meier, “The Crystal Structure of Mordenite (Pti lite),” Zeitschriftfür Kristallographie, Vol. 115, 1961, pp. 439-450. http://dx.doi.org/10.1524/zkri.1961.115.5-6.439 [26] S. van Donk, A. Broersma, O. L. J. Gijzeman, J. A. van Bokhoven, J. H. Bitter and K. P. de Jong, “Combined ,” Journal of Catalysis, Vol. 204, No. 2, Diffusion, Adsorption, and Reaction Studies of n-Hexane Hydroisomerization over Pt/H-Mordenite in an Oscillat- ing Microbalance 2001, pp. 272-280. http://dx.doi.org/10.1006/jcat.2001.3393 [27] M. H. Jordão, V. Simões, A. Montes and D. Cardoso, “Bifunction tion of n-Hexane,” Studies in Surface Science al Ni, Pt Zeolite Catalysts for the Isomeriza- and Ca- talysis, Vol. 130, 2000, pp. 2387-2392. http://dx.doi.org/10.1016/S0167-2991(00)80826-5 [28] G. J. Kim and W. S. Ahn, “Direct Synthesis and Charac- terization of High-SiO2-Content Mordenites,” Zeolites, Vol. 11, No. 7, 1991, pp. 745-750. [29] F. Cardona, N. S. Gnep, M. Guisnet, G. Szabo scimento, “Reactions Involved in the Al and P. Na- kylation of Iso- )00079-8 butane with 2-Butene and with Propene on a USHY Zeo- lite,” Applied Catalysis A: General, Vol. 128, No. 2, 1995, pp. 243-257. http://dx.doi.org/10.1016/0926-860X(95 aphtha nal of Catalysis, Vol. rted Platinum Catalysts,” Applied Catalysis [30] R.-M. Jao, T.-B. Lin and J.-R. Chang, “Light N Isomerization over Mordenite-Supported Ni-Pt Catalysts: Effects of Ni on the Catalytic Performance for Pure Feed and Sulfur-Containing Feed,” Jour 161, No. 1, 1996, pp. 222-229. [31] C. Jiménez, F. J. Romero, R. Roldán, J. M. Marinas and J. P. Gómez, “Hydroisomerization of a Hydrocarbon Feed Containing n-Hexane, n-Heptane and Cyclohexane on Zeolite-Suppo A: General, Vol. 249, No. 1, 2003, pp. 175-185. [32] M. H. Jordão, “Catalisadores Bimetálicos e Bifuncionais para Isomerização do n-Hexano: Ni-Pt Suportados na Zeólita HY,” Ph.D. Thesis, Federal Universityof São Car- los, São Carlos, 2001.
|