 Vol.3, No.1, 6-12 (2011) Health doi:10.4236/health.2011.31002 Copyright © 2011 SciRes. Openly acces sib le at http://www.scirp.org/journal/HEALTH/ Carbonic anhydrase VII–a potenti al pro gnostic marker in gliomas Fatemeh Bootorabi1,2, Joonas Haapasalo3, Elona Smith1, Hannu Haapasalo3, Seppo Parkkila1,2 1Insti tute of Med ical Technology an d School of Medicin e, Univers ity of Tampere, Tampere, Fin land 2Department of Clinical Chemistry, Cent r e for Laborator y Med icine, Tampere University Hospit al, Tampere, Finland 3Department of Pathology, Centre for labor atory Medi cine, Tampere U niversity Hospital, Tampere, Finland; *Cor responding Author : seppo.parkkila@uta.fi Received 7 December 2010; revised 20 December 2010; accepted 26 December 2010 AB STRAC T Carbonic anhydrase VII (CA VII) is a cytosolic enzyme expressed in several organs, including the human brain, but it has not been investi- gated earlier in any tumors. We designed the present study to ev alu at e C A VII exp r ession in a cohort of human diffuse astrocytomas, mixed oligoastrocytomas and oligodendrogliomas. CA VII immunostaining was correlated to clinico- pathologic findings, survival data, and expres- sion of other molecular factors, including Ki-67, p53 protein and epidermal growth factor recep- tor. CA VII-positive staining was observed in 94% of astrocytomas and 85% of oligodendrog- liomas. In the tumor specimens, strong positive areas were often located in close proximity to necrosis. The CA VII immunoreactivity showed positive correlation with tumor malignancy grades of astrocytomas (p = 0.02, chi-square test). In all tumor categories, CA VII-positive staining was often seen in the endothelial cells of neovessels in addition to the tumor cells. CA VII intensity showed no significant association with p53 nor did it correlate with the amplification of epi- dermal growth factor receptor (analyzed only in astrocytomas) or cell proliferation. Our present results show that CA VII may act as a useful biomarker in histopathologic diagnostics of gliomas. The high expression of CA VII in the tumor cells and endothelium suggests impor- tant roles for the enzyme in tumor metabolism. The results also led us to conclude that CA VII might serve as a marker of poor prognosis in diffuse astrocytomas. Keywords: Astrocytoma; Brain; Cancer; Carbonic Anhydrase; Glioblastoma; Glioma; Oligodendroglioma ; pH 1. INTRODUCTION Carbonic Anhydrase (CAs) form a family of ubiquit- ous zinc containing metalloenzymes that are able to cat- alyze the reversible hydration of carbon dioxide accord- ing to the following reaction: CO 2 + H2O ⇔ HCO 3− + H+. The CAs show different distribution in a variety of tissues where they participate in several important biological processes such as pH regulation, CO2 and HCO3− transport, respiration, ureagenesis, body fluid generation, lipogenesis, and gluconeogenesis [1,2]. The CA isozymes differ in several important characteristics, such as kinetic properties, susceptibility to inhibitors, and subcellular localization. The α-CA gene family has been repo rted to include at le ast 13 active iso for ms with d iff erent structural and catalytic properties [1-5]. CA isozymes II, IX and XII have been extensively studied for their important role as promising biomarkers in different tumors, for example astrocytic tumors [5-7]. Other important research targets focused on CAs have included hypoxia-regulation of certain CA isozymes and their potential use as targets of cancer therapy [7-13]. Although cytosolic CA VII was identified as a new CA isozyme almost twenty years ago [14], and its CA catalytic activity was already demonstrated in 1996 [15], the characteristics of this isozyme have largely remained unresolved. In fact, the most developed research area on CA VII has b een linke d to drug d evelop ment, which ha s led to the discoveries of several potent activators and inhibitors for this isozyme [16,17]. The expression of CA VII protein was first studied in the b rain tissue [18 ]. In previous publications, CA VII has never been inves- tigated in as troc ytomas nor has it been determined in any other tumor categories. Data from GeneSapiens database (http://ist.genesapiens.org/) suggested that some gliomas express high levels of CA VII mRNA. This result  F. Bootorabi et al. / Healt h 3 (2011) 6-12 Copyright © 2011 SciRes. Openly acces sib le at http://www.scirp.org/journal/HEALTH/ prompted us to design the present study to evaluate the expression of CA VII protein in diffusely infiltrating astrocytic gliomas, oligodendrogliomas and mixed oli- goastrocytomas. These tumors represent highly malig- nant ne o p las ia s d e ri ved from gli al cell s. The y c o mmo nl y show infiltrating and diffuse growth pattern, which make s the s urgical treatment inadequate in most cases. It is very important to diagnose these tumors as early as possible and to use prognostic tools in the assessment of the most suitable treatment for each patient. In the present study, we evaluate the correlation of CA VII ex- pression between astrocytomas, oligodendrogliomas and oligoastrocytomas using immunohistochemistry and compare the immunostaining results with various clini- copathological and molecular factors including cell pro- liferation, p53, and epidermal growth factor receptor (EGFR). 2. METHODS 2.1. Study Material The study material consisted of diffusely infiltrating astrocytoma and oligodendroglioma samples, which were obtained from surgically operated patients in Tam- pere University Hospital, Tampere, Finland, during 1983 -2001. First, the tumor specimens were fixed in 4% phosphate-buffered formaldehyde and processed into paraffin blocks. On the basis of hematoxylin and eosin (H&E)-stained slides a neuropathologist (HH) per- formed an evaluation of the tumors according to the WHO 2007 criteria [19,20]. According to the Finnish legislation informed consent by the patients is not re- quired in this kind of retrospective study. Most patients had died before starting the analyses. According to the national guidelines, the experiments were approved by the Ethical Committee of the Tampere University Hos- pital and the National Authority for Medicolegal Affairs and conducted according to the guidelines of the Helsin- ki Declaration. 2.2. Astrocytic Tumors The WHO criteria divide diffusely infiltrating astro- cytomas into three grades (II-IV) according to the pres- ence of atypia, mitotic activity, necrosis and endothelial proliferation. The neuropathologist selected one histo- logically representative section in each sample specimen for the CA VII immunohistochemistry. The study in- cluded 107 astrocytic tumors (grade II: 14; grade III: 11; grade IV: 82) and consisted of 90 primary tumors and 17 recurrences. Age of patients with primary tumors varied from 20 to 80 years (median 55 ± SD 13 years) and re- current tumors from 25 to 73 years (median 49 ± SD 14 years). Overall survival was kno wn for all of the patients wit h p r imary tumors (90 patients; 12 grade II, 8 grade III and 70 grade IV). The mean follow-up time for 14 sur- vivors was 67 months (range 31-165) and 76 patients died during the five-year follow-up. The tumors were radically resected if possible and most patients with high grade gliomas also received rad iotherapy. 2.3. Oligodendroglial Tumors The WHO criteria group oligodendroglial tumors into two main categor ies: pure oligodendroglio mas and mixed oligoastrocytomas, which are divided into two grades (2 and 3) according to the at ypia, presence of increased cel- lularity, and mitotic activity. From 47 cases of oligoden- droglial tumor samples which were included in our ana- lyses, there were both pure oligodendrogliomas (18 of grade 2 and 12 of grade 3) and oligoastrocytomas (11 of grade 2 and 6 of grade 3). These tumors included 35 pri- mary tumors and 12 recurrences. The ages of the patients with primary tumors varied from 8 to 76 years (mean ± SD: 43 ± 14 years), and those of the patients with recur- rent tumors varied fro m 17 to 72 year s (mean ± SD : 41 ± 14 years). Survival analysis was not performed, because overa ll survi val was only known for 26 patients. 2.4. Immunohistochemistry Five µm sections were processed for immunoperox- idase staining, which was performed using an automated Lab Vision Autostainer 480 (LabVision Corporation, Fremont, CA, USA). Automated immunostaining was performed using the Power Vision+ Poly-HRP Immuno- histochemistry kit (ImmunoVision Technologies Co) reagents. The primary rabbit anti-human CA VII serum was raised against recombinant CA VII enzyme and has shown high specificity for the CA VII enzyme [21]. The immunostaining procedure included the following steps: (1) rinsing in wash buffer; (2) treatment in 3% H2O2 in ddH2O for five minute s and rinsing with wash b uffer; (3) blocking with cow colostrum diluted 1:2 in Tris-buffered saline (TBS) contai ning 0.05% Tween-20 for 30 minutes and rinsing in wash buffer; (4) incubation with primary antibody (rabbit anti-human CA VII for 30 minutes; (5) rinsing in wash buffer three times for five minutes; (6) incubation in poly-HRP-c onjugated anti-rabbit IgG for 30 minutes and rinsing in wash buffer three times for five minutes; (7) incubation in DAB (3,3-diaminobenzidine te trahyd rochlo ride) solution (one dr op of DAB so lution A and one drop of DAB solution B in 1 ml of ddH2O) for six minutes; (8) CuSO4 treatment for five minutes to en- hance the signal; and (9) rinsing with ddH2O. All proce- dures were performed at room temperature. The mount- ing of the sections was performed using Entellan Neu (Merck; Darmstadt, Germany). The intensity (INT) of the staining was scored on a scale of 0 to 3 by three of 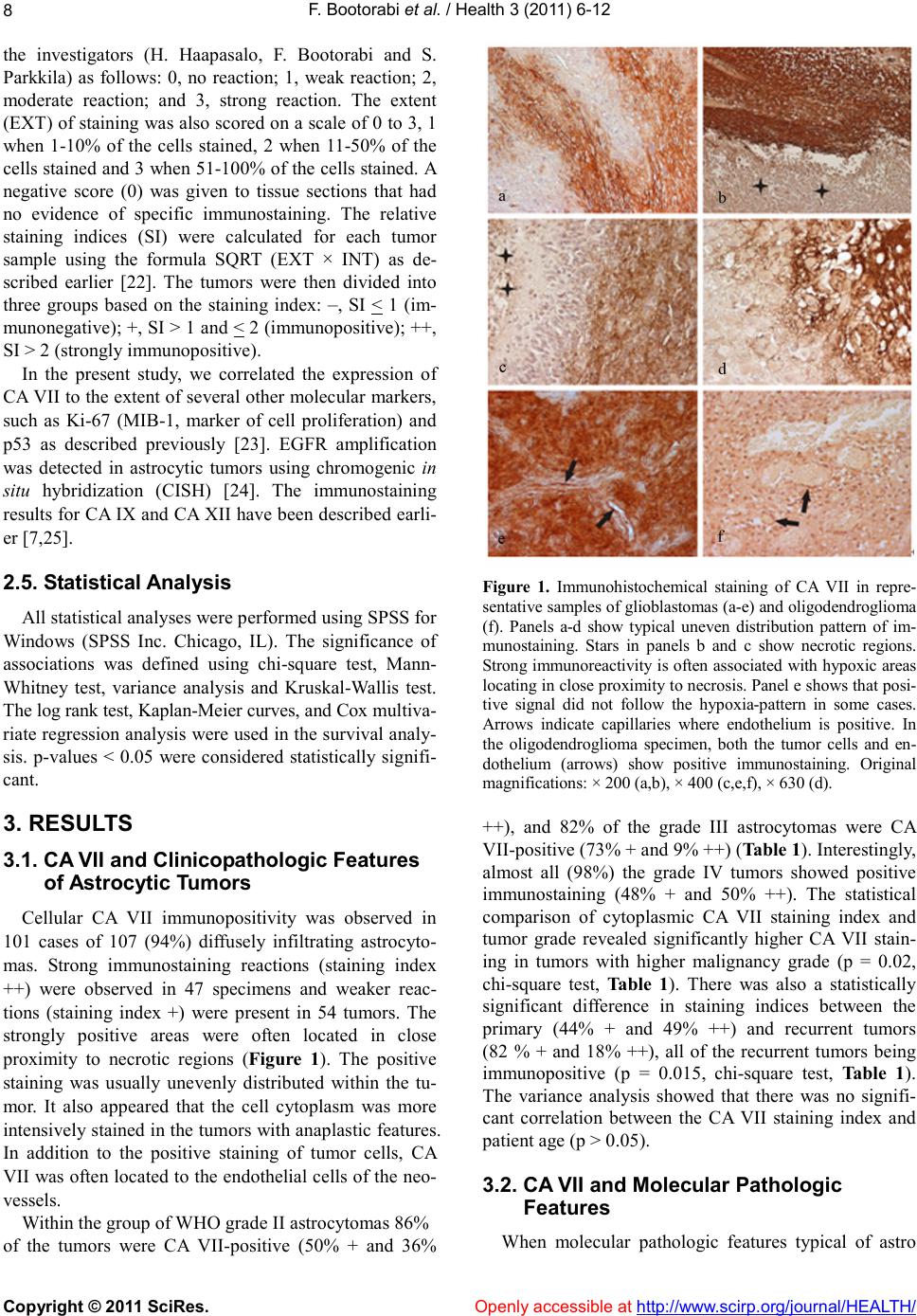 F. Bootorabi et al. / Healt h 3 (2011) 6-12 Copyright © 2011 SciRes. Openly acc es sib le at http://www.scirp.org/journal/HEALTH/ the investigators (H. Haapasalo, F. Bootorabi and S. Parkkila) as follows: 0, no reaction; 1, weak reaction; 2, moderate reaction; and 3, strong reaction. The extent (EXT) of staining was also scored on a scale of 0 to 3, 1 when 1-10% of the cells stained, 2 when 11-50% of the cells stained and 3 when 51-100% of the cells stained. A negative score (0) was given to tissue sections that had no evidence of specific immunostaining. The relative staining indices (SI) were calculated for each tumor sample using the formula SQRT (EXT × INT) as de- scribed earlier [22]. The tumors were then divided into three groups based on the staining index: –, SI < 1 (im- muno negat ive ); +, SI > 1 a nd < 2 (immunopositive); ++, SI > 2 (strongly immunopositive). In the present study, we correlated the expression of CA VII to the extent of several other molecular markers, such as Ki-67 (MIB-1, marker of cell proliferation) and p53 as described previously [23]. EGFR amplification was detected in astrocytic tumors using chromogenic in situ hybridization (CISH) [24]. The immunostaining results for CA IX and CA XII have been described earli- er [7,25]. 2.5. Statistical Analysis All statistical analyses were performed using SPSS for Windows (SPSS Inc. Chicago, IL). The significance of associations was defined using chi-square test, Mann- Whitney test, variance analysis and Kruskal-Wallis test. The log ran k test, Kapla n -Meier c urves, a nd Co x multiva- riate regression analysis were used in the survival analy- sis. p-values < 0.05 were considered statistically signifi- cant. 3. RESULTS 3.1. CA VII and Clinicopathologic Features of Astrocytic Tumors Cellular CA VII immunopositivity was observed in 101 cases of 107 (94%) diffusely infiltrating astrocyto- mas. Strong immunostaining reactions (staining index ++) were observed in 47 specimens and weaker reac- tions (staining index +) were present in 54 tumors. The strongly positive areas were often located in close proximity to necrotic regions (Figure 1). The positive staining was usually unevenly distributed within the tu- mor. It also appeared that the cell cytoplasm was more inte nsive ly s taine d i n the tu mo rs with a napla sti c fe ature s. In addition to the positive staining of tumor cells, CA VII was ofte n located to t he endothelial ce lls of the neo- vessels. Within the group of WHO grade II astrocytomas 86% of the tumors were CA VII-positive (50% + and 36% Figure 1. Immunohistochemical staining of CA VII in repre- sentative samples of glioblastomas (a-e) and oligodendroglioma (f). Panels a-d show typical uneven distribution pattern of im- munostaining. Stars in panels b and c show necrotic regions. Strong immunoreactivity is often associated with hypo xic are as locating in close proximity to necrosis. Panel e shows that posi- tive signal did not follow the hypoxia-pattern in some cases. Arrows indicate capillaries where endothelium is positive. In the oligodendroglioma specimen, both the tumor cells and en- dothelium (arrows) show positive immunostaining. Original magnifications: × 200 (a,b), × 400 (c,e,f), × 630 (d). ++), and 82% of the grade III astrocytomas were CA VII-positive (73% + and 9% ++) (Table 1). Interestingly, almost all (98%) the grade IV tumors showed positive immunostaining (48% + and 50% ++). The statistical comparison of cytoplasmic CA VII staining index and tumor grade revealed significantly higher CA VII stain- ing in tumors with higher malignancy grade (p = 0.02, chi-square test, Table 1). There was also a statistically significant difference in staining indices between the primary (44% + and 49% ++) and recurrent tumors (82 % + and 18% ++), all of the recurrent tumors being immunopositive (p = 0.015, chi-square test, Table 1). The variance analysis showed that there was no signifi- cant correlation between the CA VII staining index and patient age (p > 0.05). 3.2. CA VI I and Molecular Pathologic Features When molecular pathologic features typical of astro  F. Bootorabi et al. / Healt h 3 (2011) 6-12 Copyright © 2011 SciRes. Openly acces sib le at http://www.scirp.org/journal/HEALTH/ Table 1. CA VII staining index (SI), WHO grade and recur- rence status of the astrocytic and oliodendroglial tumors (p = p-value, chi-squar e test). CA V II imm unostaining Primary tumor / Re- cytomas were compared with CA VII immunohistoche- mistry, no association was observed between the cell proliferation (assessed by Ki-67/MIB-1 immunostaining) and CA VII expression (p > 0.05, Kruskal-Wallis test). CA VII intensity showed no significant association with p53 nor did it correlate with EGFR-amplification (p > 0.05, chi-square test). There was a significant correlation between CA VII and CA IX staining index (p = 0.034, chi-square test), whereas there was no significant rela- tionship between CA VII and CA XII staining (p > 0.05, chi-square test). 3.3. Correlation of CA VII with Prognosis in Astrocytic Tumors Overall survival data was known for all the patients with primary tumors. Patient survival was tested by log-rank test in relation to CA VII staining index. Inte- restingly, CA VII stainin g results divided the tu mors into three significantly different prognostic subsets (p = 0.017, log-rank test; Figure 2 ). When different factors, includ ing CA VII, CA IX and CA XII (all divided into three categories by staining in- dices –, + and ++) as well as histological grade (2, 3 and 4) and patient age (<50, 50-65 and >65 years) were tested in the Cox multivariate analysis, CA VII, CA IX, histological grade and patient age were found to be of independent prognostic value (Table 2). 3.4. Oligodendroglial Tumors The study materials included 47 oligodendrogliomas of which 30 cases represented pure and 17 were mixed oli Figure 2. Survival of astrocytoma patients grouped according to the CA VII immunostaining of tu- mor cells. The patients with CA VII-negative tu- mors had significantly better prognosis than those with CA VII-positive tumors (p = 0.017, log-rank test). Table 2. The independent prognostic indicators of astro cytomas as evaluated by Cox’s stepwise regression model. Histological grade (2,3,4), patient age (cut points 50 and 65 years), CA VII, and CA IX (staining index –, +, ++) were included in the analysis. Significance Exp(B) (Hazard ratio) 95% CI of Exp(B) Lower Upper Grade 0.000 3.104 1.673 5.761 CA VII 0.002 2.074 1.306 3.293 Age 0.002 1.817 1.250 2.641 CA I X 0.046 1.431 1.006 2.034 goastrocytomas. 85% of all oligodendrogliomas showed positive immunostaining for CA VII. There was no sig- nificant difference in CA VII expression levels between astrocytomas and oligodendrogliomas (p > 0.05, chi- square test). Recurrent oligodendrogliomas were more immunopositive for CA VII than the primary tumors, and the difference was found to be statistically signifi- cant (p = 0.011, chi-square test). There was no statisti- cally significant correlation between the patient age and CA VII status nor did CA VII correlate with tumor grade, cell proliferation (assessed by MIB-1), or p53 immunos- taining (p > 0.05, chi-square test). A near significant correlation (p = 0.117, chi-square test) was observed between CA VII and CA IX staining. 4. DISCUSSION Previous studies have indicated that carbonic anhy- drase isozymes, CA II , CA IX and CA XII, are promising biomarkers for certain tumors [6,7,22,26]. There are nu- merous reports showing that CA IX acts not only as a marker for particular tumors, but its presence also corre- lates to prognosis in several tumor categories, such as  F. Bootorabi et al. / Healt h 3 (2011) 6-12 Copyright © 2011 SciRes. Openly acc es sib le at http://www.scirp.org/journal/HEALTH/ brain tumors, sarcomas, and renal, lung, rectal, bladder, oral, breast, and cervical carcinomas [7,27-34]. Although studies on CA II and CA XII have been mainly focused on normal tissues, there are a few previous reports, showing their presence in cancer cells and especially in brain tumors where they have shown significant associa- tion with prognosis [6,7,25]. CA VII is a cytosolic iso- zyme which was first demonstrated in the brain tiss ue [35], where it has been linked to the regulation of GA- BAergic neuronal transmission in hippocampal neurons [36]. We recently characterized this enzyme in vitro and identified two forms of CA VII mRNA in the human brain [21]. The studies further indicated that only the full-lengt h form of CA VII is expres sed in human tissues. The novel antibodies, raised against CA VII, demon- strated that it is expressed in several other mouse tissues -particularly in the liver. The present study was designed when we found using the GeneSapiens database (http:// www.genesapiens.org), tha t CA7 gene is highly expressed in some glioblastomas. The finding suggested that CA VII could represent another CA isozyme with a potential rol e as a biomarker for gliomas. In this study, we investigated the expression of CA VII in three different categories of brain tumors: astro- cytomas, oligodendrogliomas and mixed oligoastrocy- tomas. Our findings showed that CA VII expression is higher among high grade infiltrating tumors. Interes- tingl y, CA VII immuno staining was often ver y strong in hypoxic areas adjacent to tumor necrosis. Although CA VII has not been considered a HIF-regulated isozyme contrary to CA IX [37,38] and CA XII [38], its staining pattern indicates that ce ll hypoxia co ntributes to the reg- ulation of CA VII expression by yet undefined mechan- isms. According to our results CA VII immunoreactivity did not correlate significantly with cell proliferation de- termined by MIB-1 immunostaining, nor did it show association to p53 protein expression or EGFR- amplifi- cation. Importantly, our results indicated that high CA VII expression is associated with poor prognosis in as- trocytoma patients. This result makes CA VII another C A iso zyme with a significant correlation to the patient survi val. I ndeed , the p revio us stud ies ha ve de monstrated that CA II, CA IX a nd CA X II all show similar trends as prognostic markers in diffuse astrocytomas [6,7,25,39]. The presence of several CA isozymes in astrocytomas may reflect the rapid turnover of acid metabolic products in highly malignant tumor tissues. The cytosolic isozymes, such as CA II and CA VII, can contribute to more effi- cient neutralization of cell interior, whereas the mem- brane-associated enzymes, CA IX and CA XII, partici- pate in extrusion of protons in metabolon systems to- gether with ion transport proteins [5,40,41]. These me- chanisms can provide novel opportunities for cancer therapy in which tumor cell microenvironment can be targeted by CA inhibition. Recent drug developments have already pointed out a number of different com- pounds as CA inhibitors with high efficiency [5,40]. Some of the drugs may inhibit and disturb the neoangi- ogenesis while reducing the tumor growth [42], and some compounds can also decrease the invasion capacity of tumor cells [43,44]. In conclusion, our findings demonstrate that CA VII isozyme is highly expressed in several cases of malignant brain tumors including oligodendrogliomas, oligoastro- cytomas and diffusively infiltrating astrocytomas. The positive immunostaining correlates with poor prognosis of patients with astrocytomas. The presence of several CA isozymes in malignant brain tumors may provide novel opportunities for developing cancer treatment strategies targeted to the microenvironment of cancer cells. 5. ACKNOWLEDGEMENTS The authors thank Aulikki Lehmus and Reija Randen for skilful technical assistance. This study was funded by European Union DeZ- nIT project, Academy of Finland, Sigrid Juselius Foundation, and Competitive Research Funding of the Tampere University Hospital (9L071). REFERENCE S [1] Sly, W.S. and Hu , P.Y. (1995) Human carbonic anhy- drases and carbonic anhydrase deficiencies. Annual Re- views of Biochemistry, 64, 375-401. doi:10.1146/annurev.bi.64.070195.002111 [2] Parkkila, S. and Parkkila, A.K . (1996) Carbonic anhy- drase in the alimentary tract. Roles of the different iso- zymes and salivary factors in th e mainten ance of optimal conditions in the gastrointestinal canal. Scandinavian Journal of Gastroenterology, 31, 305 -317. doi:10.3109/00365529609006403 [3] H ewe tt -Emmett, D. and Tashian, R.E. (1996) Functional diversit y, conservati on, and convergence in th e evolution of the alpha-, bet a-, an d gam ma-carbo nic anhydras e gene families. Molecular Phylogenetics and Evolution, 5, 50- 77. doi:10.1006/mpev.1996.0006 [4] Lehtonen, J., Shen, B., Vihinen, M., Casini, A., Scozza- fav a, A., Supuran, C.T., Parkkila, A.K., Saarnio, J., Ki- vela, A.J., Waheed , A., Sl y, W.S. and Parkkila, S. (2004) Characterization of CA XIII, a novel member of the car- bonic anhydrase isozyme family. Journal of Biological Chemistry, 279, 2 719-2727. doi:10.1074/jbc.M308984200 [5] Past orekova, S., Pa rkkila, S., Pasto rek, J. and Supuran, C.T. (2004) Carbon ic anhydrases: cu rrent stat e of the art, therapeutic applications and future prospects. Journal of Enzyme Inhibition and Medicinal Chemistry, 19, 199- 229. doi:10.1080/14756360410001689540 [6] Haap asal o, J., Nordfors, K., Jarvela, S., Bragge, H., Ran- tala, I., Parkkila, A.K., Haapasalo, H. and Parkkila, S.  F. Bootorabi et al. / Healt h 3 (2011) 6-12 Copyright © 2011 SciRes. Openly acces sib le at http://www.scirp.org/journal/HEALTH/ (2007) Carbonic anhydrase II in the endothelium of glial tumors: a po ten ti al target for therapy. Neuro-Oncology, 9, 308-313. doi:10.1215/15228517-2007-001 [7] Haap asal o, J.A., Nordfors, K.M., Hilvo, M., Rantala, I.J., Soini, Y., Parkkila, A.K., Pastorekova, S., Pastorek, J., Parkkila , S.M. and Haap as al o, H.K. (2006 ) Expression of carbonic anhydrase IX in astrocytic tumors predicts poor prognosis. Clinical Cancer Research, 12, 473-477. doi:10.1158/1078-0432.CCR-05-0848 [8] Sai d, H.M., Hagemann, C., Sta ab, A., Stojic, J., Kuhnel, S., Vince, G.H., Flentje, M., Roosen, K. and Vordermark, D. (2007) Expression patterns of the hypoxia-related genes osteopontin, CA9, erythropoietin, VEGF and HIF- 1alpha in human glioma in vitro and in vivo. Radiothe- rapy and Oncology, 83, 398-405. doi:10.1016/j.radonc.2007.05.003 [9] Sai d, H.M., Polat, B., Staab, A., Hagemann, C., Stein, S., Flentje, M., Theobald, M., Katzer, A. and Vordermark, D. (2008) Rapid detection of the hypoxia-regulated CA-IX and NDRG1 gene expression in different glioblastoma cells in vitro. Oncology Reports, 20, 413-419. [10] Ihnatko, R., Kubes , M., Takacova, M., Sedlakova, O., Sedlak, J., Pastorek, J., Kopacek, J. and Pastorekova, S. (2006) Extr acellu lar acid osi s elevates car bo n ic an hydrase IX in human glioblastoma cells via transcriptional modu- lation that does not depend on hypoxia. International Journal of Oncology, 29, 1025-1033. [11] Ivanov, S., Li ao , S.Y., Ivanova, A., Danil kovit ch - Miagkova, A., Tarasova, N., Weirich, G., Merrill, M.J., Proescholdt, M.A., Oldfield, E.H., Lee , J., Zavad a, J., Waheed , A., Sly, W., Lerman, M.I. and Stanbridge , E.J. (2001) Expression of hypoxia-inducible cell-surface transmembrane car- bonic anhydrases in human cancer. American Journal of Pathology, 158, 905-919. [12] Alterio, V., Hilvo, M., Di Fiore, A., Supuran, C.T., Pan, P., Parkkila , S., Scal oni, A., Pastorek, J., Pastore kova, S., Pedone, C., Scozzafa va, A., Monti, S.M. and De Simone, G. (2009) Crystal structure of the catalytic domain of the tumor-associ ated human carbonic anhydrase IX. Pro- ceedings of the National Academy o f Sciences USA, 106, 16233-16238. doi:10.1073/pnas.0908301106 [13] B ar anauskiene, L., Hilvo, M. , Matuliene, J., Golovenko, D., Man a kova, E., Dudutiene, V., Michail ovi en e, V., Torre- san, J., Jachno, J., Parkkila, S., Maresca, A., Supuran, C.T., Grazulis, S. and Matulis, D. (2010) Inhibition and binding studies of carbonic anhydrase isozymes I, II and IX with benzimidazo[1,2-c][ 1,2,3] thiad iazole-7-sul-pho- namides. Journal of Enzyme Inhibition and Medicinal Chemistry, 25, 863-870. doi:10.3109/14756360903571685 [14] Montgomery, J.C., Venta, P.J., Eddy, R.L. , Fukus hima , Y.S., S h ows , T.B. and Tashi an, R.E. (1991) Charact er i za- tion of the human gene for a newly discovered carbonic anhydrase, CA VII, and its localization to chromosome 16. Genomics, 11, 835-848. doi:10.1016/0888-7543(91)90006-Z [15] Lakkis, M.M., Bergenhem, N.C. and Tashian, R.E. (1996) Expression of mouse carbonic anhydrase VII in E. coli and demonstration of its CO2 hydrase activity. Biochem- ical and Biophysical Research Communications, 226, 268-272. doi:10.1006/bbrc.1996.1344 [16] Vullo, D., Innocenti, A., Nishimori, I., S cozzafava, A., Kaila, K. and Supuran, C.T. (2007) Carbonic anhydrase activators: activation of the human isoforms VII (cyto- solic) and XIV (transmembrane) with amino acids and amine s. Bioorganic and Medicinal Chemistry Letters, 17, 4107-4112. doi:10.1016/j.bmcl.2007.05.052 [17] Gitto, R., Agnello, S., Ferro, S., Vullo, D., Supuran, C.T. and Chimirri, A. (2010) Identification of potent and se- lective human carbonic anhydrase VII (hCA VII) inhibi- tors. Ch emMedChem, 5, 823-826. [18] Ha lmi, P., Par kkila, S. and Ho nkaniemi, J. (2006) Ex- pression of carbonic anhydrases II, IV, VII, VIII and XII in rat brain after kainic acid induced status epilepticus. Neurochemistry International, 48, 24-30. doi:10.1016/j.neuint.2005.08.007 [19] K l ei hues, P., Louis, D.N., S cheithauer, B.W., Rorke, L.B., Reifenberger, G., Bu rger, P.C. and Cavenee, W.K. (2002) The WHO classification of tumors of the nervous system. Journal of Neuropathology and Experimental Neurology, 61, 215-225. [20] Louis, D.N., O hgaki, H., Wiestler, O.D., Cavenee, W.K., Burger, P.C., Jouvet, A., Scheithauer, B.W. and K l ei hues, P. (2007) The 2007 WHO classification of tumours of the central nervous system. A cta Neuropathologica, 114, 97- 109. doi:10.1007/s00401-007-0243-4 [21] Bootorabi, F., Janis, J., Smith, E., Waheed, A., K ukku- rainen , S., Hytonen, V., Valj akka, J., Supuran, C.T., Vullo, D., Sly,W.S. and Parkkila, S. (2010) Analysis of a short- ened form of human carbonic anhydrase VII expressed in vitro compared to the full-length enzyme. Biochimie, 92, 1072-1080. [22] Leppilampi, M., Saarnio, J., Karttunen, T.J., Kivela, J., Pastorekova, S., Pastorek, J., Waheed , A., Sly and W.S., Parkkila , S. (2003) Carbonic anhydrase i sozymes IX and XII in gastric tumors. World Journal of Gastroenterology, 9, 1398-1403. [23] H aapasalo, H., Sallinen , S., Sallinen, P., Helen , P., Jaaskelainen, J., Sa lmi, T.T., Paetau , A., Paljarvi , L., Vi- sakorpi, T. and Kalimo, H. (1999) Clinicopathological correlation of cell proliferation, apoptosis and p53 in ce- rebellar pilocytic astrocytomas. Neuropathology and Ap- plied Neurobiology, 25, 134-142. doi:10.1046/j.1365-2990.1999.00157.x [24] J ar vela, S., Helin, H., Haapasalo, J., Jarvela, T., Junttila, T.T., Elenius, K., Tanner, M., Haapasalo, H. and Isola, J. (2006) Amplification of the epidermal growth factor re- ceptor in astrocytic tumours by chromogenic in situ hy- bridization: association with clinicopathological features and patient survival. Neuropathology and Applied Neu- robiology, 32, 441-450. [25] H aapasalo, J., Hilvo, M., Nordfors, K., Haapasalo, H., Parkkila , S., Hyrskyluoto, A., Rantala, I., Waheed, A., Sly, W.S., Past orekova, S., Pasto r ek, J. and Pa rkkila, A.K . (2008) Identification of an alternatively spliced isoform of carbonic anhydrase XII in diffusely infiltrating astro- cytic gliomas. N euro-Oncology, 10, 131-138. [26] Nordfors, K., Haapasalo, J., Korja, M., Niemela, A., Laine, J., Parkkila , A.K., Pasto rekova, S., Pastorek, J., Waheed, A., Sly, W.S., Parkkila, S. and Haapasal o, H. (2010) The tumour-associated car boni c anhydrases CA II, CA IX and CA XII in a group of medulloblastomas and supratentorial primitive neuroectodermal tumours: an associati on of CA IX with poor prognosis. BMC Cancer,  F. Bootorabi et al. / Healt h 3 (2011) 6-12 Copyright © 2011 SciRes. Openly acc es sib le at http://www.scirp.org/journal/HEALTH/ 10, 148. doi:10.1186/1471-2407-10-148 [27] Maseide, K., Kandel , R.A., Bell, R.S., Catton, C.N., O'Sullivan, B., Wunder, J.S., Pintilie, M., Hedley and D., Hill, R.P. (2004) Carbonic anhydrase IX as a marker for poor prognosis in soft tissue sarcoma. Clinical Cancer Research, 10, 4464-4471. doi:10.1158/1078-0432.CCR-03-0541 [28] Kon-no, H., I shii, G., Nagai , K., Yoshida, J., Nishimu ra , M., Nara, M., Fujii, T., Murata , Y., Miyamoto, H. and Ochiai, A. (2006) Carbonic anhydrase IX expression is associated with tumor progression and a poor prognosis of lung adenocarcin oma. Lung Cancer, 54, 409-418. [29] Ko rkeila, E., Talvinen, K., Jaakkola , P.M., Minn, H., Syrjanen, K., Sundstrom, J. and P yrhonen, S. (2009) Ex- pression of carbonic anhydrase IX suggests poor out- come in rectal cancer. British Journal of Cancer, 100, 874-880. doi:10.1038/sj.bjc.6604949 [30] Liao, S.Y., Darc y, K.M., Randall, L.M., Ti an, C., Mon k, B.J., Burger, R.A., Fruehauf, J.P., Peters, W.A., Stock, R.J. and Stanbridge, E.J. (2010) Prognostic relevance of carbonic anhydrase-IX in high-risk, early-stage cervical cancer: A Gynecologic Oncology Group study. Gyneco- logical Oncology, 116 , 452-458. [31] Klatte, T., Seligson, D.B., Rao, J.Y., Yu, H., De Martino M., Kawaoka, K., Wong, S.G., Belldegrun, A.S. and Pantuck, A. J. (2009) Carbonic anhydrase IX in bladder cancer: A diagnostic, prognostic, and therapeutic mole- cular marker. Cancer, 115, 1 448-1458. [32] Choi, S.W., Kim, J.Y., Park, J.Y., Cha, I.H., Kim, J. and Lee, S. (2008) Expression of carbonic anhydrase IX is associated with postoperative recurrence and poor prog- nosis in surgically treated oral squamous cell carcinoma. Human Pat h o logy, 39, 1317-1322. [33] B elldegrun, A.S . and Bevan, P. (2008) Carbonic anhy- drase IX : Role in diagnosis prognosis and cancer therapy. Introduction. British Journal of Urology International, 101, 1. [34] Hussain, S.A., Ganesan, R., Reynolds, G., Gross , L., Stevens, A., Past orek, J., Murray, P.G., Perunovic, B., Anwar, M.S., Billingham, L., J ames, N.D., Spooner, D., Poole, C.J., Rea, D.W. and Palmer, D.H. (2007) H ypox- ia-regulated carbonic anhydrase IX expression is asso- ciated with poor survival in patients with invasive breast cancer. British Journal of Cancer, 96, 104-109. [35] Lakkis, M.M., O'Shea, K.S. and Tashian, R.E. (1997) Differential expression of the carbonic anhydrase genes for CA VII (Car7 ) an d CA-RP VIII (Car8) in mouse brain. Journal of Histochemistry and Cytochemistry, 45, 657- 662. [36] Ruusuvuori, E., Li, H., H ut tu, K., Palva, J.M., Smirnov, S., Rivera, C., Kaila, K. and Voipio, J. (2004) Carbonic anhydrase isoform VII acts as a molecular switch in the development of synchronous gamma-frequency firing of hippocampal CA1 pyramidal cells. Journal of Neuros- cience, 24, 2699 -2707. doi:10.1523/JNEUROSCI.5176-03.2004 [37] Said, H.M., Staab, A., Hagemann, C., Vinc e, G.H., Katzer, A., Flentje, M. and Vordermark, D. (2007) Distinct pat- terns of hypoxic expression of carbonic anhydrase IX (CA IX) in hu man malignant glioma cell lines. Journal of Neurooncology, 81, 27-38. [38] Wykoff, C.C., Beasley, N.J., Watson, P.H., Turner, K.J., Past orek, J., Sibtain, A., Wilson, G.D., Turley, H., Talks, K.L., Maxwell, P.H., Pugh, C.W., Ratcliffe, P.J. and Har- ris, A. L. (2000) Hypoxia-inducible expression of tumor- associated carbonic anhydrases. Cancer Research, 60, 7075-7083. [39] Korkolopoulou, P., Perdiki, M., Thymara, I., Boviatsis, E., Agrogiannis, G., Kotsiakis, X., Angelidakis, D., Rologis, D., Diamantopoulou, K., Thomas-Tsagli, E., Kaklamanis, L., Gatter, K. and Patsouris, E. (2007) Expression of hy- poxia-related tissue factors in astrocytic gliomas. A mul- tivariate survival study with emphasis upon carbonic an- hydrase IX. Human Pat hology, 38 , 629-638. [40] Supuran, C.T. (2008) Carbonic anhydrases: novel thera- peutic applications for inhibitors and activators. Nature Reviews Drug Discovery, 7, 168-181. doi:10.1038/nrd2467 [41] Pastorekova, S., Par kkila, S. and Z avada, J. (2006) Tu- mor-associated carbonic anhydrases and their clinical significan ce. Advances in Clinical Chemistry, 42, 167- 216. doi:10.1016/S0065-2423(06)42005-9 [42] Xian g, Y., Ma, B., Yu, H.M. and Li, X.J. (2004) The pro- tein pro file of acetazolamide-t reated sera i n mice bearing Le wis neoplas m. Life Science, 75, 1277-1285. [43] Supuran, C.T., Briganti, F., Tilli, S., Che g widden , W.R. and Scozzafav a, A. (2001) Carbonic anhydrase inhibitors: sulfonamides as antitumor agents? Bioorganic and Medi- cinal Chemistry, 9, 703-714. doi:10.1016/S0968-0896(00)00288-1 [44] Supuran, C.T. (2003) Indisulam: An anticancer sulfona- mide in clinical development. Expert Opinion onInvesti- gational Drugs, 12, 283-287.
|