 Open Journal of Applied Sciences, 2013, 3, 323-331 http://dx.doi.org/10.4236/ojapps.2013.35042 Published Online September 2013 (http://www.scirp.org/journal/ojapps) Comparing the Effects of Interactive and Noninteractive Complementary Nutrients on Growth in a Chemostat James P. Braselton, Martha L. Abell, Lorraine M. Braselton Department of Mathematical Sciences, Georgia Southern University, Statesboro, USA Email: jbraselton@georgiasouthern.edu Received July 12, 2013; revised August 12, 2012; accepted August 19, 2013 Copyright © 2013 James P. Braselton et al. This is an open access article distributed under the Creative Commons Attribution Li- cense, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT We compare the effects of interactive and noninteractive complementary nutrients on the growth of an organism in the chemostat. We also compare these two situations to the case when the nutrients are substitutable. In previous studies, complementary nutrients have been assumed to be noninteractive. However, more recent research indicates that some complementary nutrient relationships are interactive. We show that interactive complementary and substitutable nutri- ents can lead to higher population densities than do noninteractive complementary nutrients. We numerically illustrate that if the washout rate is high, an organism can persist at higher densities when the complementary nutrients are inter- active than when they are noninteractive, which can result in the extinction of the organism. Finally, we present an ex- ample by making a small adjustment to the model that leads to a single nutrient model with an intermediate metabolite of the original substrate as the nutrient for the organism. Keywords: Chemostat; Growth; Dual Substrate; Complementary Nutrients; Substitutable Nutrients 1. Introduction We consider a basic, resource-based model of growth in the chemostat. Such models have applications in ecology to model a simple lake and in biotechnology to model the commercial bio-reactor. Experimental verification of the match between theory and experiment in the chemostat can be found in [1]. Basic growth in the chemostat is described by the dimensionless system 0 d,0 0 d d,0 0. d S Sx SSD fSS ty xfS Dxx t (1) For a detailed discussion of growth in the chemostat and a description of the constants (input of the nutrient), yS (yield constant), and D (dilution (washout) rate), see Smith and Waltman [2]. 0 S Two nutrients are complementary if they meet differ- ent needs for an organism. For example, ammonia pro- vides nitrogen while glucose provides carbon [3] (build- ing blocks of protein). Similarly, two nutrients are sub- stitutable if they meet the same needs for an organism. For example, glucose, galactose, maltose, ribose, arabi- nose, and fructose all provide energy (sugar) [4]. See Stroot et al. [5], for a recent study of Acinetobac- ter spp. bacteria in an activated sludge bioreactor system using the noninteractive Monod model for multiple nu- trients. However, other research [3,4,6-9] indicates that a model of interactive multiple limiting nutrients may be more appropriate for some situations. Of particular inter- est, Lendenmann and Egli [4] discuss several growth models appropriate for substitutable interactive nutrients and compare them to the growth of E. coli with sugar nutrients glucose, galactose, maltose, ribose, arabinose, and fructose. Whang et al. [9] perform a similar study using bacteria from the wastewater of a food-processing plant. On the other hand, Bapat et al. [10] use a Monod model to study the growth of A. mediterranei S699 with multiple interactive complementary nutrients. Champa- gne et al. [11] form a model of cometabolism with two interactive complementary nutrients in a well-mixed sys- tem. Bae and Rittmann [12] develop a dual-limiting mo- del, compare the results to experimental data, and ob- serve that they agree, which provides further evidence that a multiple nutrient limiting model might be more appropriate in some situations than a single nutrient lim- iting model. From [12], having an accurate kinetic model for dual limitation is essential for a proper design of treatment operations such as in bioremediation or con- taminated groundwater... Dual limitation also can be C opyright © 2013 SciRes. OJAppS  J. P. BRASELTON ET AL. 324 critical for predicting the fate of pollutants in certain natural environments, such as a deep lake or an ocean... To consider a single organism’s growth in the chemo- stat for two nutrients, we study the dimensionless system 0 1 11 121 1 0 2 22 121 2 12 d,,00 d d,,00 d d,,00. d Sx SSD fSSS ty Sx SSDfSSS ty xfSS Dxx t (2) If the nutrients are complementary and noninteractive and assuming Monod (or Michaelis-Menten) kinetics typical choices of f take the following forms. When as- suming that the nutrients are noninteractive, one of the nutrients is the limiting nutrient. If the nutrients are com- plementary and noninteractive, we take f to be 112 2 12 11 22 ,min , mSm S fSS KSK S , (3) The biological meaning of (3) is that one of S1 or S2 is the limiting nutrient, which is appropriate in modeling many situations [3]. If the nutrients are complementary and interactive, f has the form 112 2 12 112 2 , mS mS fSS . SK S (4) Finally, if the nutrients are supplementary, f has the form 112 2 12 112 2 , mSm S fSS . SKS (5) and the constants are described in Table 1. (Refer to [13- 15] or [16]). 2. Growth in the Chemostat Using Thieme’s results from 1992 [17], we show that System (2) is asymptotic to a single nonlinear equation. Let 0 11 1 1 1 SS y x and 0 22 2 2 1 SS y x Then, 11 D and 2 D2 so System (2) can be rewritten as 1 1 2 2 00 112 2 12 d d d d d11 , d D t D t x SxS x D ty y x (6) Because the solutions of and 11 D 22 D are 11 0 Dt Ce t as and 22 as , in the limit as , System (6) is asymp- totic to the equation t t 0 Dt Ce 00 12 12 d11 , d x SxSxD tyy x (7) Depending on whether the nutrients are complemen- tary (noninteractive or interactive) or substitutable and assuming Monod (or Michaelis-Menten) kinetics typical choices of f take the forms given by Equations (3)-(5). For all three situations, x = 0 is a boundary rest point. If f is given by (4), we the have the additional rest points: as the Equation (8) Similarly, if f is given by (5), we obtain the additional rest points: as the Equation (9) 0000 1112 22121122 12 000 0 12121 2112212 12 2 000 0 12 1122111222 1 2 4 xDKSyDKSymmSySy Dmm DmmmmS SDKSKSyy mm SySyD KSyKSy (8) 00 1112 22 11112 222 12 00 12211121212 12 00 0 1122121211211 12 2 000 2112 22111222 1 2 14 KmyKm y xKySyKySyDm m Dm mKmSKmmmSS Dm m DKSKSyyKmymmSy KmmmSyDKSyKSy 0 (9) Copyright © 2013 SciRes. OJAppS 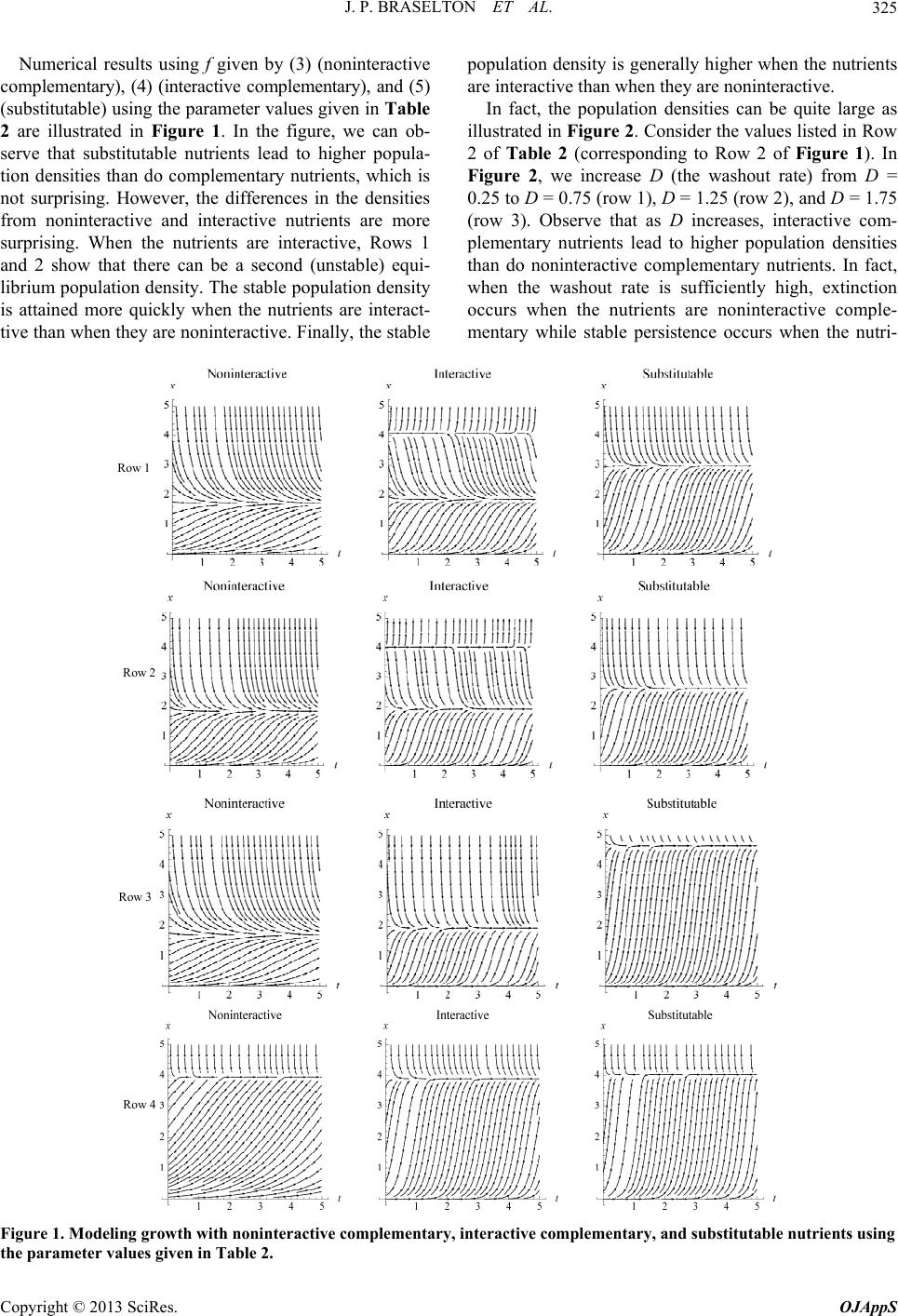 J. P. BRASELTON ET AL. 325 Numerical results using f given by (3) (noninteractive complementary), (4) (interactive complementary), and (5) (substitutable) using the parameter values given in Table 2 are illustrated in Figure 1. In the figure, we can ob- serve that substitutable nutrients lead to higher popula- tion densities than do complementary nutrients, which is not surprising. However, the differences in the densities from noninteractive and interactive nutrients are more surprising. When the nutrients are interactive, Rows 1 and 2 show that there can be a second (unstable) equi- librium population density. The stable population density is attained more quickly when the nutrients are interact- tive than when they are noninteractive. Finally, the stable population density is generally higher when the nutrients are interactive than when they are noninteractive. In fact, the population densities can be quite large as illustrated in Figur e 2. Consider the values listed in Row 2 of Tab l e 2 (corresponding to Row 2 of Figure 1). In Figure 2, we increase D (the washout rate) from D = 0.25 to D = 0.75 (row 1), D = 1.25 (row 2), and D = 1.75 (row 3). Observe that as D increases, interactive com- plementary nutrients lead to higher population densities than do noninteractive complementary nutrients. In fact, when the washout rate is sufficiently high, extinction occurs when the nutrients are noninteractive comple- mentary while stable persistence occurs when the nutri- Row 1 Row 2 Row 3 Row 4 Figure 1. Modeling growth with noninteractive complementary, interactive complementary, and substitutable nutrients using the parameter values given in Table 2. Copyright © 2013 SciRes. OJAppS  J. P. BRASELTON ET AL. 326 ow 1 Row 2 Row 3 Figure 2. Row 1: D = 0.75; Row 2: D = 1.25; Row 3: D = 1.75. Table 1. Descriptions of the constants in Equations (2)-(5). S1(0), S2(0) Input concentration of the nutrients S1 and S2 D Dilution (washout) rate of chemostat m1, m2 Maximal growth rate of ith competitor K1, K2 Michaelis-Menten (half-saturation) constants y1, y2 Yield constants f (S1, S2) Growth rate Entes are interactive and complementary. 3. An Intermediate Metabolite A particularly interesting situation occurs when one sub- stance degrades to a nutrient for the growth of an organ- ism. Specifically, Sanchez et al. [18], study the particular situation in which phenol degrades to an intermediate metabolite that is then the primary nutrient for the organ- ism (bacterium Pseudomonas putida Q5). This situation is particularly interesting because a “harmful” substance degrades to a state in which it is a nutrient for the organ- ism under consideration that is growing in the chemostat, rendered harmless, and eliminated. To model this situa- tion, System (2) is adjusted to 0 1 11 1121 1 2 2112222 1 22 d,,00 d d,, d d,0 0, d Sx SSDfSSS ty Sx DSfSSfSx S ty xfS Dxx t 00 (10) where a > 0 is a positive constant. Sanchez et al. [18], successfully fit a model of the form of System (10) using Copyright © 2013 SciRes. OJAppS  J. P. BRASELTON ET AL. 327 11 2 112 1111 ,, n ab mS S fSS SK S (11) where 22 1 11Sp pp e and 22 1 11Sn nn e and f2 = a·S2 to data obtained from their study of bacte- rium Pseudomonas putida Q5. With p = n = 1 and m1 = m1m2 (Notation in Equations (3)-(5).) and f2 = a·S2, (11) is the same as (4). Although we cannot reduce System (10) to a single equation as with System (2), we can reduce it to a system of two equations. To do so, let Then, and System (10) can rewritten as 0 112 .SSSx D 0 2 2112 222 1 22 d d d,, d d. d D t Sx DSf SSxSfSx ty xfS Dx t (12) Because the solution of is as , in the limit as , System (12) is asymp- totic to the system D t 0 Dt Ce t 0 2 2112222 1 22 d,, d d. d Sx DSfSSx SfSx ty xfS Dx t (13) For the problem to be biologically meaningful, the feasi- ble region is 0 2212 ,0,0,xS xSSSx 0. (14) Assuming Monod kinetics, we now assume that f1 takes the form given by (11) and that n = p = 1 and that 2222222 . SmSKmS The rest points of System (13) are 0, 0E 2 0 and potentially two interior rest points of the form * 22 , I ExDKmD , where Evaluated at 2 0, the Jacobian has eigenvalues λ1,2 = −D, so is always stable. E 2 0 If we eliminated S2 rather than S1, the limiting system is E 00 1 11 111 1 0 211 d, d d d Sx SSDfSSSx ty xfSS xDx t (15) with feasible region 0 1111 ,0,0,xS xSSSx 0. (16) Again, assuming that f1 takes the form given by (11) and that n = p = 1 and that 222222 SmSKS, we find that System (15) has rest points 1 0 01 0,ES Table 2. Parameter values used for Figure 1. D S1(0) y1 m1 K1 S2(0) y2 m2 K2 1. 0.254 1 6 4 2 1 7 4 2. 0.254 1 6 4 2 1 7 8 3. 0.254 2 6 1 2 1 7 8 4. 0.254 1 6 1 2 3 7 8 00 * 221 2121 00 1212211121 1 2 200 22 22121 21 00 21212122111 000 121 121 1 2 2 2 2 02 21212111 2 2 2 2 bb aa bb a a bba xDmKmmSDKS DKKKmm KSDKKSy Dm KmmSDK S KmDmDKKK mmSKS DKK SSK Sy Dm DKK Km KSyDm 12 12 21 1 b1 Dmy Km Dy Copyright © 2013 SciRes. OJAppS  J. P. BRASELTON ET AL. 328 and potentially two interior rest points of the form uated at obian has eigenvalues λ1,2 = 1 2 ** 1 , I ExS, where and the JacEval −D2 0 E, , so 2 0 E is always stable. Finally, if we had eliminated x rather than S or S, the limiting system is 0 112 11 112 1 0 2112 2112 1 0 22 112 , d d, d S SS SSDfSS ty SSSS DSfS S ty fSSS S (17) with feasible region 0 1 dS 0 211 2 0,0,0.SSS S (18) Using the same assumptions regarding f1 and m 12 1 ,SS S f2 and ade previously, the rest points of System (17) are 3 0 ** 012 1 ,,0ESSS and potentially two interior rest points of the form ** 1 , I ExSE* 122 , I SDKmD , where re 3 shows the behavior of Systems (13), (15), an Figu d (17) using the parameter values in Table 3. In all three cases, the plots indicate that all solutions tend to 0 12 1 ,,0, ,0xS SS as t. However, in the first * 212121 1 00 0 212121121221 1121 2 00 22 21 212 1 00 21212122111 000 121121 1 2 20 2121211 1 2 2 2 b bb aa bb a a bba x DmK Dmy KmDy 0 1 m mSDK SDKKKmmKSDKK Sy Km mSDKS KmDmDKKK mmSKS DK KSSKSy DmD KKKmKS 12 2 1 y * 1 212121 1 00 0 2121212121 2111 2 00 22 21 2121 00 000 212121 221111211211 2 2 202 21212111 1 2 2 b bba bbaa bba SDmK DmyKmDy KmmSD KSDmDKKKmKSy Km mSDKS mDmDK KKmmSKSDKKSS KSy DmD KKKmKSy * 1 212121 1 00 0 2121212121 2111 2 00 22 21 2121 00 000 212121 221111211211 1 2 2 202 21212111 1 2 2 b bba bbaa bba SDmK DmyKmDy KmmSD KSDmDKKKmKSy Km mSDKS mDmDK KKmmSKSDKKSS KSy DmD KKKmKSy 2 Copyright © 2013 SciRes. OJAppS  J. P. BRASELTON ET AL. 329 Row 1 Row 2 Row 3 Figure 3. Modeling growth when the nutrient is an intermediate state of the substrate using the parameter values in Table 3. Table 3. Parameter values used for Figure 3. D S1(0) y1 m1 K1a K1b m2 K2 1. 0.5 2 1 3 2 2 1 1 2. 0.5 6 1 3 2 2 1 1 3. 0.5 8 1 3 2 2 1 1 two cases there are no interior rest points. While in the third, when is increased to 8, (3,4), (3,1), and (4,1), igenvalue of the Jacobian evaluated at the rest point is λ1 = −0.08 More interesting behavior of the systems is illustrated in Figu 4 where wuseeter values in T le 4he relustrs t when (int con- tration rate), mr mh ra arin- c seduilium atescur aleetuats, te is one ule rest t, , S1, S2) = (1.76, 7.24, 1), (1.54, 9.46, 1), and (0.260, 4.56, 0.143) as shown in Table 5. However, in the first two rows of Table 5, (x, S1, S2) = (6.23, 2.76, 1) and (8.46, 2.54, 1) are stable spirals while in the third row, (x, S1, S2) = (2.92, 1.93, 0.143) is an unstable spiral. Thus, depending on the parameter values equilibrium states may be stable or unstable. Moreover, slight ad- justments to the parameter values might make a stable state unstable and vice-versa. 4. Conclusions In this paper, we have formed a model of gh in a We have used numerical results to graphically illustrate le than do complement- ta inte mentary nutrients. It is possible that if the nutrients are 0 1 S respectively, are degenerate rest points. For each, one chemostat with two interactive complementary nutrients. e 33 and the other is λ2 = 0. re e the paramab . Tfigu ilatehat 0 1 S (growt pu nce of the subst , eq 1, o oc 2 . tes)e reaibr st Inl thr siion hernstabpoin (x rowt differences in the behavior of systems modeling nonin- teractive compmentary nutrients, interactive comple- mentary nutrients, and substitutable nutrients. Not sur- prisingly, we have illustrated that substitutable nutrients lead to higher population densities ry nutrients. We have illustrated some surprising dif- ferences in the behavior of systems modeling noninterac- tive complementary nutrients and ractive comple- Copyright © 2013 SciRes. OJAppS  J. P. BRASELTON ET AL. 330 Row 2 Row 3 Row 1 Figure 4. Modeling growth when the nutrient is an intermediate state of the substrate using the parameter values in Table 4. Table 4. Parameter values used for Figure 4. D S1(0) y1 m1 K1a K1b m2 K2 interactive, the organism will attain a stable population density while if the nutrients are noninteractive, the or- ganism will become extinct. The simulations indicate that interactive complementary nutrients frequently lead to higher population densities than do noninteractive complementary nutrients. This agrees with the experi- mental results of Whang et al. [9] that show that com- plementary substrates (glucose and peptone) significantly increased hydrogen production by anaerobic hydrogen- producing bacteria. A slight adjustment to the system leads to a completely different interpretation of the model in which the nutrient the parameter values and slight adjustments to them can cause a stable state to become unstable and vice v Thises with experil res- chez et al. [18], which illustrated that slight ts of the introrreg mar et rate to 1 2) = , 0). Ma ks tu carry out many of the calculations as well as generate the 1. 0.5 10 1 3 2 2 1 1 2. 0.5 12 1 3 2 2 1 1 3. 0.25 5 1 8 2 2 2 1 Table 5. Rest points and eigenvalues of Jacobian for Figure 4. Row x-S1 x-S2 S1-S2 Eigenvalues of Jacobian (1.76,7.24) (1.76,1) (7.24,1) −0.359, 0.298 1 ± 0i .54,9.54,1 − 0 2 8.46,.54,1142 1.26 .26 56 0.29 143 (56,1.43) 0. −0. (2.92, 0.0401 ± (6:23, 2:76) (6.24,1) (2.76,1) −0.983 0.85 (1.46) (11) (9.46,) 0.373, .311 2 (8.46,.54) (1) (2) −0. ± i (00, 4.) (6, 0.) 4.230, 224 3 (2.92,1.93) 0.143) (1.93,0.143) 0.919i is an intermediate byproduct of the substrate. The simu- lations indicate the equilibrium states are very sensitive to ersa. agre thementaults of San adjustmen nlet conceation (cspondinto 0 1 S) had joffects on the converges washou (x, S, S (when the limit as (0, 0 St e 1 a Thathematic noteboohat the thors used to Copyright © 2013 SciRes. OJAppS  J. P. BRASELTON ET AL. 331 figures aby email request t B In future studies, wo exam relationships (competition, pred) are modeled nces shes re available from o Jim the aut raselton. hors sending an e hope tine how com ator-prey, and so petitive forth under circumstauch as te. REFERENCES [1] S. R. Hansen and S. P. Hubbell, “Single Nutrient Micro- bial Competition: Agreement between Experimental and Theoretical Forecast Outcomes,” Science, Vol. 20, No. 4438, 1980, pp. 1491-1493. http://dx.doi.org/10.1126/science.6767274 [2] H. L. Smith and P. Waltman, “The Theory of the Chemo- stat: Dynamics of Microbial Competition, Cambridge Studies in Mathematical Biology,” Cambridge University Press, Cambridge, 1995. http://dx.doi.org/10.1017/CBO9780511530043 [3] C. P. L. Grady, Jr., G. T. Daigger and H. C. Lim, “Bio- logical Wastewater Treatment,” Second Edition, revised and expanded, Marcel Dekker, New York, 1999. [4] U. Lendenmann and T. Egli, “Kinetic Models for the Growth of Escherichia Coli with Mixtures of Sugars Un- der Carbon-Limited Conditions,” Biotechnology and Bio- engineering, Vol. 59, No. 1, 1998, pp. 99-107. http://dx.doi.org/10.1002/(SICI)1097-0290(19980705)59: 1<99::AID-BIT13>3.0.CO;2-Y [5] G. Stroot, P. E. Saikaly and D. Berther, “Dynamic P.. O Growth Rates of Microbial Populations in Activated Sludge Systems,” Journal of Environmental Engineering, Vol. 131, No. 12, 2005, pp. 1698-1705. http://dx.doi.org/10.1061/(ASCE)0733-9372(2005)131:12 (1698) [6] edings o of Civil Engineers, New [7] J. L. Sherwoo. Skeen and N. B. 19960905)51: W. Bae and B. E. Rittmann, “Effects of Electron Accep- tor and Electron Donor on Biodegradation of CC14 by Biofilms,” Environmental Engineering: Proce the 1990 Specialty Conference, American Society f York, 1990, pp. 390-395. d, J. N. Petersen, R. S Valentine, “Effects of Nitrate and Acetate Availability on Chloroform Production during Carbon Tetrachloride De- struction,” Biotechnology and Bioengineering, Vol. 51, No. 5, 1996, pp. 551-557. http://dx.doi.org/10.1002/(SICI)1097-0290( 5<551::AID-BIT7>3.0.CO;2-B [8] D. V. Vayenas and S. Pavlou, “Coexistence of Three Microbial Populations Competing for Three COMPLE- MENTARY Nutrients in a Chemostat,” Mathematical Biosciences, Vol. 161, No. 1-2, 1999, pp. 1-13. http://dx.doi.org/10.1016/S0025-5564(99)00040-1 [9] L.-M. Whang, C.-J. Hsiao and S.-S. Cheng, “A Dual-Sub- strate Steady-State Model for Biological Hydrogen Pro- duction in an Anaerobic Hydrogen Fermentation Proc- ess,” Biotechnology and Bioengineering, Vol. 95, No. 3, 2006, pp. 492-500. http://dx.doi.org/10.1002/bit.21041 [10] P. M. Bapat, S. Bhartiya, K. V. Venkatesh and P. Wangi- kar, “Structured Kinetic Model to Represent the Utiliza- tion of Multiple Substrates in Complex Media During Rifamycin B Fermentation,” Biotechnology and Bioengi- neering, Vol. 93, No. 4, 2006, pp. 779-790. http://dx.doi.org/10.1002/bit.20767 [11] P. Champagne, P. J. Van Geel and W. J. Parker, “A Pro- posed Transient Model for Cometabolism in Ciofilm Sys- tems,” Biotechnology and Bioengineering, Vol. 60, No. 5, 1998, pp. 541-550. http://dx.doi.org/10.1002/(SICI)1097-0290(19981205)60: 5<541::AID-BIT4>3.0.CO;2-Q [12] W. Bae and B. E. Rittmann, “A Structured Model of Dual-Limitation Kinetics,” Biotechnology and Bioengi- neering, Vol. 49, No. 6, 1996, pp. 683-689. http://dx.doi.org/10.1002/(SICI)1097-0290(19960320)49: 6<683::AID-BIT10>3.3.CO;2-E [13] M. M. Ballyk and G. S. K. Wolkowicz, “Exploitive Com- petition in the Chemostat for Two Perfectly Substitutable Resources,” Mathematical Biosciences, Vol. 118, No. 2, 1993, pp. 127-180. http://dx.doi.org/10.1016/0025-5564(93)90050-K [14] G. J. Butler and G. S. K. Wolkowicz, “Exploitive Com- petition in a Chemostat for Two Complementary, and Possible Inhibitory, Resources,” Mathematical Biosci- ences, Vol. 83, No. 1, 1987, pp. 1-48. http://dx.doi.org/10.1016/0025-5564(87)90002-2 [15] S-B. Hsu, K-S. Cheng and S. P. Hubbell, “Exploitive Competition for Two Complementary Nutrients in Con- tinuous Cultures,” SIAM Journal on Applied Mathematics, Vol. 41, No. 3, 1981, pp. 422-444. http://dx.doi.org/10.1137/0141036 [16] S.-B. Hsu and Y.-H. Tzeng, “Plasmid-Bearing, Plas- mid-Free Organisms Competing for Two Complementary Nutrients in a Chemostat,” Mathematical Biosciences, Vol. 179, No. 2, 2002, pp. 183-206. http://dx.doi.org/10.1016/S0025-5564(02)00105-0 [17] H. R. Thieme, “Convergence Results and a Poincaré- Bendixson Trichotomy for Asymptotically Autonomous Differential Equations,” Journal of Mathematical Biology, Vol. 30, No. 7, 1992, pp. 755-763. http://dx.doi.org/10.1007/BF00173267 [18] J. L. Garcia-Sanchez, B. Kamp, K. A. Onysko, H. Bud- man and C. W. Robinson, “Double Inhibition Model for Degradation of Phenol by Pseudomonas Putida Q5,” Bio- technology and Bioengineering, Vol. 60, No. 5, 1998, pp. 560-567. http://dx.doi.org/10.1002/(SICI)1097-0290(19981205)60: 5<560::AID-BIT6>3.0.CO;2-L Copyright © 2013 SciRes. OJAppS
|