 Journal of Sustainable Bioenergy Systems, 2013, 3, 224-233 http://dx.doi.org/10.4236/jsbs.2013.33031 Published Online September 2013 (http://www.scirp.org/journal/jsbs) Biodiesel Production fro m Spirulina-Platensis Microalgae by In-Situ Transesterification Pr ocess H. I. El-Shimi1, Nahed K. Attia2*, S. T. El-Sheltawy1, G. I. El-Diwani2 1Chemical Engineering Department, Faculty of Engineering, Cairo University, Giza, Egypt 2Chemical Engineering and Pilot Plant Department, National Research Center, Dokki, Egypt Email: hassanshimi@gmail.com, *nahed_attia@yahoo.com Received June 8, 2013; revised July 10, 2013; accepted July 30, 2013 Copyright © 2013 H. I. El-Shimi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT This research investigates the effect of reaction variables that strongly affect the cost of biodiesel production from non-edible Spirulina-Platensis microalgae lipids, and use the acid-catalyzed in situ transesterification process. Experi- ments were designed to determine how variations in volume of reacting methanol, the concentration of an acid catalyst, time, temperature and stirring affected the biodiesel yield. The total lipid content of Spirulina-Platensis microalgae was obtained to be 0.1095 g/g biomass. The weight of the by-product glycerol obtained was used to predict the percentage yield conversion of microalgae oil biodiesel. Best results (84.7%), a yield of fatty acid methyl ester (FAME), were ob- tained at 100% (wt./wt.oil) catalyst concentration, 80 ml methanol volumes, 8 h reaction time and 65˚C reaction tem- perature with continuous stirring at 650 rpm. Properties of the produced biodiesel were measured according to EN 14214 standards. Keywords: Biodiesel; Spirulina-Platensis; Microalgae; In-Situ Transesterification 1. Introduction Energy today is the most important resources for man- kind and its sustainable development, due to the energy crisis which becomes one of the global problems con- fronting the world [1]. Major energy resources come from fuels, due to their energy content with significant amounts. Nowadays, there is a strong dependence of our life on fossil fuels such as petrol oil, coal and natural gas, since more than 80% of the world’s energy needs are from fossil fuels, whatever, in the industrial production sector, domestic uses or in the transportation sector. The problem also is that the population growth is not covered by domestic crude oil production and its derivatives [2]. In addition, the formation of fossil fuels requires millions of years, hence the petrol fuels are non-renewable. Also, change of the crude oil prices leads to global and interna- tional conflicts especially in the developing countries. Renewable energy is considered as one of the most impor- tant resources in many countries around the world, which accounts for about 10% of the world’s energy consump- tion and can be converted to other usable forms of energy like biofuels [3]. Liquid biofuels have become a green important alternative fuel that offers several advantages including its renewability, high energy content and low emission profile of carbon dioxide [4]. Liquid biofuels are classified into three generations based on the feedstocks and production technology [5]. First generation liquid biofuels—bioethanol and bio- diesel—were produced from food crops such as corn, sugarcane and vegetable oils. Since the food crops are used in the fuel production, first generation liquid biofu- els were limited to conflicting with the food supply and increasing the food crop prices. This has paved the way for second generation liquid biofuels, which were pro- duced, using waste cooking oil, non-edible plant seed oil, waste vegetable oil and animal fats [5,6]. Although sec- ond generation liquid biofuels overcame the problems faced by their first generation counterparts, increasing the fuel consumption and creating a challenge for the supply with consistent feedstock, this difficulty led to the development of third generation liquid biofuels like algae biodiesel [7]. Biodiesel (fatty acid alkyl esters, FAAE) is a green al- ternative liquid diesel fuel derived from vegetable oils or lipids by the reaction with alcohol in the presence of a catalyst. Biodiesel is used today as the basis for a clean substitute for petrol-diesel without any modification in *Corresponding author. C opyright © 2013 SciRes. JSBS  H. I. EL-SHIMI ET AL. 225 diesel engines [8]. Biodiesel is environmentally-friendly, non-toxic and biodegradable fuel, which can be made from any vegetable oils (edible or non-edible), animal fats or special strains of microalgae [9]. Microalgae has been considered recently as a prom- ising biomass feedstock with great potential for biodiesel production [10] because they reproduce themselves every few days (2 or 3 weeks), yield oil exceeding 10x the yield of the best oilseed crops, reduce emissions of a major greenhouse gas (1 kg of algal biomass requiring about 1.8 kg of CO2) and can be obtained from wastewa- ter (1 m3 of wastewater is required to produce 800 g of dry algae). In addition, microalgae as a fuel source does not conflict with the food crisis, since it is not the main food source. The production of biodiesel using microal- gae biomass as a possible feedstock has been described by Chisti Y. [11]. The production of biodiesel from microalgae oil by transesterification process has previously been demon- strated in the literature using the conventional methods [12, 13], and the process usually uses pre-extracted oil as raw material, which is usually produced [14] by mechanical pressing followed by solvent extraction to extract the remaining oil, and then its conversion to FAAE and glycerol. The transesterification reaction can be catalyzed by alkali [15-17], acidic [18], or enzymes [19-23]. The use of the alkaline catalysed transesterification technol- ogy would not be suitable for biodiesel production from microalgae oil; because of the high FFA content of mi- croalgae lipids. This is because the use of alkaline cata- lysts with high FFA containing oils would result in soap formation [24,25] and difficulties in the biodiesel separa- tion and purification downstream. The use of sulphuric acid, as reaction catalyst, has been considered as micro- algae lipid transesterification, due to its insensitivity to the FFA content of this oil feedstock, as the transes- terification and esterification reactions of biodiesel pro- duction are facilitated via acidic catalysis [12], however, acidic transesterification process is limited due to the water formation during the esterification reaction, high alcohol-to-oil ratio (about 40:1), and large amounts (5% to 25%) of catalysts may be required [26]. Also the use of enzymes as a transesterification catalyst is still under study. The biodiesel production from microalgae on an industrial scale still faces problems, mainly due to the high costs associated with the present biomass produc- tion and fuel conversion routes [24]. One of the alternatives to produce biodiesel from mi- croalgae lipids is “in-situ transesterification” or “reactive extraction” process [14,27]. This process combines the steps of lipid (oil) extraction and transesterification to produce biodiesel. Integration of these stages could minimize biodiesel production cost [28], since the use of reagents and solvents is reduced and the analysis is easier and not expensive. The method involves the simultane- ous addition of the acid catalyst and pure methanol to microalgal biomass (generally in the form of dried powder). The methanol extracts the lipids from the mi- croalgal biomass and, catalyzed by the acid, concurrently transesterifies the extracted lipids to produce fatty acid methyl esters [29,30]. The method was first demonstrated by Harrington and D’Arcy-Evans [31] with sunflower seeds as feedstock, using the in situ method, and these authors achieved an increase in biodiesel yields up to 20% compared to the conventional process. This improvement in the biodiesel yields was considered by these authors to be attributable to the improved accessibility of the oil in the biomass by the acidic medium. The in situ transesterification of mac- erated sunflower seeds was also studied by Siler- Marinkovic et al. [32] who investigated two temperature levels of 30˚C and 64.5˚C and a range of test reaction conditions: the alcohol (methanol) to oil molar ratio var- ied from 100:1 to 300:1, and the sulphuric acid catalysts concentration ranged from 16% to 100% (on the basis of the oil) and a reaction time of 1 - 4 h. Under the condi- tions studied, the best FAME yields (98.2%) based on the oil content of the sunflower seeds were obtained at a mo- lar ratio of methanol to oil of 300:1, an acid catalyst concentration of 100% and a reaction time of 1 h. The main objective of the present work is to apply the biodiesel production technology using an acid catalyst to the in-situ transesterification of microalgae (Spirulina- Platensis), where the main reaction variables that strongly affect the cost of this process were studied. These variables are: 1) the catalyst concentration (the larger the catalyst concentration, the more the material costs input); 2) the reacting alcohol volume (also, the more the alcohol volume, the more the material costs input); 3) the temperature (increasing of temperature, and increasing the process of energy requirement); 4) the reaction time (the larger the reaction time, the lower the product amounts yielded, and lower the product profit); and 5) process stirring (main energy requirement for the reaction agitation). This investigation has been carried out to provide information on the optimum operating conditions that give the best yield while also having the lowest material and energy requirements, and conse- quently lowest process costs, since the use of the in situ transesterification process as a proper biodiesel produc- tion technique is mainly driven by its possible applica- tion with relatively low cost. 2. Materials and Methods 2.1. Materials Spirulina-Platensis microalgae were supplied from the Microbiology Department, Soils Water and Environment Res. Inst., Agriculture Research Center (ARC), Giza, Egypt. This microalgae strain was collected from three Copyright © 2013 SciRes. JSBS  H. I. EL-SHIMI ET AL. 226 weeks old. The culture media used was the same of Zar- rouk’s medium [33]. The cultivation of Spirulina-Plat- ensis was in mini-tanks with dimensions similar to that used in Ref. [34]. The cultivation was carried out at 30˚C, 3.5 klx of illuminance provided by fluorescent lamps and pH of 8.5 ± 0.5. At the end of the culture cycle, algal suspensions were homogenized (Homogenizer Wisetis HG-15D) for 10 minutes at 1800 rpm; to disrupt the cells and ease the oil extraction, and filtered through Centri- fuge separator (Beckman CS-6 Centrifuge 3500 rpm, Germany) and then dried to a constant weight using solar drying beds and storedat 18˚C until use. Sulphuric acid of 98% purity is used in this study as a catalyst in the transesterification process. Methanol (99.9% purity) was used as the reacting alcohol in this study. 2.2. Method Microalgae oil was extracted using the Soxtherm extrac- tion system described by Jie Sheng et al. [35]. After re- extraction with methanol as a solvent, followed by frac- tional distillation to recover the microalgae oil, the ex- tracted oil was weighed to determine the total lipid con- tent per dry algal biomass, and then analyzed; to charac- terize the properties of Spirulina-Platensis oil. Variable sulphuric acid concentrations (0.0046, 0.0077, 0.0154 & 0.0308 mol), were used throughout this study. The acid-methanol solution was prepared freshly by mixing predetermined amounts of sulphuric acid and methanol. H2SO4 was dissolved with continuous stirring on a magnetic stirrer for 5 min. The solution was pre- pared freshly in order to maintain the catalyst activity. Dried microalgae of 15 gm was added carefully to catalyst/alcohol mixture and blended on low setting for several minutes. At this point, the simultaneous extrac- tion and transesterification reaction has been initiated; where the catalyst/alcohol solution attacked the triglyc- eride (oil) in the microalgae strain and cleaved off a fatty acid chain. The vessels containing the reaction mixtures were then heated and maintained at the temperatures of interest for specified periods. The major in situ transesterification reaction and product purification steps used are shown in “Figure 1”. 2.2.1. Settling and Separati ng After the transesterification step “Figure 1(a)”, the warm reaction mixture was allowed to cool for 20 min. The reaction mixture was filtered and the residues are washed three times by re-suspension in methanol (45 ml) for 15 min to recover any traces of FAME product left in the residues “Figure 1(b)”. Water (60 ml) was added to the filtrate, to facilitate the separation of the hydrophilic components of the extract, and then poured into a 500-mL separating funnel “Figure 1(c)” and the reaction vessel was allowed to stand for 4 h to enable its contents to settle. Further extraction of the FAME product was achieved by extracting three times for 15 min using 60 ml of hexane “Figure 1(c)”, which resulted in generation of two layers: hydrophobic layer (hexane, FAME and glycerides), and hydrophilic layer (water, glycerol and excess methanol). The reaction was demonstrated to be successful by observing the glycerin settling in the bottom soon after stopping mixing of the reactants. The top of the mixture looked lighter, and a darker layer was formed at the bottom. When the product has fully settled, two distinct layers were separated. These two layers are alkyl esters (biodiesel) and glycerin. The biodiesel on top looked as a clear, lighter in color, thin, and slippery to the touch. The glycerin settled to the bottom looked clear, darker amber color, thick, and sticky to the touch. Most of the settling occurred within the first hour. Once the glycerol and biodiesel phases have been separated, the bottom layer which contains glycerol, trace water, catalyst, and excess methanol was drawn into a pre-weighted beaker and dissolved in pure water; to purify the glycerol layer, and then subjected to a flash evaporation process “Figure 1(d)”, in which excess alcohol and water are removed. The recovered alcohol was recycled and reused. Now, the layer contains only the by-product glycerol and the catalyst, therefore the weight of pure glycerol can be detected by the well-known catalyst weight. This pro- cedure was performed in each experiment of the work, since we took a 15 g of microalgae biomass in each experiment, which expected to contain lipids of 1.6425 g, and based on just 60% reaction conversion, around 0.99 g glycerol will be obtained and can be weighted; using four digits balance. 2.2.2. Meth yl Ester (Biodiesel) Wash The top layer in the separation funnel is the produced biodiesel. This biodiesel layer was washed with water “Figure 1(e)” and filtered into a clean, dry side-arm flask; to evaporate the methanol and the hexane using a frac- tional distillation apparatus “Figure 1(f)”. The amount of collected biodiesel is difficult to be measured; since the unreacted glycerides are mixed with it, so the yield of the FAME can be calculated using the balanced equation of the transesterification reaction and then compared with the microalgae oil to monitor the extent of the conversion. With the forward reaction resulting in FAME production and the process is near to completion, the weight of the purified glycerol as a co-product (after the removal of the water and excess alcohol and omitting the weight of the catalyst used) is expected to increase until a constant value, signifying an equilibrium conversion of the micro- algae lipids to the methyl esters. Copyright © 2013 SciRes. JSBS 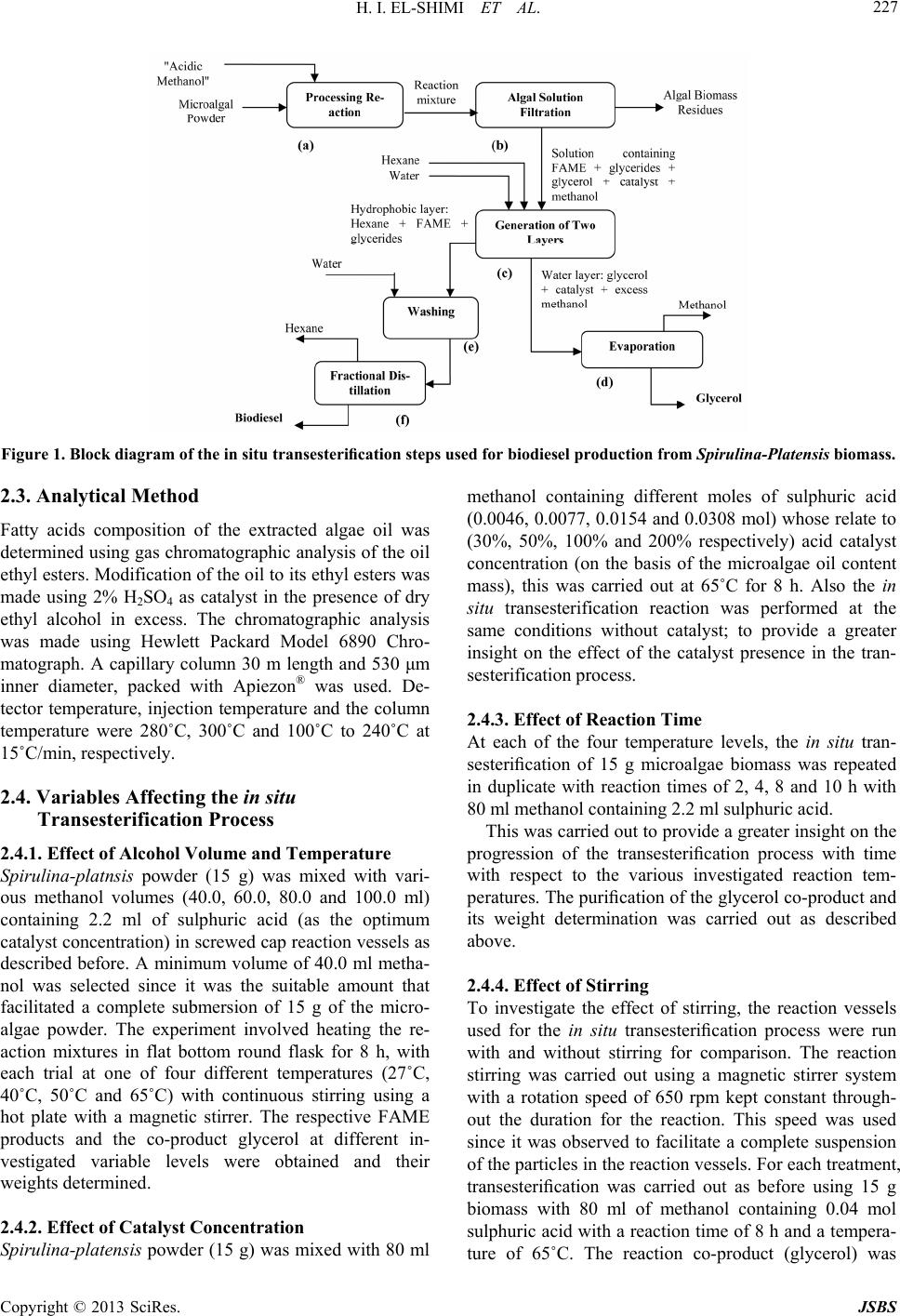 H. I. EL-SHIMI ET AL. Copyright © 2013 SciRes. JSBS 227 Figure 1. Block diagram of the in situ transesterification steps used for biodiesel production from Spirulina-Platensis biomass. 2.3. Analytical Method Fatty acids composition of the extracted algae oil was determined using gas chromatographic analysis of the oil ethyl esters. Modification of the oil to its ethyl esters was made using 2% H2SO4 as catalyst in the presence of dry ethyl alcohol in excess. The chromatographic analysis was made using Hewlett Packard Model 6890 Chro- matograph. A capillary column 30 m length and 530 μm inner diameter, packed with Apiezon® was used. De- tector temperature, injection temperature and the column temperature were 280˚C, 300˚C and 100˚C to 240˚C at 15˚C/min, respectively. 2.4. Variables Affecting the in situ Transesterification Process 2.4.1. Effect of Alcohol Volume and Temperature Spirulina-platnsis powder (15 g) was mixed with vari- ous methanol volumes (40.0, 60.0, 80.0 and 100.0 ml) containing 2.2 ml of sulphuric acid (as the optimum catalyst concentration) in screwed cap reaction vessels as described before. A minimum volume of 40.0 ml metha- nol was selected since it was the suitable amount that facilitated a complete submersion of 15 g of the micro- algae powder. The experiment involved heating the re- action mixtures in flat bottom round flask for 8 h, with each trial at one of four different temperatures (27˚C, 40˚C, 50˚C and 65˚C) with continuous stirring using a hot plate with a magnetic stirrer. The respective FAME products and the co-product glycerol at different in- vestigated variable levels were obtained and their weights determined. 2.4.2. Effect of Catalyst Concentration Spirulina-platensis powder (15 g) was mixed with 80 ml methanol containing different moles of sulphuric acid (0.0046, 0.0077, 0.0154 and 0.0308 mol) whose relate to (30%, 50%, 100% and 200% respectively) acid catalyst concentration (on the basis of the microalgae oil content mass), this was carried out at 65˚C for 8 h. Also the in situ transesterification reaction was performed at the same conditions without catalyst; to provide a greater insight on the effect of the catalyst presence in the tran- sesterification process. 2.4.3. Effect of Reaction Time At each of the four temperature levels, the in situ tran- sesterification of 15 g microalgae biomass was repeated in duplicate with reaction times of 2, 4, 8 and 10 h with 80 ml methanol containing 2.2 ml sulphuric acid. This was carried out to provide a greater insight on the progression of the transesterification process with time with respect to the various investigated reaction tem- peratures. The purification of the glycerol co-product and its weight determination was carried out as described above. 2.4.4. Effect of Stirring To investigate the effect of stirring, the reaction vessels used for the in situ transesterification process were run with and without stirring for comparison. The reaction stirring was carried out using a magnetic stirrer system with a rotation speed of 650 rpm kept constant through- out the duration for the reaction. This speed was used since it was observed to facilitate a complete suspension of the particles in the reaction vessels. For each treatment, transesterification was carried out as before using 15 g biomass with 80 ml of methanol containing 0.04 mol sulphuric acid with a reaction time of 8 h and a tempera- ture of 65˚C. The reaction co-product (glycerol) was 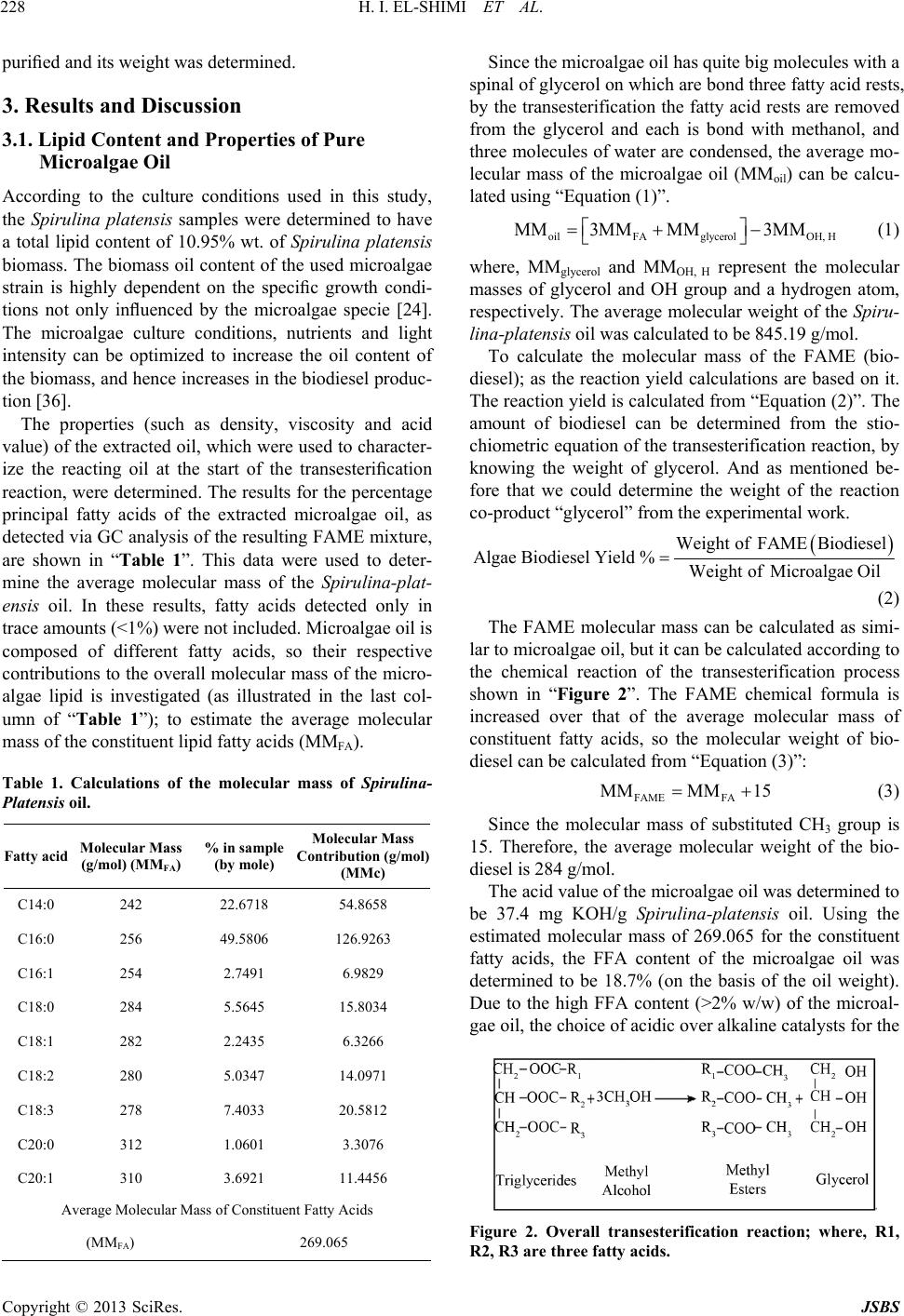 H. I. EL-SHIMI ET AL. 228 purified and its weight was determined. 3. Results and Discussion 3.1. Lipid Content and Properties of Pure Microalgae Oil According to the culture conditions used in this study, the Spirulina platensis samples were determined to have a total lipid content of 10.95% wt. of Spirulina platensis biomass. The biomass oil content of the used microalgae strain is highly dependent on the specific growth condi- tions not only influenced by the microalgae specie [24]. The microalgae culture conditions, nutrients and light intensity can be optimized to increase the oil content of the biomass, and hence increases in the biodiesel produc- tion [36]. The properties (such as density, viscosity and acid value) of the extracted oil, which were used to character- ize the reacting oil at the start of the transesterification reaction, were determined. The results for the percentage principal fatty acids of the extracted microalgae oil, as detected via GC analysis of the resulting FAME mixture, are shown in “Table 1”. This data were used to deter- mine the average molecular mass of the Spirulina-plat- ensis oil. In these results, fatty acids detected only in trace amounts (<1%) were not included. Microalgae oil is composed of different fatty acids, so their respective contributions to the overall molecular mass of the micro- algae lipid is investigated (as illustrated in the last col- umn of “Table 1”); to estimate the average molecular mass of the constituent lipid fatty acids (MMFA). Table 1. Calculations of the molecular mass of Spirulina- Platensis oil. Fatty acid Molecular Mass (g/mol) (MMFA) % in sample (by mole) Molecular Mass Contribution (g/mol) (MMc) C14:0 242 22.6718 54.8658 C16:0 256 49.5806 126.9263 C16:1 254 2.7491 6.9829 C18:0 284 5.5645 15.8034 C18:1 282 2.2435 6.3266 C18:2 280 5.0347 14.0971 C18:3 278 7.4033 20.5812 C20:0 312 1.0601 3.3076 C20:1 310 3.6921 11.4456 Average Molecular Mass of Constituent Fatty Acids (MMFA) 269.065 Since the microalgae oil has quite big molecules with a spinal of glycerol on which are bond three fatty acid rests, by the transesterification the fatty acid rests are removed from the glycerol and each is bond with methanol, and three molecules of water are condensed, the average mo- lecular mass of the microalgae oil (MMoil) can be calcu- lated using “Equation (1)”. oilFAglycerolOH, H MM3MM MM3MM (1) where, MMglycerol and MMOH, H represent the molecular masses of glycerol and OH group and a hydrogen atom, respectively. The average molecular weight of the Spiru- lina-platensis oil was calculated to be 845.19 g/mol. To calculate the molecular mass of the FAME (bio- diesel); as the reaction yield calculations are based on it. The reaction yield is calculated from “Equation (2)”. The amount of biodiesel can be determined from the stio- chiometric equation of the transesterification reaction, by knowing the weight of glycerol. And as mentioned be- fore that we could determine the weight of the reaction co-product “glycerol” from the experimental work. Weight ofFAMEBiodiesel Algae Biodiesel Yield %WeightofMicroalgae Oil (2) The FAME molecular mass can be calculated as simi- lar to microalgae oil, but it can be calculated according to the chemical reaction of the transesterification process shown in “Figure 2”. The FAME chemical formula is increased over that of the average molecular mass of constituent fatty acids, so the molecular weight of bio- diesel can be calculated from “Equation (3)”: FAME FA MMMM 15 (3) Since the molecular mass of substituted CH3 group is 15. Therefore, the average molecular weight of the bio- diesel is 284 g/mol. The acid value of the microalgae oil was determined to be 37.4 mg KOH/g Spirulina-platensis oil. Using the estimated molecular mass of 269.065 for the constituent fatty acids, the FFA content of the microalgae oil was determined to be 18.7% (on the basis of the oil weight). Due to the high FFA content (>2% w/w) of the microal- gae oil, the choice of acidic over alkaline catalysts for the Figure 2. Overall transesterification reaction; where, R1, R2, R3 are three fatty acids. Copyright © 2013 SciRes. JSBS 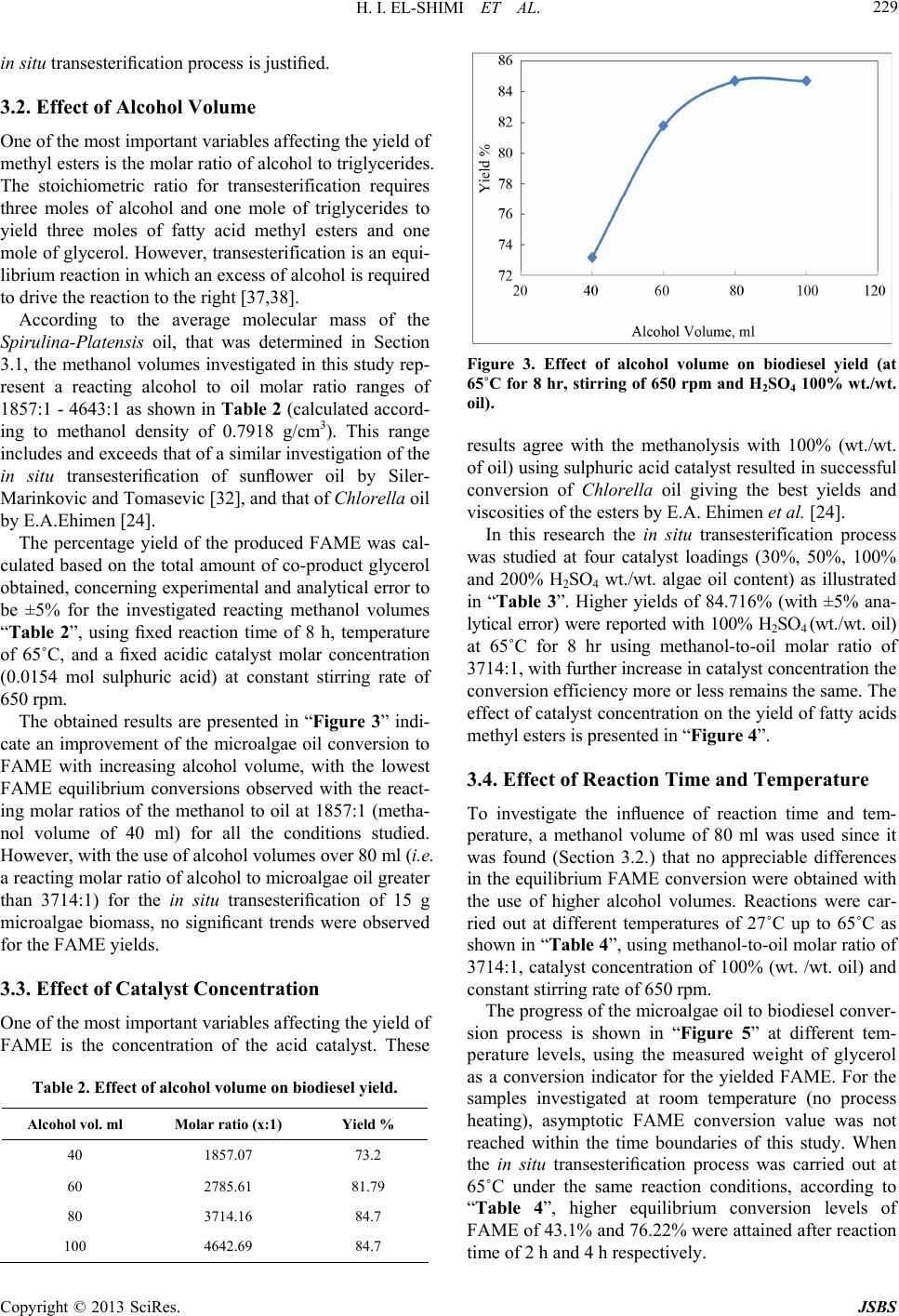 H. I. EL-SHIMI ET AL. 229 in situ transesterification process is justified. 3.2. Effect of Alcohol Volume One of the most important variables affecting the yield of methyl esters is the molar ratio of alcohol to triglycerides. The stoichiometric ratio for transesterification requires three moles of alcohol and one mole of triglycerides to yield three moles of fatty acid methyl esters and one mole of glycerol. However, transesterification is an equi- librium reaction in which an excess of alcohol is required to drive the reaction to the right [37,38]. According to the average molecular mass of the Spirulina-Platensis oil, that was determined in Section 3.1, the methanol volumes investigated in this study rep- resent a reacting alcohol to oil molar ratio ranges of 1857:1 - 4643:1 as shown in Table 2 (calculated accord- ing to methanol density of 0.7918 g/cm3). This range includes and exceeds that of a similar investigation of the in situ transesterification of sunflower oil by Siler- Marinkovic and Tomasevic [32], and that of Chlorella oil by E.A.Ehimen [24]. The percentage yield of the produced FAME was cal- culated based on the total amount of co-product glycerol obtained, concerning experimental and analytical error to be ±5% for the investigated reacting methanol volumes “Table 2”, using fixed reaction time of 8 h, temperature of 65˚C, and a fixed acidic catalyst molar concentration (0.0154 mol sulphuric acid) at constant stirring rate of 650 rpm. The obtained results are presented in “Figure 3” indi- cate an improvement of the microalgae oil conversion to FAME with increasing alcohol volume, with the lowest FAME equilibrium conversions observed with the react- ing molar ratios of the methanol to oil at 1857:1 (metha- nol volume of 40 ml) for all the conditions studied. However, with the use of alcohol volumes over 80 ml (i.e. a reacting molar ratio of alcohol to microalgae oil greater than 3714:1) for the in situ transesterification of 15 g microalgae biomass, no significant trends were observed for the FAME yields. 3.3. Effect of Catalyst Concentration One of the most important variables affecting the yield of FAME is the concentration of the acid catalyst. These Table 2. Effect of alcohol volume on biodiesel yield. Alcohol vol. ml Molar ratio (x:1) Yield % 40 1857.07 73.2 60 2785.61 81.79 80 3714.16 84.7 100 4642.69 84.7 Figure 3. Effect of alcohol volume on biodiesel yield (at 65˚C for 8 hr, stirring of 650 rpm and H2SO4 100% wt./wt. oil). results agree with the methanolysis with 100% (wt./wt. of oil) using sulphuric acid catalyst resulted in successful conversion of Chlorella oil giving the best yields and viscosities of the esters by E.A. Ehimen et al. [24]. In this research the in situ transesterification process was studied at four catalyst loadings (30%, 50%, 100% and 200% H2SO4 wt./wt. algae oil content) as illustrated in “Table 3”. Higher yields of 84.716% (with ±5% ana- lytical error) were reported with 100% H2SO4 (wt./wt. oil) at 65˚C for 8 hr using methanol-to-oil molar ratio of 3714:1, with further increase in catalyst concentration the conversion efficiency more or less remains the same. The effect of catalyst concentration on the yield of fatty acids methyl esters is presented in “Figure 4”. 3.4. Effect of Reaction Time and Temperature To investigate the influence of reaction time and tem- perature, a methanol volume of 80 ml was used since it was found (Section 3.2.) that no appreciable differences in the equilibrium FAME conversion were obtained with the use of higher alcohol volumes. Reactions were car- ried out at different temperatures of 27˚C up to 65˚C as shown in “Table 4”, using methanol-to-oil molar ratio of 3714:1, catalyst concentration of 100% (wt. /wt. oil) and constant stirring rate of 650 rpm. The progress of the microalgae oil to biodiesel conver- sion process is shown in “Figure 5” at different tem- perature levels, using the measured weight of glycerol as a conversion indicator for the yielded FAME. For the samples investigated at room temperature (no process heating), asymptotic FAME conversion value was not reached within the time boundaries of this study. When the in situ transesterification process was carried out at 65˚C under the same reaction conditions, according to “Table 4”, higher equilibrium conversion levels of FAME of 43.1% and 76.22% were attained after reaction time of 2 h and 4 h respectively. Copyright © 2013 SciRes. JSBS 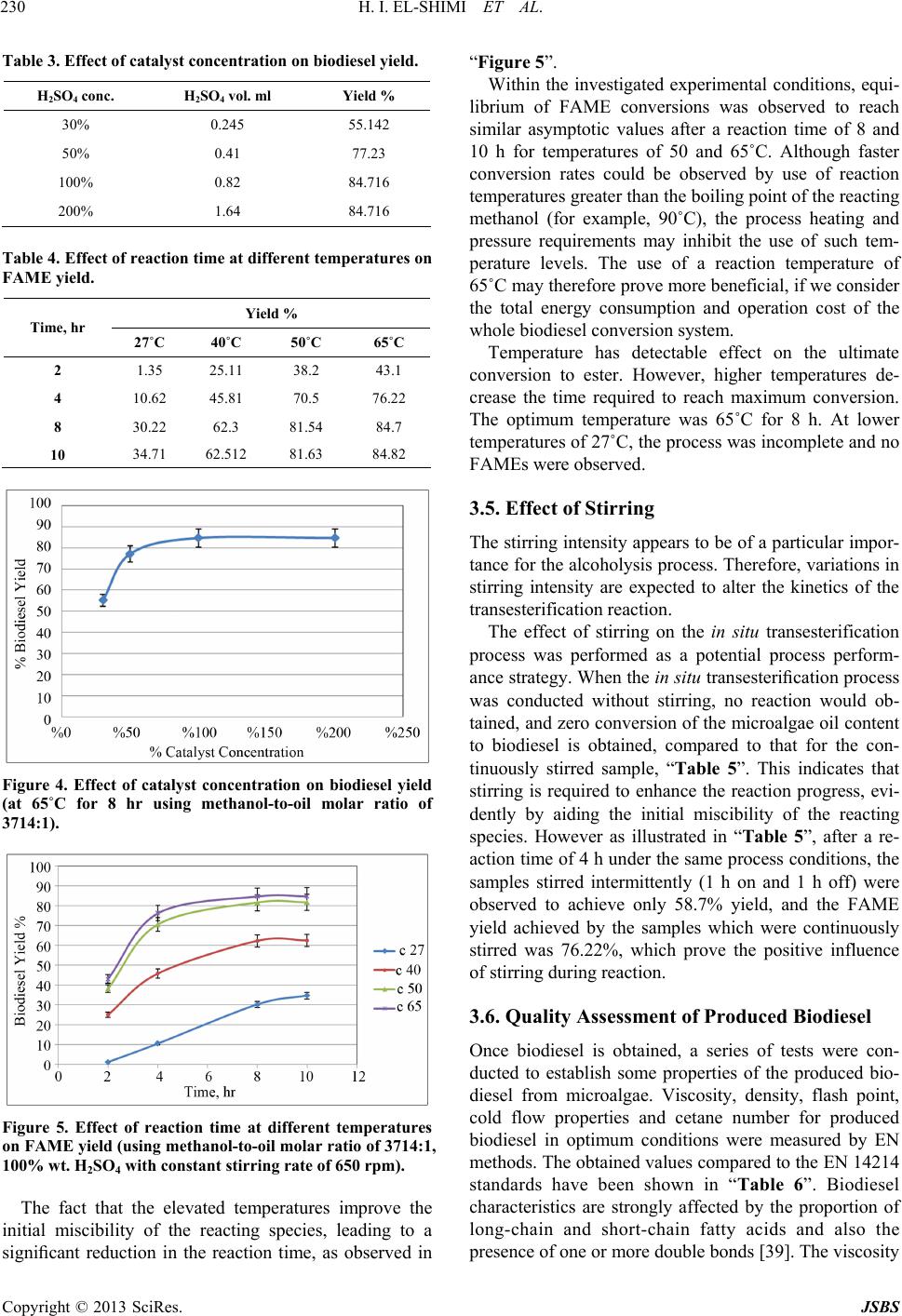 H. I. EL-SHIMI ET AL. 230 Table 3. Effect of catalyst concentration on biodiesel yield. H2SO4 conc. H2SO4 vol. ml Yield % 30% 0.245 55.142 50% 0.41 77.23 100% 0.82 84.716 200% 1.64 84.716 Table 4. Effect of reaction time at different temperatures on FAME yield. Yield % Time, hr 27˚C 40˚C 50˚C 65˚C 2 1.35 25.11 38.2 43.1 4 10.62 45.81 70.5 76.22 8 30.22 62.3 81.54 84.7 10 34.71 62.512 81.63 84.82 Figure 4. Effect of catalyst concentration on biodiesel yield (at 65˚C for 8 hr using methanol-to-oil molar ratio of 3714:1). Figure 5. Effect of reaction time at different temperatures on FAME yield (using methanol-to-oil molar ratio of 3714:1, 100% wt. H2SO4 with constant stirring rate of 650 rpm). The fact that the elevated temperatures improve the initial miscibility of the reacting species, leading to a significant reduction in the reaction time, as observed in “Figure 5”. Within the investigated experimental conditions, equi- librium of FAME conversions was observed to reach similar asymptotic values after a reaction time of 8 and 10 h for temperatures of 50 and 65˚C. Although faster conversion rates could be observed by use of reaction temperatures greater than the boiling point of the reacting methanol (for example, 90˚C), the process heating and pressure requirements may inhibit the use of such tem- perature levels. The use of a reaction temperature of 65˚C may therefore prove more beneficial, if we consider the total energy consumption and operation cost of the whole biodiesel conversion system. Temperature has detectable effect on the ultimate conversion to ester. However, higher temperatures de- crease the time required to reach maximum conversion. The optimum temperature was 65˚C for 8 h. At lower temperatures of 27˚C, the process was incomplete and no FAMEs were observed. 3.5. Effect of Stirring The stirring intensity appears to be of a particular impor- tance for the alcoholysis process. Therefore, variations in stirring intensity are expected to alter the kinetics of the transesterification reaction. The effect of stirring on the in situ transesterification process was performed as a potential process perform- ance strategy. When the in situ transesterification process was conducted without stirring, no reaction would ob- tained, and zero conversion of the microalgae oil content to biodiesel is obtained, compared to that for the con- tinuously stirred sample, “Table 5”. This indicates that stirring is required to enhance the reaction progress, evi- dently by aiding the initial miscibility of the reacting species. However as illustrated in “Table 5”, after a re- action time of 4 h under the same process conditions, the samples stirred intermittently (1 h on and 1 h off) were observed to achieve only 58.7% yield, and the FAME yield achieved by the samples which were continuously stirred was 76.22%, which prove the positive influence of stirring during reaction. 3.6. Quality Assessment of Produced Biodiesel Once biodiesel is obtained, a series of tests were con- ducted to establish some properties of the produced bio- diesel from microalgae. Viscosity, density, flash point, cold flow properties and cetane number for produced biodiesel in optimum conditions were measured by EN methods. The obtained values compared to the EN 14214 standards have been shown in “Table 6”. Biodiesel characteristics are strongly affected by the proportion of long-chain and short-chain fatty acids and also the presence of one or more double bonds [39]. The viscosity Copyright © 2013 SciRes. JSBS  H. I. EL-SHIMI ET AL. 231 Table 5. Effect of stirring on the in situ transesterification of microalgae lipids (at 65˚C for 4 hr with a H2SO4 concentra- tion of 100% and methanol volume of 80 ml). Stirring treatment Yield % No stirring 0 Intermittently stirring (1 h off, 1 h on) 58.7 Continuously stirring 76.22 Table 6. Biodiesel properties: methods, limits and values. Property Test - Method Lower limit Upper limitValue Viscosity (mm2/s @ 40˚C) EN ISO 3104 3.5 5.0 4.8 Density (kg/m3 @ 15˚C) EN ISO 3675 860 900 886 Flash Point (˚C) ISO CD 3679e >101 - 172 Cloud Point (˚C) - - - 5 Pour Point (˚C) - - - −1 Cetane number EN ISO 5165 51 - 60.73 is one of the most important properties which affects the fuel injection equipment and applied to determine the conversion of microalgae oil to methyl-esters; since the viscosity of produced biodiesel from microalgae was determined to be 4.8 mm2/s and this value is much lower than that of the crude microalgae oil which was 58 mm2/s. Viscosity and density measurements of produced bio- diesel are compliance with EN 14214 standards as shown in “Table 6”, which confirm the biodiesel quality. The flash point of produced microalgae biodiesel was 172˚C which exceeds the minimum flash point set by EN 14214 standards. This value is high as compared with about 160˚C for jatropha biodiesel [40] and much higher than that of 58˚C for petrol-diesel, which makes the biodiesel, and its blends safer fuels to handle and store near or with potential ignition sources. The cloud and pour points of the produced biodiesel are 5 and -1 respectively. These values are not better than that given in literatures for sunflower biodiesel (2 and -3 respectively) and that for biodiesel from waste vegetable oils (3 and -6 respectively); because the microalgae methyl esters are mainly composed of saturated fatty acids as illustrated in “Table 7”, and as stated by Alan Scragg [41], the unsaturated fatty acids give better cold flow properties than saturated fatty acids. Cetane number of biodiesel is generally higher than conventional diesel because it has longer fatty acids carbon chains and satu- rated molecules. Microalgae biodiesel cetane number was predicted from “Equation (4)” which conducted by A.I. Bamgboye et al. [42]. Table 7. Fatty acids composition of microalgae biodiesel. Fatty Acid % Composition (by wt.) (X1) Lauric (C12:0) 0.7 (X2) Mystic (C14:0) 20.9 (X3) Palmitic (C16:0) 48.35 (X4) Stearic (C18:0) 2.02 (X5) Palmitoleic (C16:1) 2.66 (X6) Oleic (C18:1) 2.41 (X7) Linoleic (C18:2) 5.37 (X8) Linoleuic(C18:3) 7.84 234 567 CN61.1 0.088X0.133X0.152X 0.101X0.039X 0.243X0.395X8 (4) where Xi, i= 1,2, …. 8, is the biodiesel fatty acids frac- tion. This formula gives approximate value for the biodiesel cetane number as a function of biodiesel fatty acids composition with accuracy of 90%. Fatty acids composi- tion of the produced biodiesel is shown in “Table 7”. Cetane number of microalgae biodiesel was calculated to be 60.73, which is higher compared to 45.8 for rapeseed biodiesel [43] and also better than 38 for jatropha bio- diesel [44]. The investigation of biodiesel cetane number is of high importance; since inadequate cetane numbers result in poor ignition quality, delay and excessive engine knock. 4. Conclusion This study investigated the effect of the most important reaction variables on the conversion of Spirulina-Plat- ensis microalgae oil to biodiesel using the acid-catalysed in situ transesterification process. Results show that 100% H2SO4 concentration (wt./wt oil) at 65˚C for 8 hr is the optimum investigated conditions using 15 g of bio- mass and 80 ml of the reacting methanol. The average molecular weight of the Spirulina-Platensis oil was cal- culated to be 845.19 g/mol., reduced to be 284 g/mol for the produced FAME. Without stirring, no product will be resulted. The properties of the produced fatty acid methyl esters confirm the EN 14214 standards that make the microalgae biodiesel a substitute fuel for petroleum-die- sel. 5. Acknowledgements The authors gratefully acknowledge financial support for this research by FECU, Faculty of Engineering, Cairo University (Egypt). Copyright © 2013 SciRes. JSBS  H. I. EL-SHIMI ET AL. 232 REFERENCES [1] D. M. Huang, H. N. Zhou and L. Lin, “Biodiesel: An Alternative to Conventional Fuel,” Energy Procedia, Vol. 16, Pt. C, 2012, pp. 1874-1885. doi:10.1016/j.egypro.2012.01.287 [2] A. Demirbas, “Biofuels Securing the Planet’s Future En- ergy Needs,” Energy Conversion and Management, Vol. 50, No. 9, 2009, pp. 2239-2249. doi:10.1016/j.enconman.2009.05.010 [3] V. K. Wermelinger, S. Araujo, S. Hamacher and L. F. Scavarda, “Economic Assessment of Biodiesel Produc- tion from Waste Frying Oils,” Bioresource Technology, Vol. 101, No. 12, 2010, pp. 4415-4422. doi:10.1016/j.biortech.2010.01.101 [4] L. Simasatitkul, R. Gani and A. Arpornwichanopa, “Op- timal Design of Biodiesel Production Process from Waste Cooking Palm Oil,” Procedia Engineering, Vol. 42, 2012, pp. 1292-1301. [5] S. Nagarajan, S. K. Chou, S. Y. Cao, C. Wu and Z. Zhou, “An Updated Comprehensive Techno-Economic Analysis of Algae Biodiesel,” Bioresource Technology, 2012. doi:10.1016/j.biortech.2012.11.108 [6] C.-L. Cheng, P.-Y. Che, B.-Y. Chen, W.-J. Lee, C.-Y. Lin and J.-S. Chang, “Biobutanol Production from Agricul- tural Waste by an Acclimated Mixed Bacterial Micro- flora,” Applied Energy, Vol. 100, 2012, pp. 3-9. doi:10.1016/j.apenergy.2012.05.042 [7] C. S. Jones and S. P. Mayfieldt, “Algae Biofuels: Versa- tility for the Future of Bioenergy,” Current Opinion in Biotechnology, Vol. 23, No. 3, 2012, pp. 346-351. doi:10.1016/j.copbio.2011.10.013 [8] K. Ramezani, S. Rowshanzamir and M. H. Eikani, “Cas- tor oil Transesterification Reaction: A Kinetic Study and Optimization of Parameters,” Energy, Vol. 35, No. 10, 2010, pp. 4142-4148. doi:10.1016/j.energy.2010.06.034 [9] B. B. Venu and V. G. Vaibhav, “Biodiesel Production from Renewable Feed Stocks: Status and Opportunities,” Renewable and Sustainable Energy Reviews, Vol. 16, No. 7, 2012, pp. 4763-4784. doi:10.1016/j.rser.2012.04.010 [10] F. Delrue, P.-A. Setier, C. Sahut, L. Cournac, A. Roubaud, G. Peltier and A.-K. Froment, “An Economic, Sustain- ability, and Energetic Model of Biodiesel Production from Microalgae,” Bioresource Technology, Vol. 111, 2012, pp. 191-200. doi:10.1016/j.biortech.2012.02.020 [11] Y. Chisti, “Biodiesel from Microalgae,” Biotechnology Advances, Vol. 25, No. 3, 2007, pp. 294-306. doi:10.1016/j.biotechadv.2007.02.001 [12] N. Nagle and P. Lemke, “Production of Methyl Ester Fuels from Microalgae,” Applied Biochemistry and Bio- technology, Vol. 24-25, No. 1, 1990, pp. 355-361. doi:10.1007/BF02920259 [13] X. Miao and Q. Wu, “Biodiesel Production from Hetero- trophic Microalgal Oil,” Bioresource Technology, Vol. 97, No. 6, 2006, pp. 841-846. doi:10.1016/j.biortech.2005.04.008 [14] S. A. Abo El-Enin, N. K. Attia, N. N. El-Ibiari, G. I. El- Diwani and K. M. El-Khatib, “In-Situ Transesterification of Rapeseed and Cost Indicators for Biodiesel Produc- tion,” Renewable and Sustainable Energy Reviews, Vol. 18, 2013, pp. 471-477. doi:10.1016/j.rser.2012.10.033 [15] P. Chitra, P. Venkatachalam and A. Sampathrajan, “Op- timisation of Experimental Conditions for Biodiesel Pro- duction from Alkali-Catalysed Transesterification of Jat- ropha curcus Oil,” Energy Sustainable Development, Vol. 9, No. 3, 2005, pp. 13-18. doi:10.1016/S0973-0826(08)60518-9 [16] Y. Zhang, M. A. Dub, D. D. McLean and M. Kates, “Bio- diesel Production from Waste Cooking Oil: 2. Economic Assessment and Sensitivity Analysis,” Bioresource Tech- nology, Vol. 90, No. 3, 2003, pp. 229-240. doi:10.1016/S0960-8524(03)00150-0 [17] S. Gryglewicz, “Rapeseed Oil Methyl Esters Preparation Using Heterogeneous Catalysts,” Bioresource Technology, Vol. 70, No. 3, 1999, pp. 249-253. doi:10.1016/S0960-8524(99)00042-5 [18] S. Furuta, H. Matsuhashi and K. Arata, “Biodiesel Fuel Production with Solid Super Acid Catalysis in fixed Bed Reactor under Atmospheric Pressure,” Catalyst Commu- nication, Vol. 5, No. 12, 2004, pp. 721-723. doi:10.1016/j.catcom.2004.09.001 [19] H. Noureddini, X. Gao and R. S. Philkana, “Immobilized Pseudomonas cepacia Lipase for Biodiesel Fuel Produc- tion from Soybean Oil,” Bioresource Technology, Vol. 96, No. 7, 2005, pp. 769-777. doi:10.1016/j.biortech.2004.05.029 [20] S. Hama, H. Yamaji, M. Kaieda, M. Oda, A. Kondo and H. Fukuda, “Effect of Fatty Acid Membrane Composition on Whole-Cell Biocatalysts for Biodiesel-Fuel Produc- tion,” Biochemical Engineering Journal, Vol. 21, No. 2, 2004, pp. 155-160. doi:10.1016/j.bej.2004.05.009 [21] M. Oda, M. Kaieda, S. Hama, H. Yamaji, A. Kondo and E. Izumoto, “Facilitator Effect of Immobilized Lipase- Producing Rhizopus oryzae Cells on Acyl Migration in Biodiesel-Fuel Production,” Biochemical Engineering Journal, Vol. 23, No. 1, 2004, pp. 45-51. doi:10.1016/j.bej.2004.10.009 [22] W. Du, Y. Xu, D. Liu and J. Zeng, “Comparative Study on Lipase-Catalyzed Transformation of Soybean Oil for Biodiesel Production with Different Acyl Acceptors,” Journal of Molecular Catalysis B: Enzymatic, Vol. 30, No. 3-4, 2004, pp. 125-129. doi:10.1016/j.molcatb.2004.04.004 [23] C. J. Shieh, H. F. Liao and C. C. Lee, “Optimization of Lipase-Catalyzed Biodiesel by Response Surface Meth- odology,” Bioresource Technology, Vol. 88, No. 2, 2003, pp. 103-106. doi:10.1016/S0960-8524(02)00292-4 [24] E. A. Ehimen, Z. F. Sun and C. G. Carrington, “Variables Affecting the in Situ Transesterification of Microalgae Lipids,” El-Sevier Fuel, Vol. 89, No. 3, 2010, pp. 677- 684. doi:10.1016/j.fuel.2009.10.011 [25] S. Al-Zuhair, “Production of Biodiesel: Possibilities and Challenges,” Biofuels Bioprod Biorefining, Vol. 1, No. 1, 2007, pp. 57-66. doi:10.1002/bbb.2 [26] N. Kumar, “Biodiesel Production Technology and Feed- stocks for India,” Expert Lecture at Workshop on Moving Copyright © 2013 SciRes. JSBS  H. I. EL-SHIMI ET AL. Copyright © 2013 SciRes. JSBS 233 toward Sustainable Energy Systems: Exploring Global Pathways to a Common Destination Organized by Uni- versity of Minnesota, Minneapolis, 2006. [27] M. J. Haas, K. M. Scott, W. N. Marmer and T. A. Foglia, “In Situ Alkaline Transesterification: An Effective Method for the Production of Fatty Acid Esters from Vegetable Oils,” Journal of the American Oil Chemist’s Society, Vol. 81, No. 1, 2004, pp. 83-89. doi:10.1007/s11746-004-0861-3 [28] G. Hincapié, F. Mondragón and D. López, “Conventional and in Situ Transesterification of Castor Seed Oil for Bio- diesel Production,” Fuel, Vol. 90, No. 4, 2011, pp. 1618- 1623. doi:10.1016/j.fuel.2011.01.027 [29] R. Halim, M. K. Danquah and P. A. Webley, “Extraction of Oil from Microalgae for Biodiesel Production: A Re- view,” Biotechnology Advances, Vol. 30, No. 3, 2012, pp. 709-732. doi:10.1016/j.biotechadv.2012.01.001 [30] B. D. Wahlen, R. M. Willis and L. C. Seefeldt, “Biodiesel Production by Simultaneous Extraction and Conversion of Total Lipids from Microalgae, Cyanobacteria, and Wild Mixed-Cultures,” Bioresource Technology, Vol. 102, No. 3, 2011, pp. 2724-2730. doi:10.1016/j.biortech.2010.11.026 [31] K. J. Harrington and C. D’Arcy-Evans, “A Comparison of Conventional and in Situ Methods of Transesteri- fication of Seed Oil from a Series of Sunflower Culti- vars,” Journal of the American Oil Chemists’ Society, Vol. 62, No. 6, 1985, pp. 1009-1013. doi:10.1007/BF02935703 [32] S. Siler-Marinkovic and A. Tomasevic, “Transesterifica- tion of Sunflower Oil in Situ,” Fuel, Vol. 77, No. 12, 1998, pp. 1389-1391. doi:10.1016/S0016-2361(98)00028-3 [33] M. R. Andrade and J. A. V. Costa, “Mixotrophic Cultiva- tion of Microalga Spirulina platensis Using Molasses as Organic Substrate,” Biochemical Engineering Laboratory, Department of Chemistry, Federal University Foundation of Rio Grande (FURG), Rio Grande, 2006. [34] L. H. Pelizer, E. D. G. Danesi, C. de O. Rangel, C. E. N. Sassano, J. C. M. Carvalho, S. Sato and I. O. Moraes, “In- fluence of Inoculum Age and Concentration in Spirulina platensis Cultivation,” Departamento de Tecnologia Bio- quımico-Farmaceutica, Faculdade de Ciencias Farmaceu- ticas, Universidade de Sao Paulo, Sao Paulo, 2001. [35] J. Sheng, R. Vannela and B. E. Rittmann, “Evalution of Methods to Extract Andquantify Lipids from Synechocys- tis PCC 6803,” Center for Environmental Biotechnology, The Biodesign Institute, Arizona University, Tucson, 2011. [36] Q. Hu, M. Sommerfeld, E. Jarvis, M. Ghiradi, M. Posewitz, M. Siebert and A. Darzins, “Microalgal Triacy- glycerols as Feedstock for Biofuel Production: Perspec- tives and Advances,” The Plant Journal, Vol. 54, No. 4, 2008, pp. 621-639. doi:10.1111/j.1365-313X.2008.03492.x [37] A. A. Refaat, “Optimization of Biodiesel Production Us- ing Different Techniques,” Master Thesis, Cairo Univer- sity, Cairo, 2009. [38] X. Meng, G. Chen and Y. Wang, “Biodiesel Production from Waste Cooking Oil via Alkali Catalyst and Its En- gine Test,” Fuel Processing Technology, Vol. 89, No. 9, 2008, pp. 851-857. doi:10.1016/j.fuproc.2008.02.006 [39] R. Zanzi, “Biodiesel from Microalgae,” B.Sc. Report Submitted to Chemical Engineering and Technology De- partment, Royal School of Technology, Stockholm, 2010. [40] G. El-Diwani, N. K. Attia and S. I. Hawash, “Develop- ment and Evaluation of Biodiesel Fuel and Byproducts from Jatropha Oil,” International Journal of Environ- mental Science and Technology, Vol. 6, No. 2, 2009, pp. 219-224. [41] A. Scragg, “Biofuels: Production, Application and De- velopment,” 2009. [42] A. I. Bamgboye and A. C. Hansen, “Prediction of Cetane Number of Biodiesel Fuel from the Fatty Acid Methyl Ester (FAME) Composition,” International Agrophysics, Vol. 22, No. 1, 2008, pp. 21-29. [43] J. M. Encinar, J. F. Gonzalez, A. Pardal and G. Martinez, “Transesterification of Rapeseed Oil with Methanol in the Presence of Various Co-Solvents,” Proceedings Venice 2010, Third International Symposium on Energy from Biomass and WasteVenice, Italy, 8-11 November 2010. [44] K. Sivaramakrishnan and P. Ravikumar, “Determination of Cetane Number of Biodiesel and It’s Influence on Phy- sical Properties,” ARPN Journal of Engineering and Ap- plied Sciences, Vol. 7, No. 2, 2012, pp. 205-211.
|