 International Journal of Clinical Medicine, 2013, 4, 409-416 http://dx.doi.org/10.4236/ijcm.2013.49074 Published Online September 2013 (http://www.scirp.org/journal/ijcm) 409 Intraoperative Glycaemia Following Paracetamol with and without Glucose: A Randomized-Controlled Trial Ricardo Mota Pereira1*, Fátima Gonçalves1, João Costa2,3,4, Filomena Couto5, Carolina Sá1, Isabel Neves1, Lucindo Ormonde1,4 1Department of Anaesthesiology, Santa Maria University Hospital, Lisbon, Portugal; 2Laboratory of Clinical Pharmacology and Therapeutics, Faculty of Medicine, University of Lisbon, Lisbon, Portugal; 3Evidence-Based Medicine Centre, Faculty of Medicine, University of Lisbon, Lisbon, Portugal; 4Instituto de Medicina Molecular (IMM), Faculty of Medicine, University of Lisbon, Lisbon, Portugal; 5Department of Gynaecology, Santa Maria University Hospital, Lisbon, Portugal. Email: *nricardopereira@gmail.com Received August 3rd, 2013; revised August 31st, 2013; accepted September 6th, 2013 Copyright © 2013 Ricardo Mota Pereira et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Hyperglycaemia is conversely a risk factor for perioperative complications. We are currently using a ge- neric 3.3 g glucose containing formula of intravenous 1000 mg paracetamol for perioperative analgesia. Our main goal was to compare the trends of glycaemic values after administration of a generic 3.3 g glucose containing formula with a non-glucose containing branded formula of intravenous 1000 mg paracetamol. Methods: A exploratory proof-of-con- cept randomized clinical trial was conducted with 150 patients scheduled for elective gynaecologic. Patients were ran- domly assigned into three groups: control group (saline); active-control group: intraoperative administration of a branded non-glucose containing 1000 mg paracetamol formula; experimental group: intraoperative administration of a generic 3.3 g glucose containing 1000 mg paracetamol formula. The primary outcome was mean change from baseline in glaucoma. In case significant differences were found, the following secondary outcomes were explored: the proportion of patients with high glycaemia values (>150 mg/dL) and the proportion of patients with negative glycaemic variation. Results: Mean glycaemia change was higher after generic 3.3 g glucose containing paracetamol formula both in comparison to placebo (16.3 mg/dL [95% CI: 6.1 to 26.6]) and active-control (19.1 mg/dL [8.2 to 30.0] groups. Similar results were found in the intention-to-treat analysis. In only the experimental group, patients had high glycaemic values (11.3%). Conclusions: This study showed that in non-diabetic, under non-cardiac surgery, administration of a generic glucose-containing formula of intravenous 1000 mg paracetamol was associated with poorer glycaemic control. These results raise the question of a possible increased risk among these patients. Further studies using diabetic patients are recommended. Keywords: Paracetamol; Hyperglycaemia; Gyneacologic Surgical Procedures; Randomized Controlled Trial 1. Introduction It has been demonstrated that inadequate glycaemic con- trol in surgical patients increases perioperative morbidity and mortality [1]. While most studies focus on neurosur- gical [2], cardiac [3-5] and critical care patients [6], and therefore further investigation is required, some of the outcome key findings are most probably applicable to general surgical patients [5]. Acute hyperglycaemia is associated with several dele- terious effects such as suppressed immune function, in- creased systemic vascular resistance, dehydration, elec- trolyte and acid-base imbalance and central nervous sys- tem dysfunction [7]. Although patients with diabetes mellitus (DM) are at higher risk for perioperative com- plications [8], the occurrence of intraoperative acute hy- perglycaemia in non-DM patients is also considered [9, 10] to be a strong and independent predictor of poorer outcome (sepsis, pneumonia, surgical wound infection). Furthermore, individuals with previous unknown hyper- glycaemia are at even higher risk than those with pre- diagnosed DM [2]. Recent studies found that 21% to 34% of patients who underwent surgery had uncontrolled blood glucose level (Blood Glycaemia (BG) > 150 mg/dl), particularly in the immediate postoperative period (< 72 hours) [11]. Many of these patients may miss a DM diagnosis as only two *Corresponding author. Copyright © 2013 SciRes. IJCM  Intraoperative Glycaemia Following Paracetamol with and without Glucose: A Randomized-Controlled Trial 410 thirds of those have a pre-established DM diagnosis [12]. Paracetamol (acetaminophen) is one of the safest and more cost-effective [13] non-opioid analgesic when ad- ministered in analgesic doses. Paracetamol is considered an atypical nonsteroidal anti-inflammatory drug (NSAID) [14], given the nonspecific and weak inhibition of COX- 3 [15], with central and peripheral effects. In our hospital, it is currently in use a 3 g glucose con- taining formula of intravenous 1000 mg paracetamol for perioperative analgesia. To evaluate what is the impact of this glucose containing paracetamol formula in the in- traoperative glycaemic control among non-diabetic gen- eral surgical patients, we conducted an exploratory proof- of-concept randomized clinical trial. 2. Methods All patients provided their written informed consent for participating in this study, which was approved by the Ethics Committee of our Hospital (Santa Maria Univer- sity Hospital, Lisbon). This was an academic trial with- out any direct or indirect funding. 2.1. Study Population This study was conducted in the gynaecologic surgery department of Santa Maria University Hospital (Lisbon, Portugal). Non-diabetic female patients scheduled for elective gynaecologic procedure were consecutively re- cruited. Inclusion criteria were: 1) written informed con- sent; 2) age between 18 and 80 years-old; 3) body mass index (BMI) < 30 Kg/m2; 4) fasting glycaemic values > 60 mg/dL and <126 mg/dL; 5) American Society of An- aesthesiology (ASA) classification ≤ 2; and 6) 8-hour fasting period. Patients with a diagnosis of DM or glu- cose impairment were excluded. 2.2. Study Design Patients were randomly assigned to one of three groups: 1) Placebo-control group (saline); 2) Paracetamol 1000 mg in a non-glucose containing formula (active-control group), and; 3) Paracetamol 1000 mg in a 3 g glucose containing formula (experimental group). All intervena- tions were delivered in the last third of the surgical pro- cedure (15 minutes infusion). Random sequence generation was independently done by the principal investigator (RMP) that did not partici- pate in the patients’ recruitment or evaluation. Opaque sealed envelopes in a closed box were used to retain the random codes and successively replaced until 150 con- secutive patients were obtained. Hence, each new patient had an equal chance of being allocated to one of the three treatment groups. Allocation concealment was achieved by making patients and assessment investigator (FC) blind to treatment assignment. The investigators (FG, FC) who administered the treatment were the only subjects that were not blind to treatment assignment, however they didn’t participated further in the study. Primary outcome was defined as the mean change from baseline in blood glucose. If significant differences were found between groups, the following secondary outcomes were investigated: proportion of patients with high glycaemia values (>150 mg/dL) in the second meas- urement and proportion of patients with negative varia- tion in glycaemia between the two measurements. The same investigator (FC) assessed all patients and perform- ed all measurements. Fingerprick capillary glucose was determined at the beginning of the surgery (baseline gly- caemia) and 10 minutes after treatment infusion ending (post-interventional glycaemia), using the Precision Xceed Pro point-of-care glucometer (Abbott), which was calibrated daily. All test-trips used were taken from the same lot. For the capillary glucose measurement, the se- cond finger from the opposite arm in which the drug was administrated, was chosen. In none of the glycemic measurements was found an invalid value, making un- necessary a second attempt. 2.3. Anaesthesia Protocol After an 8-hour preoperative fasting, all patients were pre-medicated with midazolam 0.05 mg/kg. General an- aesthesia was induced with single doses of fentanyl (5 ug/kg) and propofol (2 mg/kg) given slowly as bolus injections. Traqueal intubation was facilitated with ro- curonium (0.4 mg/kg) and anaesthesia maintained with sevoflurane vaporized in air and oxygen mixture (FiO2 0.4) titrated to achieve stable hemodynamics. 2.4. Statistical Analysis and Data Synthesis This was an exploratory trial and we planned to enrol 40 patients per treatment group. Assuming that the response within each subject group is normally distributed with standard deviation (SD) of 20 mg/dL, this sample size will allow detecting a true difference in the mean re- sponse of treatment groups (experimental, placebo-con- trol and active-control) of 15 mg/dL with a probability (power) of 82% (analysis of variance with Bonferroni correction). We considered that such difference is of po- tential clinical significance. The Type I error probability associated with this test of the null hypothesis that the population means of the experimental and controls groups were equal was 0.05. The mean change from baseline in glycaemia values between groups (primary outcome: dependent variable) was compared using a full factorial generalized linear model (GLM) with treatment group as main effect and including terms for age, fasting period (Nulla per os: NTO), surgery duration and baseline glycaemia. Interac- Copyright © 2013 SciRes. IJCM 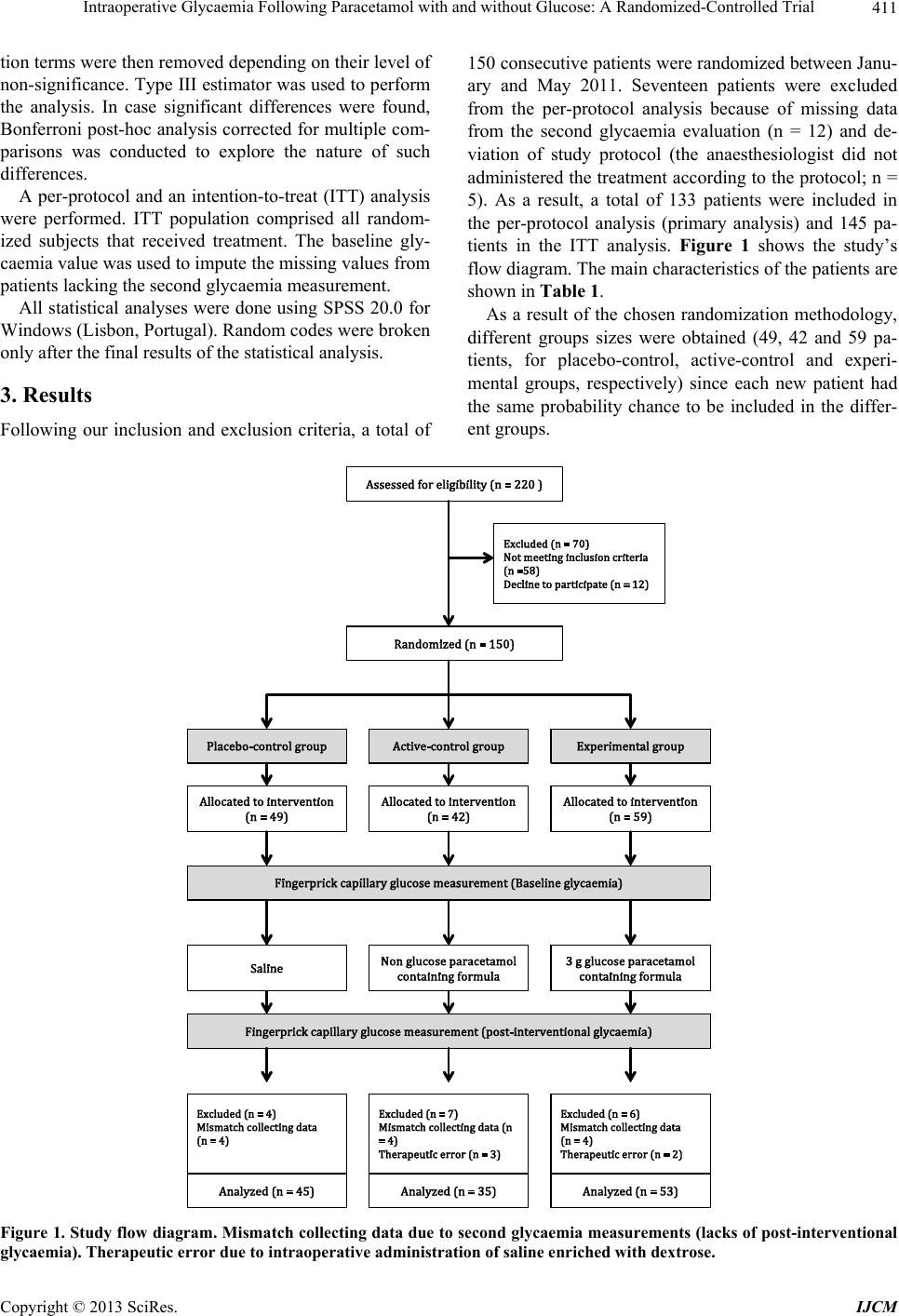 Intraoperative Glycaemia Following Paracetamol with and without Glucose: A Randomized-Controlled Trial Copyright © 2013 SciRes. IJCM 411 tion terms were then removed depending on their level of non-significance. Type III estimator was used to perform the analysis. In case significant differences were found, Bonferroni post-hoc analysis corrected for multiple com- parisons was conducted to explore the nature of such differences. 150 consecutive patients were randomized between Janu- ary and May 2011. Seventeen patients were excluded from the per-protocol analysis because of missing data from the second glycaemia evaluation (n = 12) and de- viation of study protocol (the anaesthesiologist did not administered the treatment according to the protocol; n = 5). As a result, a total of 133 patients were included in the per-protocol analysis (primary analysis) and 145 pa- tients in the ITT analysis. Figure 1 shows the study’s flow diagram. The main characteristics of the patients are shown in Table 1. A per-protocol and an intention-to-treat (ITT) analysis were performed. ITT population comprised all random- ized subjects that received treatment. The baseline gly- caemia value was used to impute the missing values from patients lacking the second glycaemia measurement. All statistical analyses were done using SPSS 20.0 for Windows (Lisbon, Portugal). Random codes were broken only after the final results of the statistical analysis. As a result of the chosen randomization methodology, different groups sizes were obtained (49, 42 and 59 pa- tients, for placebo-control, active-control and experi- mental groups, respectively) since each new patient had the same probability chance to be included in the differ- ent groups. 3. Results Following our inclusion and exclusion criteria, a total of Figure 1. Study flow diagram. Mismatch collecting data due to second glycaemia measurements (lacks of post-interventional glycaemia). Therapeutic error due to intraoperative administration of saline enriched with dextrose. 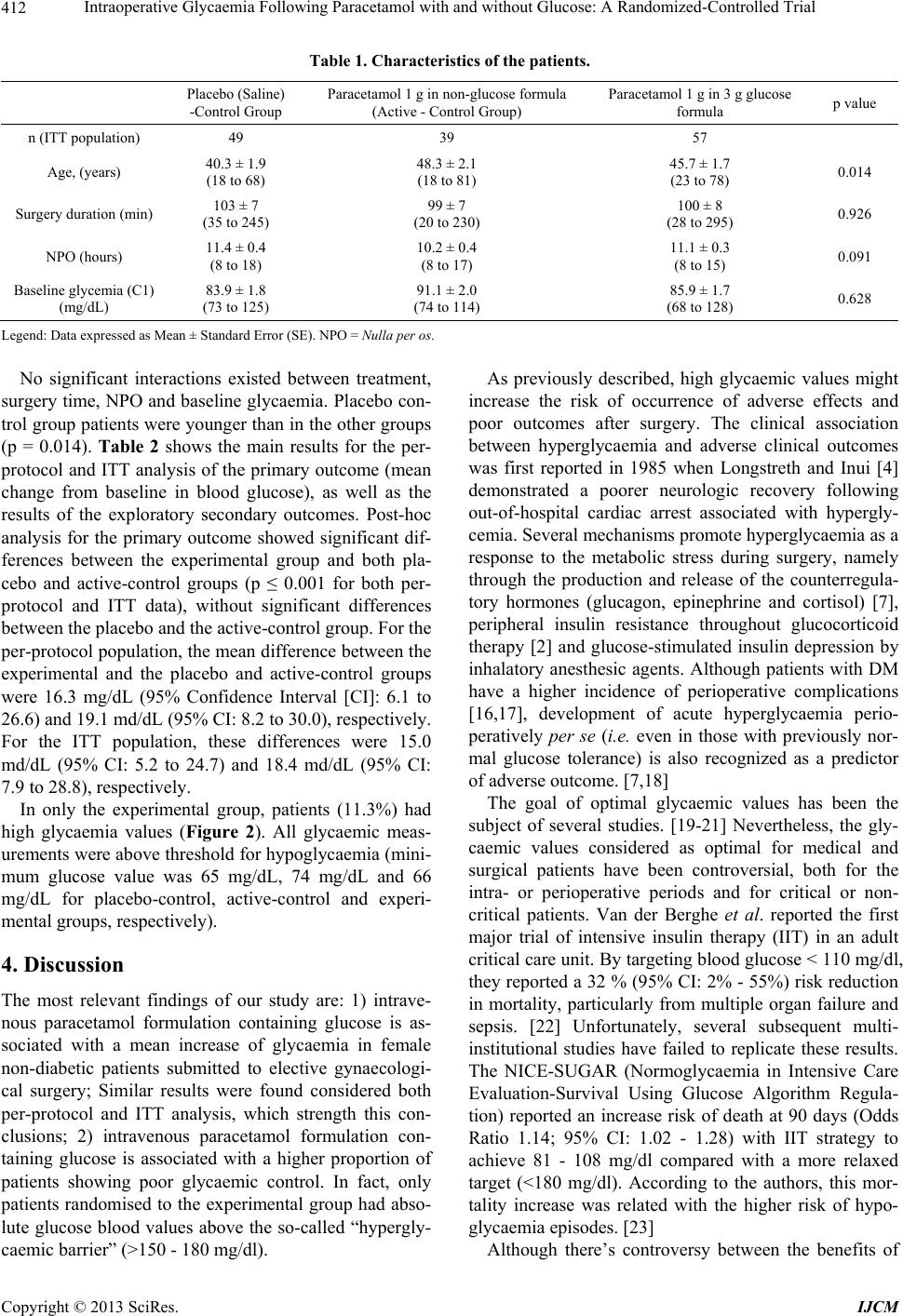 Intraoperative Glycaemia Following Paracetamol with and without Glucose: A Randomized-Controlled Trial 412 Table 1. Characteristics of the patients. Placebo (Saline) -Control Group Paracetamol 1 g in non-glucose formula (Active - Control Group) Paracetamol 1 g in 3 g glucose formula p value n (ITT population) 49 39 57 Age, (years) 40.3 ± 1.9 (18 to 68) 48.3 ± 2.1 (18 to 81) 45.7 ± 1.7 (23 to 78) 0.014 Surgery duration (min) 103 ± 7 (35 to 245) 99 ± 7 (20 to 230) 100 ± 8 (28 to 295) 0.926 NPO (hours) 11.4 ± 0.4 (8 to 18) 10.2 ± 0.4 (8 to 17) 11.1 ± 0.3 (8 to 15) 0.091 Baseline glycemia (C1) (mg/dL) 83.9 ± 1.8 (73 to 125) 91.1 ± 2.0 (74 to 114) 85.9 ± 1.7 (68 to 128) 0.628 Legend: Data expressed as Mean ± Standard Error (SE). NPO = Nulla pe r os. No significant interactions existed between treatment, surgery time, NPO and baseline glycaemia. Placebo con- trol group patients were younger than in the other groups (p = 0.014). Table 2 shows the main results for the per- protocol and ITT analysis of the primary outcome (mean change from baseline in blood glucose), as well as the results of the exploratory secondary outcomes. Post-hoc analysis for the primary outcome showed significant dif- ferences between the experimental group and both pla- cebo and active-control groups (p ≤ 0.001 for both per- protocol and ITT data), without significant differences between the placebo and the active-control group. For the per-protocol population, the mean difference between the experimental and the placebo and active-control groups were 16.3 mg/dL (95% Confidence Interval [CI]: 6.1 to 26.6) and 19.1 md/dL (95% CI: 8.2 to 30.0), respectively. For the ITT population, these differences were 15.0 md/dL (95% CI: 5.2 to 24.7) and 18.4 md/dL (95% CI: 7.9 to 28.8), respectively. In only the experimental group, patients (11.3%) had high glycaemia values (Figure 2). All glycaemic meas- urements were above threshold for hypoglycaemia (mini- mum glucose value was 65 mg/dL, 74 mg/dL and 66 mg/dL for placebo-control, active-control and experi- mental groups, respectively). 4. Discussion The most relevant findings of our study are: 1) intrave- nous paracetamol formulation containing glucose is as- sociated with a mean increase of glycaemia in female non-diabetic patients submitted to elective gynaecologi- cal surgery; Similar results were found considered both per-protocol and ITT analysis, which strength this con- clusions; 2) intravenous paracetamol formulation con- taining glucose is associated with a higher proportion of patients showing poor glycaemic control. In fact, only patients randomised to the experimental group had abso- lute glucose blood values above the so-called “hypergly- caemic barrier” (>150 - 180 mg/dl). As previously described, high glycaemic values might increase the risk of occurrence of adverse effects and poor outcomes after surgery. The clinical association between hyperglycaemia and adverse clinical outcomes was first reported in 1985 when Longstreth and Inui [4] demonstrated a poorer neurologic recovery following out-of-hospital cardiac arrest associated with hypergly- cemia. Several mechanisms promote hyperglycaemia as a response to the metabolic stress during surgery, namely through the production and release of the counterregula- tory hormones (glucagon, epinephrine and cortisol) [7], peripheral insulin resistance throughout glucocorticoid therapy [2] and glucose-stimulated insulin depression by inhalatory anesthesic agents. Although patients with DM have a higher incidence of perioperative complications [16,17], development of acute hyperglycaemia perio- peratively per se (i.e. even in those with previously nor- mal glucose tolerance) is also recognized as a predictor of adverse outcome. [7,18] The goal of optimal glycaemic values has been the subject of several studies. [19-21] Nevertheless, the gly- caemic values considered as optimal for medical and surgical patients have been controversial, both for the intra- or perioperative periods and for critical or non- critical patients. Van der Berghe et al. reported the first major trial of intensive insulin therapy (IIT) in an adult critical care unit. By targeting blood glucose < 110 mg/dl, they reported a 32 % (95% CI: 2% - 55%) risk reduction in mortality, particularly from multiple organ failure and sepsis. [22] Unfortunately, several subsequent multi- institutional studies have failed to replicate these results. The NICE-SUGAR (Normoglycaemia in Intensive Care Evaluation-Survival Using Glucose Algorithm Regula- tion) reported an increase risk of death at 90 days (Odds Ratio 1.14; 95% CI: 1.02 - 1.28) with IIT strategy to achieve 81 - 108 mg/dl compared with a more relaxed target (<180 mg/dl). According to the authors, this mor- tality increase was related with the higher risk of hypo- glycaemia episodes. [23] Although there’s controver y between the benefits of s Copyright © 2013 SciRes. IJCM 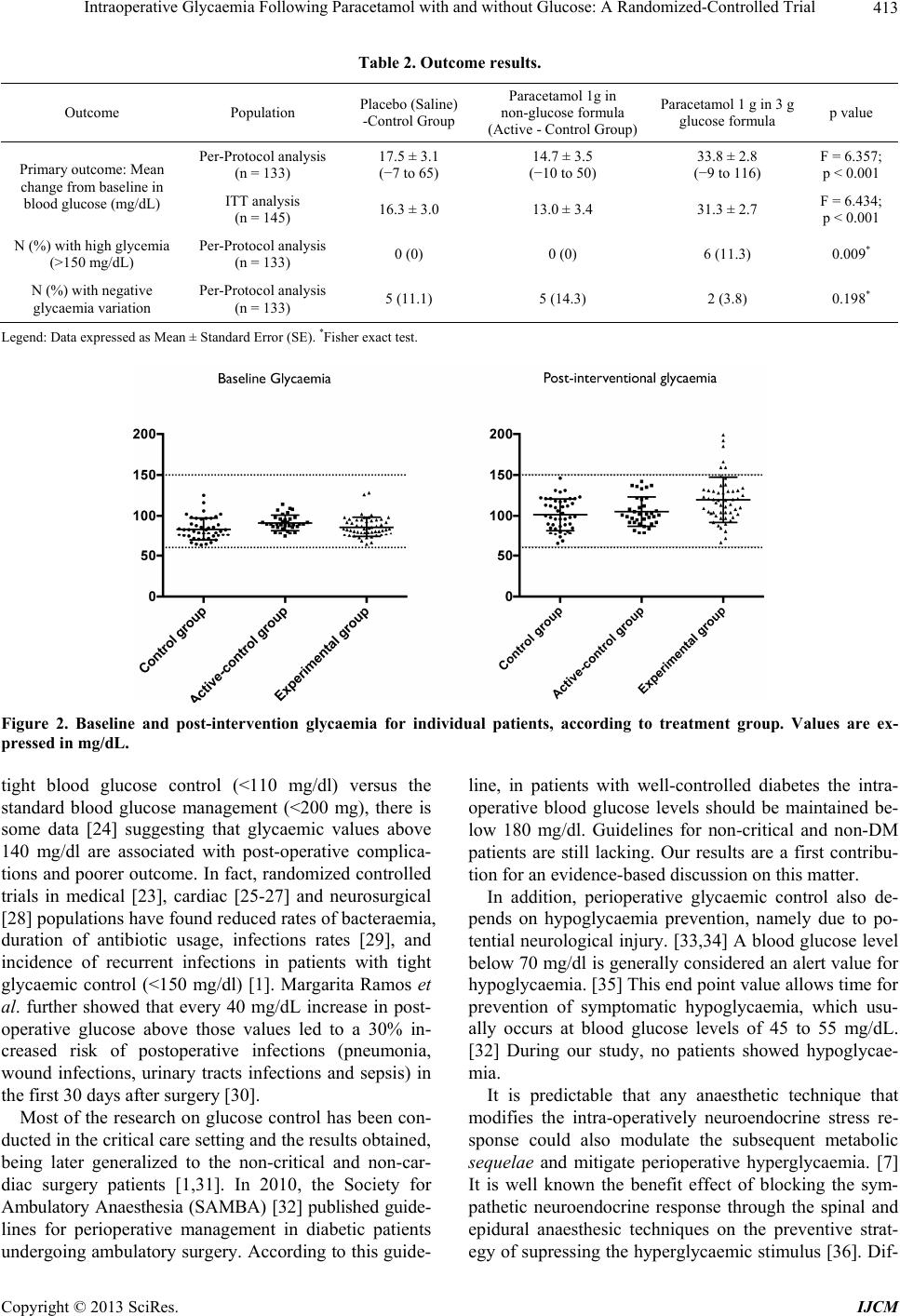 Intraoperative Glycaemia Following Paracetamol with and without Glucose: A Randomized-Controlled Trial 413 Table 2. Outcome results. Outcome Population Placebo (Saline) -Control Group Paracetamol 1g in non-glucose formula (Active - Control Group) Paracetamol 1 g in 3 g glucose formula p value Per-Protocol analysis (n = 133) 17.5 ± 3.1 (−7 to 65) 14.7 ± 3.5 (−10 to 50) 33.8 ± 2.8 (−9 to 116) F = 6.357; p < 0.001 Primary outcome: Mean change from baseline in blood glucose (mg/dL) ITT analysis (n = 145) 16.3 ± 3.0 13.0 ± 3.4 31.3 ± 2.7 F = 6.434; p < 0.001 N (%) with high glycemia (>150 mg/dL) Per-Protocol analysis (n = 133) 0 (0) 0 (0) 6 (11.3) 0.009* N (%) with negative glycaemia variation Per-Protocol analysis (n = 133) 5 (11.1) 5 (14.3) 2 (3.8) 0.198* Legend: Data expressed as Mean ± Standard Error (SE). *Fisher exact test. Figure 2. Baseline and post-intervention glycaemia for individual patients, according to treatment group. Values are ex- pressed in mg/dL. tight blood glucose control (<110 mg/dl) versus the standard blood glucose management (<200 mg), there is some data [24] suggesting that glycaemic values above 140 mg/dl are associated with post-operative complica- tions and poorer outcome. In fact, randomized controlled trials in medical [23], cardiac [25-27] and neurosurgical [28] populations have found reduced rates of bacteraemia, duration of antibiotic usage, infections rates [29], and incidence of recurrent infections in patients with tight glycaemic control (<150 mg/dl) [1]. Margarita Ramos et al. further showed that every 40 mg/dL increase in post- operative glucose above those values led to a 30% in- creased risk of postoperative infections (pneumonia, wound infections, urinary tracts infections and sepsis) in the first 30 days after surgery [30]. Most of the research on glucose control has been con- ducted in the critical care setting and the results obtained, being later generalized to the non-critical and non-car- diac surgery patients [1,31]. In 2010, the Society for Ambulatory Anaesthesia (SAMBA) [32] published guide- lines for perioperative management in diabetic patients undergoing ambulatory surgery. According to this guide- line, in patients with well-controlled diabetes the intra- operative blood glucose levels should be maintained be- low 180 mg/dl. Guidelines for non-critical and non-DM patients are still lacking. Our results are a first contribu- tion for an evidence-based discussion on this matter. In addition, perioperative glycaemic control also de- pends on hypoglycaemia prevention, namely due to po- tential neurological injury. [33,34] A blood glucose level below 70 mg/dl is generally considered an alert value for hypoglycaemia. [35] This end point value allows time for prevention of symptomatic hypoglycaemia, which usu- ally occurs at blood glucose levels of 45 to 55 mg/dL. [32] During our study, no patients showed hypoglycae- mia. It is predictable that any anaesthetic technique that modifies the intra-operatively neuroendocrine stress re- sponse could also modulate the subsequent metabolic sequelae and mitigate perioperative hyperglycaemia. [7] It is well known the benefit effect of blocking the sym- pathetic neuroendocrine response through the spinal and epidural anaesthesic techniques on the preventive strat- egy of supressing the hyperglycaemic stimulus [36]. Dif- Copyright © 2013 SciRes. IJCM  Intraoperative Glycaemia Following Paracetamol with and without Glucose: A Randomized-Controlled Trial 414 ferently, propofol and opioids do not have a major effect on glucose metabolism. [2] In this study, all patients were submitted to surgery under balanced general anaes- thesia and with the same anaesthetic protocol, according to patients’ weight. Therefore, our study is not likely biased due to confounding anaesthesic variables. A variety of measurement techniques are currently in use. Arterial samples are considered more accurate [37]. It is known that capillary testing should be avoided in patients with haematocrit levels <25% or >60%, in shock, with severe dehydration and when vasoactive agents such as norepinephrine are given [38-40]. However, for most surgical patients, phlebotomy or arterial access is not routine [41]. In hemodynamically stable patients the cap- illary glucose meter correlates well with laboratory ref- erence values [7] and is strongly correlated with arterial samples [41]. Also, the laboratory plasma values gener- ally provide little additional information for non-ICU patients and typically lower the mean glucose [42]. In this study, all patients were hemodynamically stable and non-critically ill, submitted to minor/moderate gynaeco- logic procedures. Therefore, we think that the glycaemia measurement technique used in our study does not rep- resent a major limitation. Furthermore, to minimize bias, the same investigator using the same capillary glucose meter and technique, performed all measurements. In our hospital, paracetamol is included in the analge- sic strategy for all patients (medical and surgical). Sev- eral peripheral and central mechanisms of action have been suggested to explain the paracetamol analgesic properties including selective inhibition of cyclooxy- genase activity in the CNS, spinal interaction with 5-HT3 receptors [14], inhibition of neurons excited by substance P and activation of suprasegmental descending inhibitory pathways [15]. Due to the analgesic properties, parace- tamol plays a key role in suppressing the surgery-induced pain adrenergic stimulation, and this suppression could, in theory, blunt the adrenergic hyperglycaemic response. In our opinion, this is the most plausible explanation for the findings in the paracetamol (without glucose) group, which had the lowest increase in glycaemia. Finally, as with most studies, this also presented some limitations. First, this was an exploratory trial, which aimed to address the question if paracetamol with glu- cose is associated with an increased risk of poorer gly- caemic control. We have not evaluated the clinical con- sequences of this poorer glycaemic control. Second, sample size was relatively small and we included only female patients undergoing minor to moderate gynaeco- logic procedures. This precludes the external validity of the findings to other non-diabetic populations and gender. Third, female patients underwent different types of sur- geries. We have not performed post-hoc subgroup analy- sis because of low power. The possibility exists that the results could also differ according to the type of surgery. Fourth, the paracetamol brand used in the experimental (Paracetamol-APS®) and active-control (Perfalgan®) groups were different. Although these are thought to be equiva- lent, we cannot rule out bias emerging from this differ- ence. 5. Conclusion In conclusion, our results strongly suggest that admini- stration of a glucose containing formula of paracetamol may increase the risk of perioperative hyperglycemia in non-diabetic patients submitted to non-cardiac surgery. However, this was an exploratory “proof-of-concept” trial and, although the results were robust, conclusions and implications for practice should be thought with cau- tion. Future clinical research should address the postop- erative outcomes and potential consequences of this poorer glycaemic control. 6. Acknowledgements Specific author contributions: RMP contributed to the concept and design, data analysis, and interpretation of the data; wrote the first draft of the manuscript; critically revised the manuscript; and gave final approval of the submitted manuscript. FG contributed to the concept, design, and data acquisition; critically revised the manu- script; and gave final approval of the submitted manu- script. JC contributed to data analysis, and interpretation of the data; critically revised the manuscript; and gave final approval of the submitted manuscript. IN made con- tribution in the concept and design; and gave final ap- proval of the submitted manuscript. FC contributed to data acquisition. LO gave final approval of the submitted manuscript. Financial support and sponsorship: This was an academic project without any direct or indirect funding. Conflicts of interest: The authors do not have any potential conflict of interest to declare. Acknowledgement: Cochrane Coordinating Centre in Portugal. REFERENCES [1] T. A. Raju, M. C. Torjman and M. E. Goldberg, “Pe- rioperative Blood Glucose Monitoring in the General Sur- gical Population,” Journal of Diabetes Science and Tech- nology, Vol. 3 No. 6, 2009, pp. 1282-1287. [2] D. A. Godoy, M. Di Napoli, A. Biestro and R. Lenhardt, “Perioperative Glucose Control in Neurosurgical Patients,” Anesthesiology Research and Practice, 2013, 1-13. [3] P. Lecomte, L. Foubert, F. Nobels, et al., “Dynamic Tight Glycemic Control During and after Cardiac Surgery Is Effective, Feasible, and Safe,” Anesthesia & Analgesia, Vol. 107, No. 1, 2008, pp. 51-58. Copyright © 2013 SciRes. IJCM  Intraoperative Glycaemia Following Paracetamol with and without Glucose: A Randomized-Controlled Trial 415 doi:10.1213/ane.0b013e318172c557 [4] W. T. Longstreth and T. S. Inui, “High Blood Glucose Level on Hospital Admission and Poor Neurological Re- covery after Cardiac Arrest,” Annals of Neurology, Vol. 15, No. 1, 1984, pp. 59-63. doi:10.1002/ana.410150111 [5] A. E. Duncan, A. Abd-Elsayed, A. Maheshwari, M. Xu, E. Soltesz and C. G. Koch, “Role of Intraoperative and Post- operative Blood Glucose Concentrations in Predicting Outcomes after Cardiac Surgery,” Anesthesiology, Vol. 112, No. 4, 2010, pp. 860-871. doi:10.1097/ALN.0b013e3181d3d4b4 [6] D. Lena, P. Kalfon, J.-C. Preiser and C. Ichai, “Glycemic Control in the Intensive Care Unit and during the Postop- erative Period,” Anesthesiology, Vol. 114, No. 2, 2011, pp. 438-444. doi:10.1097/ALN.0b013e3182078843 [7] S. Akhtar, P. G. Barash and S. E. Inzucchi, “Scientific Principles and Clinical Implications of Perioperative Glucose Regulation and Control,” Anesthesia & Analge- sia, Vol. 110, No. 2, 2010, pp. 478-497. doi:10.1213/ANE.0b013e3181c6be63 [8] O. Alexandre, P. Lecomte and Y. Le Manach, “Poor In- traoperative Blood Glucose Control Is Associated with a Worsened Hospital Outcome after Cardiac Surgery in Diabetic Patients,” Anesthesiology, Vol. 103, No. 4, 2010, pp. 687-694. [9] G. V. Bochicchio, L. Salzano, M. Joshi, K. Bochicchio and T. M. Scalea, “Admission Preoperative Glucose Is Predictive of Morbidity and Mortality in Trauma Patients Who Require Immediate Operative Intervention,” The American Journal of Surgery, Vol. 71, No. 2, 2005, pp. 171-174. [10] G. V. Bochicchio, J. Sung, M. Joshi, et al., “Persistent Hyperglycemia Is Predictive of Outcome in Critically Ill Trauma Patients,” Journal of Trauma, Vol. 58, No. 5, 2005, pp. 921-924. doi:10.1097/01.TA.0000162141.26392.07 [11] S. Ganai, M. K. F. Lee, et al., “Adverse Outcomes of Geriatric Patients Undergoing Abdominal Surgery Who Are at High Risk for Delirium,” Archives of Surgery, Vol. 142, No. 11, 2007, pp. 1072-1078. doi:10.1001/archsurg.142.11.1072 [12] G. E. Umpierrez, S. D. Isaacs, N. Bazargan, X. You, L. M. Thaler and A. E. Kitabchi, “Hyperglycemia: An Inde- pendent Marker of In-Hospital Mortality in Patients with Undiagnosed Diabetes,” The Journal of Clinical Endo- crinology & Metabolism, Vol. 87, No. 3, 2002, pp. 978- 982. doi:10.1210/jc.87.3.978 [13] E. P. Krenzelok and M. A. Royal, “Confusion: Aceta- minophen Dosing Changes Based on NO Evidence in Adults,” Drugs in R&D, Vol. 12, No. 2, 2012, pp. 45-48 [14] K. Toussaint, X. C. Yang, M. A. Zielinski, et al., “What Do We (Not) Know about How Paracetamol (Acetami- nophen) Works?” Journal of Clinical Pharmacy and The- rapeutics, Vol. 35, No. 6, 2010, pp. 617-638. doi:10.1111/j.1365-2710.2009.01143.x [15] H. F. Miranda, M. M. Puig, J. C. Prieto and G. Pinardi, “Synergism between Paracetamol and Nonsteroidal Anti- Inflammatory Drugs in Experimental Acute Pain,” Pain, Vol. 121, No. 1, 2006, pp. 22-28. doi:10.1016/j.pain.2005.11.012 [16] S. E. Siegelaar, J. Hermanides, H. M. Oudemans-van Straaten, et al., “Mean Glucose during ICU Admission Is Related to Mortality by a U-Shaped Curve in Surgical and Medical Patients: A Retrospective Cohort Study,” Criti- cal Care, Vol. 14, No. 6, 2010, p. R224. doi:10.1186/cc9369 [17] G. E. Umpierrez, D. Smiley, A. Zisman, et al., “Random- ized Study of Basal-Bolus Insulin Therapy in the Inpa- tient Management of Patients with Type 2 Diabetes (RA BBIT 2 Trial),” Diabetes Care, Vol. 30, No. 9, 2007, pp. 2181-2186. doi:10.2337/dc07-0295 [18] R. Hirose, F. Xu, K. Dang, et al., “Transient Hyperglyce- mia Affects the Extent of Ischemia-Reperfusion-Induced Renal Injury in Rats,” Anesthesiology, Vol. 108, No. 3, 2008, pp. 402-414. doi:10.1097/ALN.0b013e318164cff8 [19] A. M. Sheehy and R. A. Gabbay, “An Overview of Pre- operative Glucose Evaluation, Management, and Peri- operative Impact,” Journal of Diabetes Science and Te- chnology, Vol. 3, No. 6, 2009, pp. 1261-1269. [20] L. F. Meneghini, “Perioperative Management of Diabetes: Translating Evidence into Practice,” Cleveland Clinic Journal of Medicine, Vol. 76, No. 4, 2009, pp. S53-S59. doi:10.3949/ccjm.76.s4.09 [21] A. Gautnam, A. Balusch, A. D. Kaye and E. A. Frost, “Modern Strategies for the Anesthesic Management of the Patient with Diabetes,” M.E.J. Anesthesia, Vol. 20, No. 2, 2009, pp. 187-197. [22] G. Van Den Berghe, P. Wouters, F. Weekers and C. Ver- waest, “Intensive Insulin Therapy in Critically Ill Patients,” The New England Journal of Medicine, Vol. 345, No. 19, 2001, pp. 1356-1367. [23] S. Finfer, FRCP, FJFICM, et al., “Intensive versus Con- ventional Glucose Control in Critically Ill Patients,” The New England Journal of Medicine, Vol. 360, No. 13, 2009, pp. 1283-1297. doi:10.1056/NEJMoa0810625 [24] E. S. Moghissi, M. T. Korytkowski, M. Di Nardo, et al., “American Association of Clinical Endocrinologists and American Diabetes Association Consensus Statement on Inpatient Glycemic Control,” Diabetes Care, Vol. 32, No. 6, 2009, pp. 1119-1131. doi:10.2337/dc09-9029 [25] J. Steven and S. Nicolson, “Perioperative Management of Blood Glucose during Open Heart Surgery in Infants and Children,” Pediatric Anesthesia, Vol. 21, No. 5, 2011, pp. 530-537. doi:10.1111/j.1460-9592.2011.03587.x [26] G. Y. Gandhi, G. A. Nuttall, M. D. Abel, et al., “Intraop- erative Hyperglycemia and Perioperative Outcomes in Cardiac Surgery Patients,” Mayo Clinic Proceedings, Vol. 80, No. 7, 2005, pp. 862-866. doi:10.4065/80.7.862 [27] F. Puskas, H. P. Grocott, W. D. White, J. P. Mathew, M. F. Newman and S. Bar-Yosef, “Intraoperative Hypergly- cemia and Cognitive Decline after CABG,” The Annals of Thoracic Surgery, Vol. 84, No. 5, 2007, pp. 1467-1473. doi:10.1016/j.athoracsur.2007.06.023 [28] F. Bilotta and G. Rosa, “Glucose Management in the Neurosurgical Patient: Are We yet Any Closer?” Current Opinion in Anaesthesiology, Vol. 23, No. 5, 2010, pp. 539-543. doi:10.1097/ACO.0b013e32833e150a Copyright © 2013 SciRes. IJCM  Intraoperative Glycaemia Following Paracetamol with and without Glucose: A Randomized-Controlled Trial Copyright © 2013 SciRes. IJCM 416 [29] L. S. Kao, D. Meeks, V. A. Moyer and K. P. Lally “Peri- Operative Glycaemia Control Regimens for Preventing Surgical Site Infection in Adults,” Cochrane Database of Systematic Reviews, John Wiley & Sons, Ltd, New York, p. 12. [30] M. Ramos, Z. Khalpey, S. Lipsitz, et al., “Relationship of Perioperative Hyperglycemia and Postoperative Infections in Patients Who Undergo General and Vascular Surgery,” Transactions of the Meeting of the American Surgical Association, Vol. 126, 2008, pp. 228-234. doi:10.1097/SLA.0b013e31818990d1 [31] A. K. M. Lipshutz and M. A. Gropper, “Perioperative Glycemic Control: An Evidence-Based Review,” Anes- thesiology, Vol. 110, No. 2, 2009, pp. 408-421. [32] G. P. Joshi, F. Chung, M. A. Vann, et al., “Society for Ambulatory Anesthesia Consensus Statement on Peri- operative Blood Glucose Management in Diabetic Pa- tients Undergoing Ambulatory Surgery,” Anesthesia & Analgesia, Vol. 111, No. 6, 2010, pp. 1378-1387. doi:10.1213/ANE.0b013e3181f9c288 [33] D. Kansagara, R. Fu, M. Freeman, F. Wolf and M. Hel- fand, “Intensive Insulin Therapy in Hospitalized Patients: A Systematic Review,” Annals of Internal Medicine, Vol. 154, No. 4, 2011, pp. 268-282. doi:10.7326/0003-4819-154-4-201102150-00008 [34] C. Ryan, A. Vega and A. Drash, “Cognitive Deficits in Adolescents Who Developed Diabetes Early in Life,” Pe- diatrics, Vol. 75, No. 5, 1985, pp. 921-927. [35] P. E. Cryer, L. Axelrod, A. B. Grossman, S. R. Heller, V. M. Montori, E. R. Seaquist and F. J. Service “Evaluation and Management of Adult Hypoglycemic Disorders: An Endocrine Society Clinical Practice Guidelines,” The Jour- nal of Clinical Endocrinology & Metabolism, Vol. 94, 2009, pp. 709-728. [36] C. H. Jensen, P. Berthelsen, C. Kühl and H. Kehlet, “Ef- fect of Epidural Analgesia on Glucose Tolerance during Surgery,” Acta Anaesthesiologica Scandinavica, Vol. 24, No. 6, 1908, pp. 472-474. doi:10.1111/j.1399-6576.1980.tb01586.x [37] N. K. Skjaervold, E. Solligård, D. R. Hjelme and P. Aa- dahl, “Continuous Measurement of Blood Glucose: Vali- dation of a New Intravascular Sensor,” Anesthesiology, Vol. 114, No. 1, 2011, pp. 120-125. doi:10.1097/ALN.0b013e3181ff4187 [38] H. F. Pidcoke, C. E. Wade, E. A. Mann, et al., “Anemia Causes Hypoglycemia in Intensive Care Unit Patients Due to Error in Single-Channel Glucometers: Methods of Reducing Patient Risk,” Critical Care Medicine, Vol. 38, No. 2, 2010, pp. 471-476. doi:10.1097/CCM.0b013e3181bc826f [39] E. A. Mann, J. Salinas, H. F. Pidcoke, S. E. Wolf, J. B. Holcomb and C. E. Wade, “Error Rates Resulting from Anemia Can Be Corrected in Multiple Commonly Used Point-of-Care Glucometers,” Journal of Trauma, Vol. 64, No. 1, 2008, pp. 15-20. [40] M. J. Rice, A. D. Pitkin and D. B. Coursin, “Glucose Measurement in the Operating Room: More Complicated than It Seems,” Anesthesia & Analgesia, Vol. 110, No. 1, 2010, pp. 1058-1065. [41] F. Akinbami, S. Segal, J. L. Schnipper, M. Stopfkuchen- Evans, J. Mills and S. O. Rogers, “Tale of Two Sites: Capillary versus Arterial Blood Glucose Testing in the Operating Room,” AJS, Vol. 203, No. 4, 2012, pp. 423- 427. [42] J. L. Schnipper, M. Magee, K. Larsen, S. E. Inzucchi and G. Maynard, “Society of Hospital Medicine Glycemic Control Task Force Summary: Practical Recommenda- tions for Assessing the Impact of Glycemic Control Ef- forts,” Journal of Hospital Medicine, Vol. 3, No. S5, 2008,pp. 66-75. doi:10.1002/jhm.356
|