 Open Journal of Medicinal Chemistry, 2013, 3, 74-86 http://dx.doi.org/10.4236/ojmc.2013.33010 Published Online September 2013 (http://www.scirp.org/journal/ojmc) 6-Azido-Galactosyl Imidate as a Building Block for Preparation of 1-(4-Aminobutyl)-, Di-, Tri- and Tetra-Saccharides Kun-I Lin1,2, Li-Wu Chiang1, Cheng-Tse Pan1, Ho-Lien Huang1, Yuan-Hsiao Su1, Shui-Tein Chen3, Ying-Cheng Huang4*, Chung-Shan Yu1,5* 1Department of Biomedical Engineering and Environmental Sciences, National Tsing-Hua University, Hsinchu, Taiwan 2Department of Obstetrics and Gynecology, Chang Bing Show Chwan Memorial Hospital, Changhua, Taiwan 3Institute of Biological Chemistry, Academia Sinica, Taipei, Taiwan 4Department of Neurosurgery, Chang Gung Memorial Hospital at Linkou, Chang Gung University, Taoyuan, Taiwan 5Institute of Nuclear Engineering and Science, National Tsing Hua University, Hsinchu, Taoyuan, Taiwan Email: *csyu@mx.nthu.edu.tw Received May 19, 2013; revised June 17, 2013; accepted July 1, 2013 Copyright © 2013 Kun-I Lin et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT 6-azidogalactosyl imidate has been used as a donor to generate 1-(4-aminobutyl)-6-aminogalactose, 6-aminothiotolyl- glycosides of disaccharide, trisaccharide and tetrasaccharide that incorporates 6-azido group and 1-(4-tolyl)thio group. Trisaccharide and tetrasaccharide were obtained from lactosyl-based acceptor. The anomeric 1-(4-tolyl)thio group could be used to conjugate with sphingosine analogs to provide the alpha-Gal Sph analogs for library extension from the azido group. Keywords: Glycosylation; Chemoselective; Imidate; Building Block; Lactoside 1. Introduction Glycoconjugates have been well known for their diversi- fied functions in molecular recognition. For example, glycolipids are involved in numerous immune-related diseases such as cancer progression [1]. Recently, alpha galactosyl ceramide (α-GalCer, KRN7000) has been in- tensively studied owing to their immune stimulation ef- fects that may be useful for development of cancer vac- cines [2]. Because the sugar components play crucial roles in the recognition events, structural modification on the sugar moieties might be capable of discovering more potential GalCer analogs. To broaden the diversities of saccharides, the library approach has become a promis- ing method [3-6]. The library of oligosaccharide could be generated through combinatorial methods by varying the sugar components, modifying with diverse functional groups as well as performing parallel synthesis or mix- ture-based synthesis [6-8]. We recently reported a preparation and analysis of li- braries of amide derived from a core amine with carbox- ylic acids via a parallel solution phase synthesis (psps) [9-11]. The library members could be directly analyzed for their antitumoral cytotoxicities in a cell-based assay [12]. Instead of the chromatographic purification but by using a serial dilution to 1000 fold, toxicities of the re- sidual reagents and solvents could be leveled off. En- couraged by the success in discovering a number of po- tential bioactive compounds [13,14], we are interested in preparing various galactosyl-containing saccharides that had been functionalized with azido group (Figure 1). The azido group could be potentially reduced to amine for further elaboration to amide libraries. 2. Experimental Section 2.1. Apparatus and the General Treatment of the Reagents All preparations were routinely conducted in dried glassware under nitrogen. CH2Cl2, toluene, and pyridine were dried over CaH2. THF was treated with FeSO4 to remove peroxide, followed by drying over Na. MeOH was dried over Mg and distilled. Dimethyl amino pyri- *Corresponding authors. C opyright © 2013 SciRes. OJMC 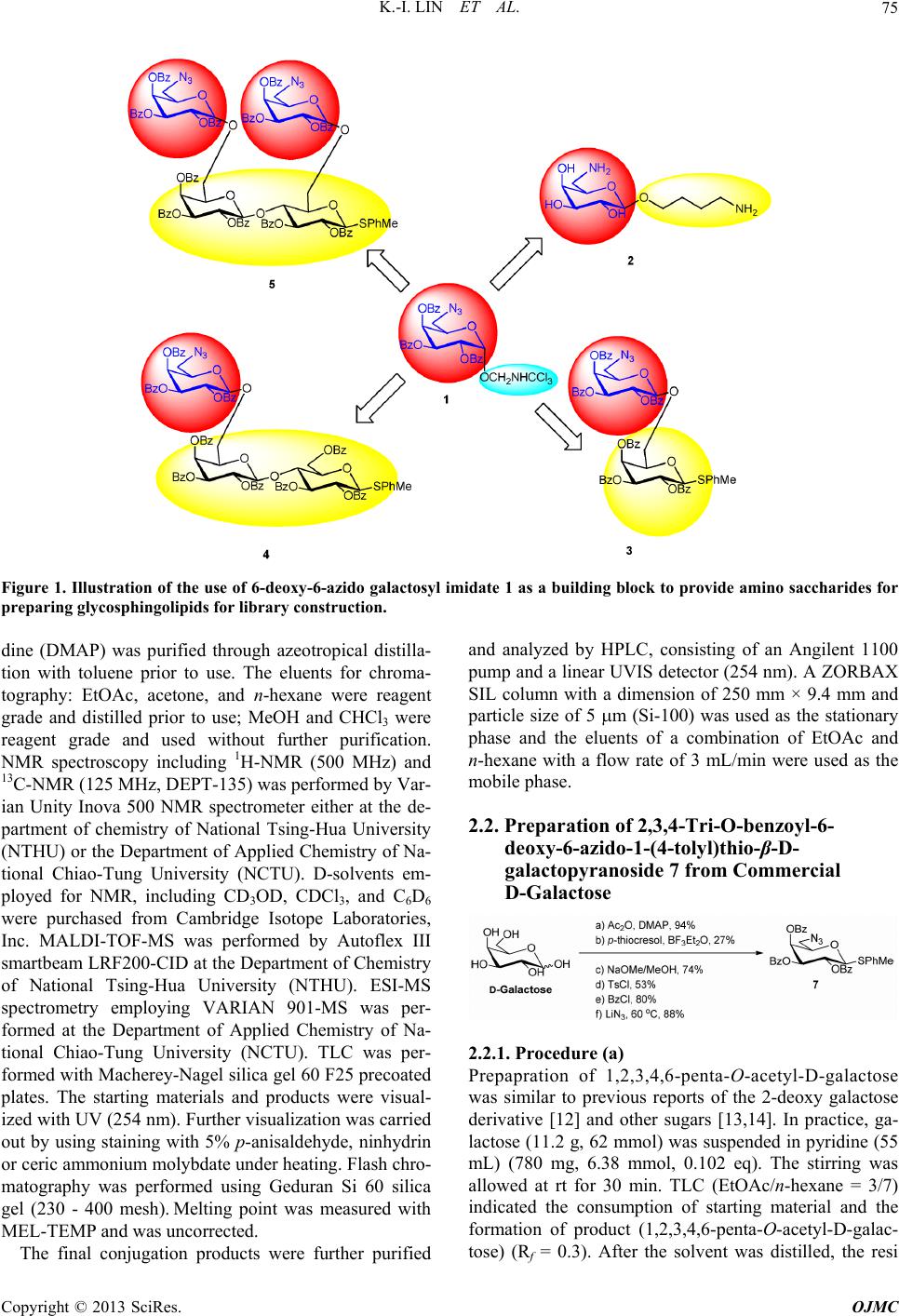 K.-I. LIN ET AL. 75 Figure 1. Illustration of the use of 6-deoxy-6-azido galactosyl imidate 1 as a building block to provide amino saccharides for preparing glycosphingolipids for library construction. dine (DMAP) was purified through azeotropical distilla- tion with toluene prior to use. The eluents for chroma- tography: EtOAc, acetone, and n-hexane were reagent grade and distilled prior to use; MeOH and CHCl3 were reagent grade and used without further purification. NMR spectroscopy including 1H-NMR (500 MHz) and 13C-NMR (125 MHz, DEPT-135) was performed by Var- ian Unity Inova 500 NMR spectrometer either at the de- partment of chemistry of National Tsing-Hua University (NTHU) or the Department of Applied Chemistry of Na- tional Chiao-Tung University (NCTU). D-solvents em- ployed for NMR, including CD3OD, CDCl3, and C6D6 were purchased from Cambridge Isotope Laboratories, Inc. MALDI-TOF-MS was performed by Autoflex III smartbeam LRF200-CID at the Department of Chemistry of National Tsing-Hua University (NTHU). ESI-MS spectrometry employing VARIAN 901-MS was per- formed at the Department of Applied Chemistry of Na- tional Chiao-Tung University (NCTU). TLC was per- formed with Macherey-Nagel silica gel 60 F25 precoated plates. The starting materials and products were visual- ized with UV (254 nm). Further visualization was carried out by using staining with 5% p-anisaldehyde, ninhydrin or ceric ammonium molybdate under heating. Flash chro- matography was performed using Geduran Si 60 silica gel (230 - 400 mesh). Melting point was measured with MEL-TEMP and was uncorrected. The final conjugation products were further purified and analyzed by HPLC, consisting of an Angilent 1100 pump and a linear UVIS detector (254 nm). A ZORBAX SIL column with a dimension of 250 mm × 9.4 mm and particle size of 5 m (Si-100) was used as the stationary phase and the eluents of a combination of EtOAc and n-hexane with a flow rate of 3 mL/min were used as the mobile phase. 2.2. Preparation of 2,3,4-Tri-O-benzoyl-6- deoxy-6-azido-1-(4-tolyl)thio-β-D- galactopyranoside 7 from Commercial D-Galactose 2.2.1. Procedure (a) Prepapration of 1,2,3,4,6-penta-O-acetyl-D-galactose was similar to previous reports of the 2-deoxy galactose derivative [12] and other sugars [13,14]. In practice, ga- lactose (11.2 g, 62 mmol) was suspended in pyridine (55 mL) (780 mg, 6.38 mmol, 0.102 eq). The stirring was allowed at rt for 30 min. TLC (EtOAc/n-hexane = 3/7) indicated the consumption of starting material and the formation of product (1,2,3,4,6-penta-O-acetyl-D-galac- tose) (Rf = 0.3). After the solvent was distilled, the resi Copyright © 2013 SciRes. OJMC  K.-I. LIN ET AL. 76 due was purified by column chromatography with eluents of EtOAc/n-hexane = 3/7 to give product (1,2,3,4,6-pen- ta-O-acetyl-D-galactose) in 94% yield (25 g). 2.2.2. Procedure (b) Preparation of 2,3,4,6-tetra-O-acetyl-1-(4-tolyl)thio-D-ga- lactoside has been reported and was similar to previous reports of the 2-deoxy galactose derivative and other sugars [15-19]. In practice, starting material (1,2,3,4,6- penta-O-acetyl-D-galactose) (25 g, 0.067 mol) was dis- solved in CH2Cl2 (50 mL) followed by cooling down to 0˚C. p-Thiocresol (8.7 g, 0.07 mol) and BF3Et2O (17 mL, 0.134 mol) were added, sequentially. The solution turned yellow. After 1.5 h, the color changed to purple. After a further stirring for 19 h, the solution turned black. TLC (EtOAc/n-hexane = 37) indicated the formation of a number of by-products. The mixture was partitioned by using 0.5 N HCl (20 mL) and saturated NaHCO3 (60 mL × 3), sequentially. The organic layer was dried with Na2SO4(s), followed by filtration. The filtrate was con- centrated under reduced pressure. The residue obtained was recrystallized from EtOAc/n-hexane to give product (2 ,3,4,6-tetra-O-ace-tyl-1-(4-tolyl)thio-D-galactoside) in 27% yield (7 g). 2.2.3. Procedure (c) Preparation of 1-(4-tolyl)thio-D-galactoside was similar to the previous reports of the 2-deoxy galactose deriva- tive and other sugars [15-24]. To a mixture of starting material (2,3,4,6-tetra-O-acetyl-1-(4-tolyl)thio-D-galac- toside) (6.8 g, 0.015 mol) and MeOH (20 mL) was added NaOMe (700 mg, 0.012 mol). The stirring was allowed for 1 h. TLC (MeOH/CH2Cl2 = 14) indicated the con- sumption of starting material (Rf = 0.83) and the forma- tion of product (Rf = 0.55). The mixture was treated with ion exchange resin of Amberlite IR-120 (H+ form). The resin was removed by gravitational filtration. The filtrate was concentrated under reduced pressure to generate the product in 74% yield (2.8 g). 2.2.4. Procedure (d) Preparation of 6-O-(p-tolylsulfonyl)-1-(4-tolyl) thio-D- galactoside was similar to the previous reports [25,26]. To a mixture of the starting material (p-tolyl 1-thio-D-ga- lactoside) (2.8 g, 9.7 mmol) and pyridine (3.5 mL) was added TsCl (2.05 g, 10.6 mmol, 1.1 eq). The stirring was allowed at rt for 1 h. TLC (MeOH/CHCl3 = 1/19) indi- cated the incomplete formation of product (Rf = 0.2) and the incomplete consumption of starting material (Rf = 0.1). To the above mixture was added TsCl (1 g, 5.3 mmol, 0.54 eq). The crude product was purified using column chromatography with eluents of MeOH/CHCl3 = 1/19 to afford the product in 53% yield (2.3 g). 2.2.5. Procedure (e) To a mixture of the starting material 6-O-(p-tolylsulfo- nyl)-1-(4-tolyl)thio-D-g alactoside) (1.9 g, 0.0043 mmol) and pyridine (5 mL) was added BzCl (1.3 mL, 0.014 mmol, 3.25 eq) at 0˚C [27]. The ice bath was removed and the stirring was allowed for 1.5 h. TLC indicated the consumption of starting material (Rf = 0.07, EtOAc/n-he- xane = 1/1) and the formation of product (2, 3, 4-tri- O-benzoyl-6-O-(p-tolylsulfonyl)-1-(4-tolyl)thio-D-galac- toside) (Rf = 0.61, EtOAc/n-hexane = 23). The mixture was quenched by addition of MeOH. The solvent was distilled and EtOAc (3 mL) was added to the residue. The organic layer was extracted with 1 N HCl (2 mL), NaHCO3(aq) (2 mL) and water (2 mL), sequentially. The organic layer was dried with Na2SO4(s) followed by filtra- tion. The filtrate was concentrated under reduced pres- sure. The residue obtained was purified using column chromatography with eluents of EtOAc/n-hexane = 1/4 to provide product (2,3,4-tri-O-benzo yl-6- O-(p-tolylsulfo- nyl)-1-(4-tolyl)thio-D-g alactoside) in 80% yield (2.6 g). 2.2.6. Procedure (f) The aqueous solution of LiN3 [25] (67 mmol, 20.3 eq) was coevaporated with toluene under reduced pressure. To a mixture of starting material (2,3,4-tri-O-benzoyl- 6-O-(p-tolyl sulfonyl)-1-(4-tolyl)thio-D-galactoside) (2.5 g, 3.3 mmol) and DMF (4 mL) was added the above so- lution of LiN3 in DMF (1 mL). The stirring was al- lowed at 80˚C for 17 h. TLC (EtOAc/n-hexane = 1/4) indicated the consumption of starting material (Rf = 0.12) and the formation of product 7 (Rf = 0.4). The mixture was concentrated under reduced pressure. Water (15 mL) and EtOAc (30 mL) were added for partition. The or- ganic layer was dried with Na2SO4(s) followed by filtra- tion. The filtrate was concentrated under reduced pres- sure. The residue obtained was purified using column chromatography to provide product 7 in 88% yield (1.3 g). 1H-NMR (500 MHz, C6D6) δ 2.03 (s, 3H, CH3, HSPhMe), 2.57 (dd, 1H, J6a,6b = 13.0, J6a,5 = 3.5 Hz, H6a), 3.05 (dd, 1H, J6b,6a = 13.0, J6b,5 = 8.5 Hz, H6b), 3.26 (dd, 1H, J5,6b = 8.5, J5,6a = 3.5 Hz, H5), 4.59 (dd, 1H, J1,2 = 10.0, 4J1,3 = 2.5 Hz, H1), 5.52 (ddd, 1H, J3,2 = 10.0, J3,4 = 3.5, 4J3,1 = 1.5 Hz, H3), 6.02 (d, 1H, J4,3 = 3.5 Hz, H4), 6.09 (dd, 1H, J2,3 =10.0, J2,1 = 10.0 Hz, H2), 6.71 (t, 2H, J = 7.5 Hz, Harom), 6.82 (t, 1H, J = 7.0 Hz, Harom), 6.95 (t, 5H, J = 7.0 Hz, Harom), 6.98 - 7.03 (m, 3H, Harom), 7.71 (d, 2H, J = 8.5 Hz, Harom), 7.91 - 7.98 (m, 4H, Harom), 7.99 - 8.01 (m, 4H, Harom), 8.09 - 8.14 (m, 2H, Harom); 13C-NMR (125 MHz, C6D6) δ 21.13 (CH3, CSPhMe), 51.12 (N3CH2), 68.28 (CH), 69.34 (CH), 73.50 (CH), 76.50 (CH), 85.55 (CH), 125.64 (CH, Carom), 127.48 (CH, Carom), 128.29 (CH, Carom), 128.43 (CH, Carom), 128.51 (CH, Carom), 128.61 (CH, Carom), 128.77 (CH, Carom), 129.28 (CH, Carom), 129.35 (C, Carom), 129.51 (C, Carom), 129.84 Copyright © 2013 SciRes. OJMC  K.-I. LIN ET AL. 77 (CH, Carom), 129.95 (CH, Carom), 130.02 (CH, Carom), 130.13 (C, Carom), 130.23 (CH, Carom), 133.17 (CH, Carom), 133.21 (CH, Carom), 133.41 (CH, Carom), 135.66 (CH, Carom), 138.81 (CH, Carom), 165.39 (OCO), 165.42 (OCO), 165.65 (OCO). 2.3. Preparation of 2,3,4-Tri-O-benzoyl-1-(2,2,2- trichloroenthanimidate)-6-deoxy-6-azido-α- D-galactopyranose 1 To a solution of compound 7 (448 mg, 0.71 mmol) in acetone (5 mL) was added N-bromosuccinimide [14,25] (152 mg, 0.39 mmol). After 30 min, the mixture was treated with EtOAc (5 mL), followed by partition using NaHCO3 (5 mL) and brine (5 mL), sequentially. The or- ganic layer was dried with Na2SO4 followed by filtra- tion. The filtrate was concentrated under reduced pres- sure. The residue obtained was purified using column chromatography with eluents of EtOAc/n-hexane = 1/4 to afford 2,3,4-tri-O-benzoyl-6-deoxy-6-azido-α-D-galacto- pyranose in 78% yield (287 mg). Anal. C27H23N3O8, M (calcd.) = 517.2 (m/z), ESI + Q-TOF: M = 518.1 (m/z), [M + K]+ = 557.1; 1H-NMR (500 MHz, C6D6) δ 2.59 (d, 1H, J6a,6b = 12.0 Hz, H6a), 3.09 (dd, 1H, J6b,6a = 13.0, J6b,5 = 9.0 Hz, H6b), 4.04 (bs, 1H, H5), 5.46 (bs, 1H), 5.75 (bs, 1H), 5.97 (dd, 1 H, J = 10.0, J = 3.5 Hz), 6.19 (t, 1 H, J = 10.5, J = 3.0 Hz), 6.72 (t, 2H, J = 8.0, J = 8.0 Hz, Harom), 6.83 - 7.12 (m, 8H, Harom), 7.92-8.12 (m, 5H, Harom). To the above intermediate (430 mg, 0.82 mmol) in CH2Cl2 (5 mL) was added CCl3CN (904 uL, 9.0 mmol, 11 eq) and 1,8-diazabicyclo[5.4.0]undec-7-ene (62 uL, 0.41 mmol, 0.5 eq), sequentially [25,28]. After 30 min, TLC (EtOAc/n-hexane = 1:4) indicated the consumption of the starting material (Rf = 0.35) and the formation of the product 1 (Rf = 0.46). After concentration under re- duced pressure, the residue obtained was purified using column chromatography with eluents of EtOAc/n-hexane 1:9 to provide compound 1 in 94% yield (512 mg). 1H-NMR (500 MHz, CDCl3) δ 3.36 (dd, 1H, J6a,6b (gem) = 12.5, J6a,5 = 5.0 Hz, H6a), 3.58 (dd, 1H, J6b,6a (gem) = 13.0, J6b,5 = 8.0 Hz, H6b), 4.59 (dd, 1H, J5,6b = 7.5, J5,6a = 5.0 Hz, H5), 5.92 (dd, 1H, J3,4 = 3.5, J3,2 = 9.5 Hz, H3), 5.96-6.01 (m, 2H, H2 and H4), 6.87 (d, 1H, J1,2 = 4 Hz, H1), 7.22 - 7.51 (m, 8H, Harom), 7.63 (t, 1H, J = 8.0, J = 7.0 Hz, Harom), 7.77 (d, 2H, J = 7.0 Hz, Harom), 7.93 (d, 2H, J = 7.0 Hz, Harom), 8.07 (d, 2H, J = 7.0 Hz, Harom), 8.67 (s, 1H, HNH). 2.4. Preparation of 4-Azidobutan-1-ol 8 2.4.1. Preparation of TfN3 [29]* NaN3 (9.7 g, 150 mmol) was dissolved in H2O (7 mL) at 0˚C. A solution of Tf2O (5 mL, 30 mmol) in CH2Cl2 (15 mL) was added. The biphasic mixture was stirred vigor- ously for 1 hr. The organic layer was collected and the aqueous layer was back-extracted with CH2Cl2 (60 mL). The organic layers were combined and washed with saturated NaHCO3 (27 mL) twice to give TfN3. *Caution: TfN3 should be manipulated in a solution. Explosion may occur when drying. 2.4.2. Azido Transfer Reaction To a solution of commercial 4-aminobutan-1-ol (924 L, 890 mg, 10 mmol) in H2O (40 mL) was added K2CO3 (2 g, 15 mmol), CuSO4 (16 mg, 0.1mmol) and the above solution of TfN3, sequentially. The mixture was brought about to a homogeneous solution by addition of MeOH (100 mL). After 18 h, the mixture was partitioned be- tween saturated NaHCO3 (aq., 30 mL) and CH2Cl2 (80 mL). The organic layer was concentrated under reduced pressure at 30˚C to provide the crude yellow oil product 8 in 70% yield (800 mg). Anal. C4H9N3O, M (calcd.) = 115.1 (m/z), ESI + Q-TOF:M = 115.1 (m/z), [M + H]+ = 116.1; 1H-NMR (500 MHz, CDCl3) δ 1.59 (m, 4H, Hbutyl), 3.25 (dd, 2H, J = 6.5 Hz, N3CH2, Hbutyl), 3.59 (dd, 2H, J = 6.0 Hz, CH2OH, Hbutyl); 13C-NMR (125 MHz, CDCl3) δ 24.92 (CH2, Cbutyl), 29.20 CH2, Cbutyl), 50.83 (CH2N3, Cbutyl), 61.24 (CH2OH, Cbutyl). Analytic data also can be found in literature [30,31]. 2.5. Preparation of 4’-Azidobutyl 2,3,4-Tri-O-benzoyl-6-deoxy-6-azido-β-D-gal actopyranoside 9 A mixture of the donor 1 (512 mg, 0.77 mmol) and the acceptor 8 (266 mg, 2.31 mmol, 3 eq) was distilled azeotropically with toluene at 50˚C for three times fol- lowed by concentration under reduced pressure for 1 h. The mixture was transferred to a two-necked round-bot- tom flask charging with CH2Cl2 (5 mL), followed by addition of 4 Å MS (680 mg). The mixture was stirred at 0˚C under N2 for 30 min. After addition of TMSOTf (32 μL, 0.15 mmol, 0.2 eq), the ice bath was removed and the stirring was allowed at rt for 10 min. The solution was concentrated under reduced pressure. The residue ob- tained was purified using column chromatography with eluents of EtOAc/n-hexane = 3.5/6.5 to provide com- pound 9 in 86% yield (400 mg). The product was further purified using HPLC with eluents of EtOAc/n-hexane = 2/8 to provide compound 9, tR = 15.6 min. Anal. C31H30N6O8, M (calcd.) = 614.2 (m/z), ESI+Q-TOF: M = 614.1 (m/z), [M + Na]+ = 637.1; 1H-NMR (500 MHz, C6D6) δ 1.15 - 1.32 (m, 4H, Hbutyl), 2.55 (t, 2H, J = 6.5 Hz, N3CH2, H4 (butyl)), 2.60 (dd, 1H, J6a’,6b’ (gem) = 13.5, J6a’,5’ = 3.5 Hz, H6a’), 3.18 (dd, 1H, J6b’,6a’ (gem) = 13.0, J6b’,5’ = 9.0 Hz, H6b’), 3.22-3.26 (m, 1H, Hbutyl), 3.34 (dd, 1H, J5’,6b’ = 9.0, J5’,6a’ = 3.5 Hz, H5’), 3.83 - 3.87 (m, 1H, Hbutyl), 4.42 (d, 1H, J1’,2’ = 8.0 Hz, H1’), 5.63 (dd, 1H, Copyright © 2013 SciRes. OJMC  K.-I. LIN ET AL. 78 J3’,2’ = 11.0, J3’,4’ = 3.5 Hz, H3’), 5.77 (d, 1H, J4’,3’ = 3.5 Hz, H4’), 6.23 (dd, 1H, J2’,3’ = 10.5, J2’,1’ = 8.0 Hz, H2’), 6.71 (t, 1 H, J = 8.0, J = 7.5 Hz, 2H, Harom), 6.83 (t, 1 H, J = 8.0, J = 7.0 Hz, 1H, Harom), 6.90 - 7.06 (m, 6H, Harom), 7.97 (d, 2H, J = 7.5 Hz, Harom), 8.08 (d, 4H, J = 8.5 Hz, Harom); 13C-NMR (125 MHz, C6D6) δ 25.52 (CH2, Cbutyl), 26.68 (CH2, Cbutyl), 50.78 (CH2N3), 51.00 (CH2N3), 69.29 (CH2OH, Cbutyl), 69.38 (CH), 70.26 (CH), 72.05 (CH), 73.56 (CH), 101.65 (CH), 128.29 (CH, Caro m), 128.47 (CH, Carom), 128.60 (CH, Carom), 128.86 (CH, Carom), 129.30 (CH, Carom), 129.37 (CH, Carom), 129.85 (CH, Carom), 129.93 (CH, Carom), 130.02 (CH, Carom), 130.19 (CH, Carom), 133.22 (CH, Carom), 133.51 (CH, Carom), 165.45 (OCO), 165.64 (OCO), 165.81 (OCO). 2.6. Preparation of 4’-Azidobutyl 6-deoxy-6-azido-β-D-galactopyranoside 10 To a mixture of compound 9 (60 mg, 0.09 mmol) in MeOH (1 mL) was added NaOMe (4 mg, 0.07 mmol, 0.8 eq). The mixture was stirred at rt for 30 min. After the treatment with ion exchange resin (H+), the mixture was treated with gravitational filtration. The filtrate obtained was concentrated under reduced pressure. The residue obtained was purified using column chromatography with eluents of MeOH/CHCl3 = 1/9 to provide compound 10 in 71% yield (21 mg). 1H-NMR (500 MHz, CD3OD) δ 1.62 (m, 4H, Hbutyl), 3.20 (dd, 1H, J6a’,6b’(gem) = 11.6, J6a’,5’ = 3.0 Hz, H6a’), 3.23 - 3.24 (m, 3H), 3.39 - 3.66 (m, 5H), 3.83 - 3.86 (m, 1H), 4.17 (d, 1H, J1,2 = 6.8 Hz, H1); 13C- NMR (125 MHz, CD3OD) δ 26.72 (CH2, Cbutyl), 27.91 (CH2, Cbutyl), 52.24 (CH2N3), 52.57 (CH2N3), 70.04 (OCH2, Cbutyl), 70.85 (CH), 72.31 (CH), 74.71 (CH), 75.79 (CH), 104.82 (CH). 2.7. Preparation of 4’-Aminobutyl 6-deoxy-6-amino-β-D-galactopyranoside 2 To compound 10 (21 mg, 0.07 mmol) in MeOH (5 mL) was added 10% Pd/C (10 mg, 50%). The mixture was charged with a ballon containing H2. The stirring was allowed for 2 h as monitored by TLC (MeOH/HCCl3/ NH3 = 5/5/2). The mixture was filtered through a celite pad. The filtrate obtained was concentrated under re- duced pressure to afford compound 2 in 70% yield (12 mg). Anal. C10H22N2O5, M (calcd.) = 250.2 (m/z), ESI + Q-TOF: M = 250.2 (m/z), [M + Na]+ = 273.2; 1H-NMR (500 MHz, CD3OD) δ 1.59 - 1.66 (m, 4H, Hbutyl), 2.71 (t, J = 6.5 Hz, 2H, CH2NH2, H4 (butyl)), 2.79 (dd, 1H, J6a’,6b’(gem) = 13.5 Hz, J6a’,5’ = 4.0 Hz, H6a’), 2.95 (dd, 1H, J6b’,6a’(gem) = 13.0, J6b’,5’ = 7.5 Hz, H6b’), 3.41 - 3.58 (m, 3H, H2’ and H3’ and H5’), 3.59 (dt, Jgem = 10.0, J = 6.5 Hz, 1H, H1a(butyl)), 3.77 (d, 1H, J = 3.0 Hz, H4’), 3.90 (dt, Jgem = 10.0, J = 6.5 Hz, 1H, H1b(butyl)), 4.21 (d, 1H, J1,2 = 8.0 Hz, H1’). 2.8. Preparation of 2,3,4-tri-O-benzoyl-1- (4-tolyl)thio-β-D-galactopyranoside 11 Compound 6 (700 mg, 2.45 mmol) and DMAP (189 mg, 1.6 mmol, 0.63 eq) was dissolved in pyridine (3 mL). A solution of TBDMSCl (738 mg, 4.9 mmol, 2 eq) in CH2Cl2 was added [24]. The stirring was allowed for 20 h. TLC (MeOH/CHCl3 = 1/9) indicated the consumption of compound 6 (Rf = 0.24). The mixture was cooled down by ice bath. BzCl (1.7 mL, 2.1 g, 14.7 mmol, 6 eq) was added. The mixture was then stirred for 0.5 h. TLC (EtOAc/n-hexane = 1/3) indicated the formation of the intermediate compound (Rf = 0.75). The mixture was concentrated under reduced pressure. The residue was purified using column chromatography with gradients of EtOAc/n-hexane = 1/19 → 1/9 to provide the intermedi- ate product, 2,3,4-tri-O-benzoyl- 6-O-(tert-butyldimethyl- silyl)-1(4-tolyl)-thio-β-D-galactopyranoside, in 56% yi- eld (976 mg). The obtained intermediate compound (170 mg, 0.24 mmol) was dissolved in THF (5 mL). AcOH (1 mL) and HF-pyridine (700 μL) were added, sequentially. The stir- ring was allowed for 10 min. TLC (EtOAc/n-hexane = 1/3) indicated the consumption of the intermediate com- pound (Rf = 0.63) and the formation of product 11 (Rf = 0.27). The reaction was quenched by washing with satu- rated NaHCO3(aq) (5 mL). The organic layer was dried with Na2SO4 followed by filtration with a celite pad. The filtrate was concentrated under reduced pressure. The residue obtained was purified using column chromatog- raphy with eluents of EtOAc/n-hexane = 1/3 to provide product 11 in 88% yield (126 mg). 1H-NMR (500 MHz, C6D6) δ 2.03 (s, 3H, CH3, HSPhMe), 3.62 (dd, 1H, J5,6b = 7.0, J5,6a = 6.5 Hz, H5), 4.12 (dd, 1H, J6a,6b = 11.0, J6a,5 = 6.0 Hz, H6a), 4.32 (dd, 1H, J6b,6a = 11.0, J6b,5 = 7.0 Hz, H6b), 4.64 (d, 1H, J1,2 = 10.0 Hz, H1), 5.62 (dd, 1H, J3,2 = 10.0, J3,4 = 3.5 Hz, H3), 6.01 (d, 1H, J4,3 = 3.5 Hz, H4), 6.11 (dd, 1H, J2,3 = 10.0, J2,1 = 10.0 Hz, H2), 6.67 - 7.10 (m, 12H, Harom), 7.64 - 8.15 (m, 7H, Harom). 2.9. Preparation of 2,3,4-tri-O-benzoyl-6-azido-6- deoxy-β-D-galactopyranosyl-(1→6)-2,3,4-tri- O-benzoyl-1-(4-tolyl)thio-galactopyranoside 3 A mixture of the donor 1 (13 mg, 0.02 mmol) and the acceptor 11 (17 mg, 0.028 mmol, 1.3 eq) were azeo- tropically distilled with toluene at 50˚C for three times, which was followed by concentration under reduced pressure for 1 h. The mixture was dissolved in a two- necked round-bottom flask charging CH2Cl2 (5 mL) and 4 Å MS (30 mg). The stirring was allowed at rt for 30 min, followed by cooling down with an ice bath. An ali- quot of TMSOTf (100 uL, 0.006 mmol) generated from TMSOTf (17 μL) in CH2Cl2 (1 mL) was added [16, Copyright © 2013 SciRes. OJMC  K.-I. LIN ET AL. 79 30-34]. The bath was then removed. After 10 min, TLC (EtOAc/n-hexane 1:3) indicated the formation of the product 3 (Rf = 0.34) and the consumption of the donor 1 (Rf = 0.62). The mixture was concentrated under reduced pressure. The residue obtained was purified using col- umn chromatography with eluents of EtOAc/n-hexane = 1/3 to provide compound 3 in 85% yield (19 mg). The sample was further purified using HPLC with eluents of EtOAc/n-hexane = 3/7. tR = 15.88 min. Anal. C61H51N3O15S, M (calcd.) = 1097.3 (m/z), ESI + Q-TOF: M = 1097.3 (m/z), [M + Na]+= 1120.3 (3.8%), 1121.3 (2.3%), equi- valent to the calculated isotopic ratio 100:67; 1H-NMR (500 MHz, C6D6) δ 2.06 (s, 3H, CH3, HSPhMe), 2.57 (dd, 1H, J6a’,6b’ = 13.0, J6a’,5’ = 4.0 Hz, H6a’(donor)), 2.93 (dd, 1H, J6b’,6a’ = 13.0, J6b’,5’ = 8.0 Hz, H6b’(donor)), 3.29 (dd, 1H, J5’,6b’ = 8.0, J5’,6a’ = 4.0 Hz, H5’(donor)), 3.67 (dd, 1H, J5,6b = 7.0, J5,6a = 5.0 Hz, H5(acceptor)), 3.96 (dd, 1H, J6b,6a = 11.0, J6b,5 = 7.0 Hz, H6b(acceptor)), 4.01 (dd, 1H, J6a,6b = 11.0, J6a,5 = 5.0 Hz, H6a(acceptor)), 4.64 (d, 1H, J1,2 = 10.0 Hz, H1(acceptor)), 4.66 (d, 1H, J1’,2’ = 8.0 Hz, H1’(donor)), 5.58 (dd, 1H, JAA = 10.0, JAE = 3.5 Hz, HAxial), 5.63 (dd, 1H, JAE = 10.0, JAE = 3.5 Hz, HAxial), 5.77 (d, 1H, JEA = 3.5 Hz, HEquatorial), 6.02 (d, 1H, JEA = 3.5 Hz, HEquatorial), 6.09 (dd, 1H, J2,3 = 10.0, J2,1 = 10.0 Hz, H2(acceptor)), 6.26 (dd, 1H, J2’,3’ =10.0, J2’,1’ = 8.5 Hz, H2’(donor)), 6.68 - 6.76 (m, 4H, Harom), 6.80 - 6.86 (m, 2H, Harom), 6.90 - 7.14 (m, 14H, Harom), 7.68 (d, 2H, J = 7.5 Hz, Harom), 7.92 - 7.96 (m, 2H, Harom), 7.99 - 8.01 (m, 4H, Harom), 8.08 - 8.10 (m, 2H, Harom), 8.16 - 8.21 (m, 4H, Harom); 13C-NMR (125 MHz, C6D6) δ 21.15 (CH3, CSPhMe), 50.48 (CH2), 67.82 (CH2), 68.58 (CH), 69.13 (CH), 69.20 (CH), 70.35 (CH), 72.19 (CH), 73.23 (CH), 73.42 (CH), 77.06 (CH), 85.52 (CH), 101.19 (CH), 125.64 (CH, Carom), 128.29 (CH, Carom), 128.47 (CH, Carom), 128.54 (CH, Carom), 128.63 (CH, Carom), 128.89 (CH, Carom), 129.28 (CH, Carom), 129.34 (C, Carom), 129.43 (C, Carom), 129.57 (C, Carom), 129.86 (C, Carom), 129.93 (CH, Carom), 129.98 (CH, Carom), 130.04 (C, Carom), 130.16 (C, Carom), 130.20 (C, Carom), 130.31 (CH, Carom), 132.98 (CH, Carom), 133.09 (CH, Carom), 133.20 (CH, Carom), 133.54 (CH, Carom), 135.17 (CH, Carom), 138.61 (CH, Carom), 165.35 (OCO), 165.45 (OCO), 165.58 (OCO), 165.63 (OCO), 165.74 (OCO). 2.10. Preparation of 2,2’,6,6’-tetra-O-benzoyl- 3’,4’-O-isopropylidene-1-(4-tolyl)thio-β-D- lactoside 13 and 2,2’,3,6,6’-penta-O- benzoyl-3’,4’-O-isopropylidene-1- (4-tolyl)thio-β-D-lactoside 14 To a mixture of 12 (1.2 g, 2.46 mmol), pyridine (17 mL) and toluene (22.9 mL) was added BzCl (2.4 mL, 19.7 mmol, 8 eq) at 0˚C. After removal of the ice bath, the mixture was stirred at rt for 30 min. TLC (MeOH/CHCl3 = 1/9) indicated the consumption of 12 (Rf = 0.5) and the formation of a product mixture (Rf = 0.96). The reaction was quenched by addition of MeOH. TLC (EtOAc/n-he- xane = 1/9) indicated that the mixture consists of two products i.e. product 13 (Rf = 0.19) and product 14 (Rf = 0.24). The mixture was concentrated under reduced pres- sure. The residue obtained was purified using column chromatography with gradients of EtOAc/n-hexane = 1/4 → EtOAc/n-hexane = 1/3 to afford product 13 in 38% yield (358 mg) and product 14 in 2% yield (47.1 mg) and a mixture of 13 and 14 (642 mg). The crude yield to compound 13 and 14 was 60% and 30%, respectively. Compound 13: 1H-NMR (500 MHz, CDCl3) δ 1.33 (s, 3H, CH3, Hisopropylidene), 1.58 (bs, 1H, HOH), 1.61 (s, 3H, CH3, Hisopropylidene), 2.16 (s, 3H, CH3, HSPhMe), 3.60 - 3.70 (m, 2H), 3.96 (dd, 1H, J3,2 = 9.0, J3,4 = 8.0 Hz, H3), 4.16 (dd, 1H, J = 12.0, J = 4.5 Hz), 4.20 - 4.28 (m, 2H), 4.35 - 4.45 (m, 3H), 4.62 (d, 1H, J1’,2’ = 8.5 Hz, H1’), 4.65 (d, 1H, J1,2 = 10.5 Hz, H1), 4.83 (dd, 2H, J = 12.5, J = 2.5 Hz), 5.12 (dd, 1H, J2,3 = 9.0, J2,1 = 10.5 Hz, H2), 5.32 (dd, 1H, J2’,3’ = 7.5, J2’,1’ = 8.5 Hz, H2’), 6.78 (d, 2H, J = 8.5 Hz), 7.11 - 7.47 (m, 12H), 7.52 - 7.61 (m, 2H), 7.85 (d, 2H, J = 7.0 Hz), 7.95 (d, 2H, J = 7.0 Hz), 8.02 (d, 2H, J = 8.5 Hz), 8.06 (d, 2H, J = 8.5 Hz); 13C-NMR (125 MHz, CDCl3) δ 21.04 (CH3), 26.27 (CH3), 27.61 (CH3), 62.59 (CH2), 63.67 (CH2), 71.47 (CH), 72.11 (CH), 73.07 (CH), 73.43 (CH), 74.92 (CH), 75.77 (CH), 77.00 (CH), 82.17 (CH), 85.63 (CH), 101.52 (CH), 111.29 (C, Cisopropylidene), 125.29 (CH, Carom), 127.78 (C, Carom), 128.21 (CH, Carom), 128.32 (CH, Carom), 128.33 (CH, Carom), 128.84 (C, Carom), 129.03 (C, Carom), 129.06 (CH, Carom), 129.39 (CH, Carom), 129.69 (CH, Carom), 129.75 (CH, Carom), 129.80 (CH, Carom), 129.95 (CH, Carom), 130.04 (C, Carom), 133.06 (CH, Carom), 133.08 (CH, Carom), 133.22 (CH, Carom), 133.41 (CH, Carom), 133.79 (CH, Carom), 138.16 (C, Carom), 165.19 (OCO, CBz), 165.26 (OCO, CBz), 165.44 (OCO, CBz), 165.54 (OCO, CBz). Compound 14: 1H-NMR (500 MHz, CDCl3) δ 1.23 (s, 3H, CH3, Hisopropylidene), 1.50 (s, 3H, CH3, Hisopropylidene), 2.21 (s, 3H, CH3, HSPhMe), 3.62 (dd, 1H, J = 11.5, J = 7.5 Hz), 3.75 - 3.87 (m, 2H), 4.03 - 4.13 (m, 2H), 4.16 - 4.25 (m, 2H), 4.43 (dd, 1H, J = 12.0, J = 5.0 Hz), 4.55 (d, 1H, J1’,2’ = 8.0 Hz, H1’), 4.62 (d, 1H, J = 10.5 Hz), 4.77 (d, 1H, J1,2 = 9.5 Hz, H1), 5.10 (dd, 1H, J2’,3’ = 7.5, J2’,1’ = 8.0 Hz, H2’), 5.34 (dd, 1H, J3,2 = 10.0, J3,4 = 10.0 Hz, H3), 5.69 (dd, 1H, J2,3 = 10.0, J2,1 = 9.5 Hz, H2), 6.78 (d, 2H, J = 7.5 Hz), 7.22 - 7.61 (m, 17H), 7.88 - 7.95 (m, 6H), 7.97 (d, 2H, J = 7.5 Hz), 8.04 (d, 2H, J = 7.0 Hz); 13C-NMR (125 MHz, C6D6) δ 20.97 (CH3), 26.33 (CH3), 27.61 (CH3), 62.94 (CH2), 63.19 (CH2), 71.00 (CH), 71.54 (CH), 73.49 (CH), 74.30 (CH), 74.35 (CH), 75.86 (CH), 76.98 (CH), 77.52 (CH), 85.78 (CH), 100.89 (CH), 110.84 (C, Cisopropylidene), 127.91 (CH, Carom), 128.12 (CH, Carom), 128.24 (CH, Carom), 128.29 (CH, Carom), 128.59 (CH, Carom), 128.67 (CH, Carom), 128.75 (CH, Carom), 128.99 (CH, Carom), 129.69 (CH, Carom), 129.87 (CH, Carom), 129.91 (CH, Carom), 130.09 (CH, Copyright © 2013 SciRes. OJMC  K.-I. LIN ET AL. 80 Carom), 130.20 (CH, Carom), 130.26 (C, Carom), 133.43 (C, Carom), 130.73 (C, Carom), 130.76 (C, Carom), 132.85 (CH, Carom), 133.17 (CH, Carom), 133.44 (CH, Carom), 134.23 (CH, Carom), 138.08 (C, Carom), 164.96 (OCO, CBz), 165.50 (OCO, CBz), 165.87 (OCO, CBz), 165.98 (OCO, CBz). 2.11. 2,3,4,-tri-O-benzoyl-6-O-(tert- butyldimethylsilyl)-β-D-galactopyranosyl- (1→4)-2,3,6-tri-O-benzoyl-1- (4-tolyl)thio-β-D-glucopyranoside 16 and 2,3,4,-tri-O-benzoyl-6-O-(tert- butyldimethylsilyl)-β-D-galactopyranosyl- (1→4)-2,3-di-O-benzoyl-6-O-(tert- butyldimethylsilyl)-1-(4-tolyl)thio-β-D- glucopyranoside 17 To a mixture of p-tolyl-1-thio-β-D-lactose 15 (300 mg, 0.67 mmol), DMAP (59 mg, 0.42 mmol) and pyridine (3 mL) was added a solution of TBDMSCl (222 mg, 1.48 mmol) in CH2Cl2 (2 mL). The stirring was allowed for 20 h. Followed by cooling down with an ice bath, BzCl (3 mL, 5.36 mmol, 8 eq) was added. After 10 min, the ice bath was removed and the mixture was stirred for 30 min. The mixture was concentrated under reduced pressure. The residue obtained was purified using column chro- matography with eluents of EtOAc/n-hexane = 1/9 to provide a mixture of compound 16 and compound 17 in 56% yield (448 mg) with a ratio of 2:1. Compound 16: 1H-NMR (500 MHz, CDCl3) δ 0.17 (s, 6H, Si-(CH3)2, HTBDMS), 0.91 (s, 9H, C(CH3)3, HTBDMS), 2.31 (s, 3H, CH3, HSPhCH3), 3.39 (dd, 1H, J6a,6b(gem) = 10.0 Hz, H6a), 3.54 (dd, 1H, J6b’,6a’(gem) = 11.5, J6b’,5’ = 7.0 Hz, H6b’), 3.72 - 3.82 (m, 2H, H6b and H5), 3.83 (dd, 1H, J6a’,6b(gem) = 11.5, J6a’,5’ = 6.0 Hz, H6a’), 3.97 (dd, 1H, J5’,6a’ = 6.0, J5’,6b’ = 7.0 Hz, H5’), 4.22 (dd, 1H, J4,3 = 9.5, J4,5 = 9.5 Hz, H4), 4.74 (d, 1H, J1’,2’ = 10.0 Hz, H1’), 5.04 (d, 1H, J1,2 = 8.0 Hz, H1), 5.31 (dd, 1H, J2’,1’ = 10.0, J2’,3’ = 10.0 Hz, H2’), 5.37 (dd, J3’,2’ = 10.0, J3’,4’ = 3.0 Hz, H3’), 5.64 (dd, J2,1 = 8.0, J2,3 = 10.0 Hz, H2), 5.69 (dd, J3,2 = 10.0, J3,4 = 9.5 Hz, H3), 5.73 (d, 1H, J4’,3’ = 3.0 Hz, H4’), 7.02 - 7.09 (m, 4H, Harom), 7.24 - 8.08 (m, 30H, Harom). Compound 17: 1H-NMR (500 MHz, CDCl3) δ −0.29 (s, 3H, Si-CH3, HTBDMS), −0.20 (s, 3H, Si-CH3, HTBDMS), 0.13 (s, 3H, Si-CH3, HTBDMS), 0.14 (s, 3H, Si-CH3, HTBDMS), 0.74 (s, 9H, C(CH3)3, HTBDMS), 0.99 (s, 9H, C(CH3)3, HTBDMS), 2.30 (s, 3H, CH3, HSPhCH3), 2.74 (dd, 1H, J6b,6a(gem) = 10.0, J6b,5 = 9.5 Hz, H6b), 3.21 (dd, 1H, J6a,6b = 10.0, J6a,5 = 5.0 Hz, H6a), 3.37 (d, 1H, J = 10.0 Hz) , 3.64 (ddd, 1H, J = 5.0, J = 9.5, J = 9.5 Hz, H5a), 3.81 - 3.71 (m, 2H), 4.13 (dd, 1H, J4,3 = 9.5, J4,5 = 9.5 Hz, H4), 4.73 (d, 1H, J1’,2’ = 10.0 Hz, H1’), 4.94 (d, 1H, J1,2 = 8.0 Hz, H1), 5.29 (dd, 1H, J2’,1’ = 10.0, J2’,3’ = 10.0 Hz, H2’), 5.37 (dd, J3’,2’ = 10.0, J3’,4’ = 3.0 Hz, H3’), 5.57 (dd, J2,1 = 8.0, J2,3 = 10.0 Hz, H2), 5.62 (dd, J3,2 = 10.0, J3,4 = 9.5 Hz, H3), 5.73 (d, 1H, J4’,3’ = 3.0 Hz, H4’), 7.02 - 8.08 (m, 29H, Harom). 2.12. Preparation of 2,3,4,-tri-O-benzoyl-β-D- galactopyranosyl-(1→4)-2,3,6-tri-O- benzoyl-1-(4-tolyl)thio-β-D-glucopyranoside 18 and 2,3,4,-tri-O-benzoyl-β-D- galactopyranosyl-(1→4)-2,3-di-O-benzoyl- 1-(4-tolyl)thio-β-D-glucopyranoside 19 To a mixture of 16 and 17 (448 mg, 0.37 mmol) and THF (5 mL) was added HF-pyridine (2.5 mL) and AcOH (3.5 mL), sequentially. The mixture was stirred for 30 min. The mixture was then partitioned between CH2Cl2 (25 mL) and saturated aqueous NaHCO3 (7 mL). The organic layer was filtered through a celite pad. The filtrate was concentrated under reduced pressure. The residue ob- tained was purified using column chromatography with gradients of EtOAc/n-hexane = 1/9 → 1/4 to provide compound 18 and compound 19 in total 79% yield (286 mg). Compound 19: 1H-NMR (500 MHz, CDCl3) δ 1.85 (bs, 2H, OH), 2.30 (s, 3H, CH3, HSPhCH3), 2.67 (dd, 1H, J = 12.5, J = 7.0 Hz), 2.84 (dd, 1 H, J = 12.0, J = 6.5 Hz), 3.68 - 3.74 (m, 2H), 3.80 (dd, 1 H, J = 12.5, J = 2.0 Hz), 4.16 (dd, 1H, J4,3 = 9.5, J4,5 = 9.5 Hz, H4), 4.80 (d, 1H, J1’,2’ = 10.0 Hz, H1’), 4.88 (d, 1H, J1,2 = 8.0 Hz, H1), 5.36 (dd, 1H, J2’,1’ = 10.0, J2’,3’ = 10.0 Hz, H2’), 5.40 (dd, J3’,2’ = 10.0, J3’,4’ = 3.0 Hz, H3’), 5.54 (d, 1H, J4’,3’ = 3.0 Hz, H4’), 5.63 (dd, J3,2 = 10.0, J3,4 = 9.5 Hz, H3), 5.71 (dd, J2,1 = 8.0, J2,3 = 10.0 Hz, H2), 7.01 - 8.20 (m, 29H, Harom). Structure of compound 18 was indirectly confirmed from the following trisaccharide 3. 2.13. 2,3,4-tri-O-benzoyl-6-deoxy-6-azido-β- D-galactopyranosyl-(1→6)-2,3,4-tri-O- benzoyl-β-D-galactopyranosyl-(1→4)-2,3,6- tri-O-benzoyl-1-(4-tolyl)thio-β-D- glucopyranoside 4 and 2,3,4-tri-O-benzoyl- 6-deoxy-6-azido-β-D-galactopyranosyl- (1→6)-2,3,4-tri-O-benzoyl-β-D- galactopyranosyl-(1→4)-[2,3,4-tri-O- benzoyl-6-deoxy-6-azido-β-D- galactopyranosyl]-(1→6)-2,3-di-O- benzoyl-1-(4-tolyl)thio-β-D- glucopyranoside 4 A mixture of crude compounds 18 and 19 (50 mg, 0.046 mmol, 0.6 eq) and the donor 1 (50 mg, 0.075 mmol) were distilled azeotropically using toluene at 50˚C for three times, which was followed by concentration under re- duced pressure for 1 h. The mixture was transferred to a two-necked round-bottom flask charging with CH2Cl2 (5 mL), followed by a stirring at rt for 30 min. After cooling down with an ice bath, an aliquot of TMSOTf (100 L, equivalent to 0.015 mmol), generated from TMSOTf (30 Copyright © 2013 SciRes. OJMC  K.-I. LIN ET AL. 81 L) in CH2Cl2 (1 mL), was added. After removal of the bath, the mixture was stirred at rt for 10 min. TLC (EtOAc/n-hexane 1:1) indicated the formation of product 3 (Rf = 0.70) and product 4 (Rf = 0.80) and the consump- tion of the donor 1 (Rf = 0.82). After concentration under reduced pressure, the residue was purified using column chromatography with eluents of EtOAc/n-hexane = 3.5/6.5 to provide compound 3 (35 mg) and compound 4 (22 mg) in a total yield of 70%. The samples were further purified using HPLC with eluents of EtOAc/n-hexane = 3/7 to afford compound 4 at tR of 15.2 min and com- pound 3 at t R of 18.0 min, respectively. Compound 3: 1H-NMR (500 MHz, C6D6) δ 1.98 (s, 3H, CH3, HSPhMe), 2.66 (dt, 1H, J = 11.5, J = 7.0 Hz), 2.82 - 2.94 (m, 2H), 3.06 (dd, 1H, J = 10.0, J = 3.5 Hz), 3.31 - 3.42 (m, 3H), 3.95 (dd, 1H, J(gem) = 12.0, J = 4.0 Hz, CHCH2), 4.03 (d, 1H, J(gem) = 11.0 Hz, CHCH2), 4.30 (dd, 1H, J4,3 = 9.0, J4,2 = 9.0 Hz, H4(Glc)), 4.49 (d, 1H, J = 10.0 Hz, Hanomeric), 4.80 (d, 1H, J = 8.0 Hz, Hanomeric), 4.97 (d, 1H, J = 8.0 Hz, Hanomeric), 5.56 (dd, 1H, J = 10.5, J = 3.5 Hz, H3(Gal)), 5.72 (dd, 1 H, J = 10.0, J = 3.5 Hz, H3(Gal)), 5.77 (dd, 1 H, J = 10.0, J = 9.5 Hz, H2(Gal)), 5.78 (d, 1H, J = 3.5 Hz, H4(Gal)), 5.82 (dd, 1 H, J = 9.0, J = 8.5 Hz, H3(Glc)), 5.85 (d, 1 H, J = 3.5 Hz, H4(Gal)), 6.22 (dd, 1H, J = 10.0, J = 8.5 Hz), 6.29 (dd, 1H, J = 10.5, J = 8.0 Hz), 6.68 - 7.19 (m, 31H, Harom), 7.55 (d, 2H, J = 8.0 Hz, Harom), 7.92 - 8.03 (m, 6H, Harom), 8.08 - 8.28 (m, 10H, Harom); 13C-NMR (500 MHz, C6D6) δ 20.93 (CH3), 51.02 (N3CH2), 60.04 (CH2), 68.81 (CH), 68.98 (CH2), 69.35 (CH), 70.81 (CH), 70.89 (CH), 71.40 (CH), 72.16 (CH), 72.65 (CH), 73.55 (CH), 74.10 (CH), 75.23 (CH), 75.85 (CH), 78.65 (CH), 86.07 (CH), 101.07 (CH), 102.39 (CH), 125.64 (CH, Carom), 127.80 (CH, Carom), 127.91 (C, Carom), 128.09 (C, Carom), 128.29 (CH, Carom), 128.43 (CH, Carom), 128.52 (CH, Carom), 128.69 (CH, Carom), 128.85 (CH, Carom), 128.99 (CH, Carom), 129.13 (CH, Carom), 129.25 (CH, Carom), 129.36 (C, Carom), 129.44 (C, Carom), 129.78 (C, Carom), 129.89 (CH, Carom), 129.94 (CH, Carom), 129.96 (CH, Carom), 130.01 (CH, Carom), 130.07 (CH, Carom), 130.12 (CH, Carom), 130.15 (CH, Carom), 130.29 (CH, Carom), 130.31 (CH, Carom), 130.67 (C, Carom), 132.74 (CH, Carom), 133.02 (CH, Carom), 133.11 (CH, Carom), 133.31 (CH, Carom), 133.41 (CH, Carom), 133.44 (CH, Carom), 133.63 (CH, Carom), 133.69 (CH, Carom), 134.14 (CH, Carom), 138.40 (C, Carom), 165.27 (OCO), 165.57 (OCO), 165.71 (OCO), 165.69 (OCO), 165.78 (OCO), 165.85 (OCO), 166.33 (OCO). Compound 4: Anal. C108H90N6O29S, M (calcd.) = 1967.55065 (100.0%), 1966.54730 (83.2%), 1968.55401 (59.5%), 1969.55736 (23.4%), 1970.56072 (6.8%), 1969.55490 (5.8%), 1968.55154 (4.8%), 1969.54645 (4.4%), 1968.54309 (3.7%), 1970.55825 (3.5%), 1970.54980 (2.6%), 1968.54769 (2.2%), 1967.54433 (1.9%), 1971.56407 (1.6%), 1971.56161 (1.4%), 1968.55683 (1.4%), 1969.55104 (1.3%), 1968.55487 (1.2%), 1967.55347 (1.1%), 1971.55316 (1.0%) (m/z), MALDI-Tof: M = 1966.5 (m/z), [M + Na]+= 1989.3; 1H-NMR (500 MHz, C6D6) δ 1.98 (s, 3H, CH3, HSPhCH3), 2.66 (dd, 1H, J(gem) = 13.0, J = 7.0 Hz), 2.72 - 2.79 (m, 2H), 2.83 (dd, 1 H, J = 10.0 J = 4.5 Hz), 3.05 - 3.12 (m, 1H), 3.16 (t, 1H, J = 9.0 Hz), 3.21 (t, 1H, J = 6.0 Hz), 3.25 - 3.35 (m, 2H), 3.39 (dd, 1H, J = 8.5, 5.0 Hz), 3.90 (dd, 1H, J(gem) = 11.0, J= 4.0 Hz), 4.06 (d, 1H, J = 10.5 Hz), 4.12 (d, 1H, J = 8.0 Hz, Hanomeric), 4.13 (t, 1H, J = 10.0, 10.0 Hz, H4(Glc)), 4.50 (d, 1H, J = 9.5 Hz, Hanomeric), 4.71 - 4.79 (m, 2H, Hanomeric), 5.52 (dd, 1H, JAA = 10.5, JAE = 3.5 Hz, HAxial), 5.61 (dd, 1H, JAA = 10.5, JAE = 4.0 Hz, HAxial), 5.69 (t, 1H, JAA = 9.5, JAA = 9.5 Hz, HAxial), 5.69 (dd, 1H, JAA = 10.5, JAE = 4.0 Hz, HAxial), 5.82 (d, 1H, JEA = 3.0 Hz, HEquatorial), 5.86 (t, 1H, JAA = 9.0, JAA = 9.0 Hz, HAxial), 5.87 (d, 1H, JEA = 3.0 Hz, HEquatorial), 6.06 (dd, 1 H, JAA = 10.0, JAA = 7.5 Hz, HAxial), 6.10 (d, 1 H, JEA = 3.0 Hz, HEquatorial), 6.11 (dd, 1H, JAA = 10.5, JAA = 8.0 Hz, HAxial), 6.27 (dd, 1H, JAA =10.5, JAA =8.0 Hz, HAxial), 6.68 - 7.14 (m, 37H, Harom), 7.57 (d, 2H, J = 8.0 Hz, Harom), 7.96 - 8.26 (m, 20H, Harom); 13C-NMR (125 MHz, C6D6) δ 20.94 (CH3), 49.89 (N3CH2), 50.97 (N3CH2), 66.12 (CH2), 67.87 (CH), 68.25 (CH2), 68.53 (CH), 69.40 (CH), 70.46 (CH), 70.58 (CH), 71.05 (CH), 71.35 (CH), 71.69 (CH), 72.13 (CH), 72.23 (CH), 72.37 (CH), 73.58 (CH), 75.27 (CH), 76.66 (CH), 78.43 (CH), 85.94 (CH), 100.98 (CH), 101.54 (CH), 102.12 (CH), 125.64 (C, Carom), 128.45 (CH, Carom), 128.50 (CH, Carom), 128.63 (CH, Carom), 128.68 (CH, Carom), 128.86 (CH, Carom), 128.90 (CH, Carom), 129.12 (CH, Carom), 129.28 (CH, Carom), 129.32 (C, Carom), 129.41 (C, Carom), 129.68 (C, Carom), 129.74 (C, Carom), 129.96 (CH, Carom), 130.06 (CH, Carom), 130.19 (CH, Carom), 130.26 (CH, Carom), 130.41 (CH, Carom), 130.98 (C, Carom), 132.52 (CH, Carom), 133.02 (CH, Carom), 133.19 (CH, Carom), 133.26 (CH, Carom), 133.31 (CH, Carom), 133.55 (CH, Carom), 133.94 (CH, Carom), 134.22 (CH, Carom), 138.37 (C, Carom), 165.24 (OCO), 165.28 (OCO), 165.36 (OCO), 165.47 (OCO), 165.54 (OCO), 165.61 (OCO), 165.77 (OCO), 165.81 (OCO). 3. Results and Discussion Preparation of the imidate 1 was accomplished by using a common protocol via first deprotection of the anomeric hydroxy group of the thioglycoside 7 followed by intro- duction of the trichloroacetamide (Scheme 1) [28]. Ac- ceptor 8 was obtained from 4-amino-1-butanol via a di- azo transfer reaction [29]. The subsequent glycosylation for the donor 1 and acceptor 8, deprotection of the hy- droxy groups and reduction of both amino groups of glycosylated product 9 proceeded effortlessly. The de- sired amino sugar 2 could be prepared in an acceptable Copyright © 2013 SciRes. OJMC 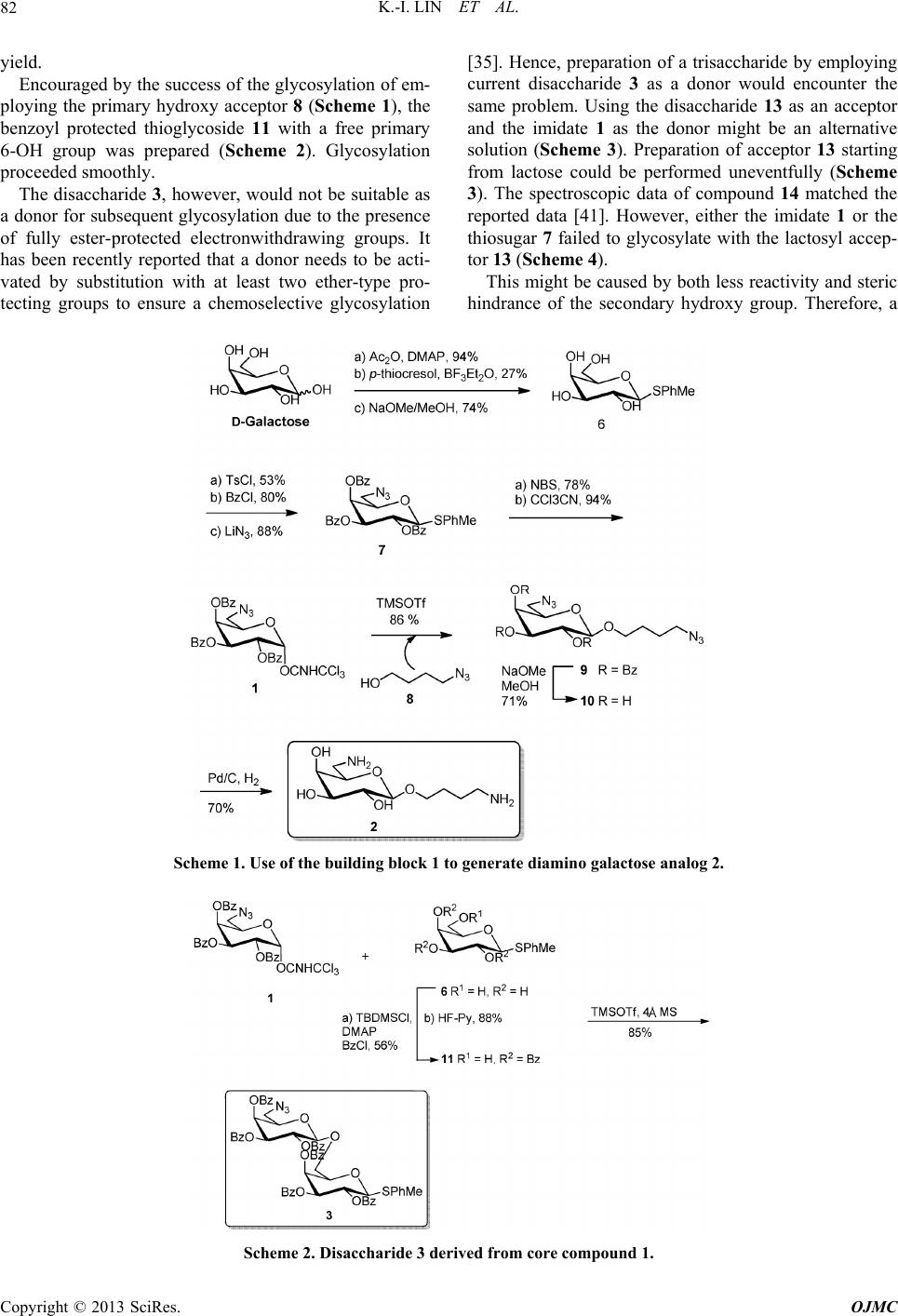 K.-I. LIN ET AL. Copyright © 2013 SciRes. OJMC 82 [35]. Hence, preparation of a trisaccharide by employing current disaccharide 3 as a donor would encounter the same problem. Using the disaccharide 13 as an acceptor and the imidate 1 as the donor might be an alternative solution (Scheme 3). Preparation of acceptor 13 starting from lactose could be performed uneventfully (Scheme 3). The spectroscopic data of compound 14 matched the reported data [41]. However, either the imidate 1 or the thiosugar 7 failed to glycosylate with the lactosyl accep- tor 13 (Scheme 4). yield. Encouraged by the success of the glycosylation of em- ploying the primary hydroxy acceptor 8 (Scheme 1), the benzoyl protected thioglycoside 11 with a free primary 6-OH group was prepared (Scheme 2). Glycosylation proceeded smoothly. The disaccharide 3, however, would not be suitable as a donor for subsequent glycosylation due to the presence of fully ester-protected electronwithdrawing groups. It has been recently reported that a donor needs to be acti- vated by substitution with at least two ether-type pro- tecting groups to ensure a chemoselective glycosylation This might be caused by both less reactivity and steric indrance of the secondary ydroxy group. Therefore, a h h Scheme 1. Use of the building block 1 to generate diamino galactose analog 2. Scheme 2. Disaccharide 3 derived from core compound 1. 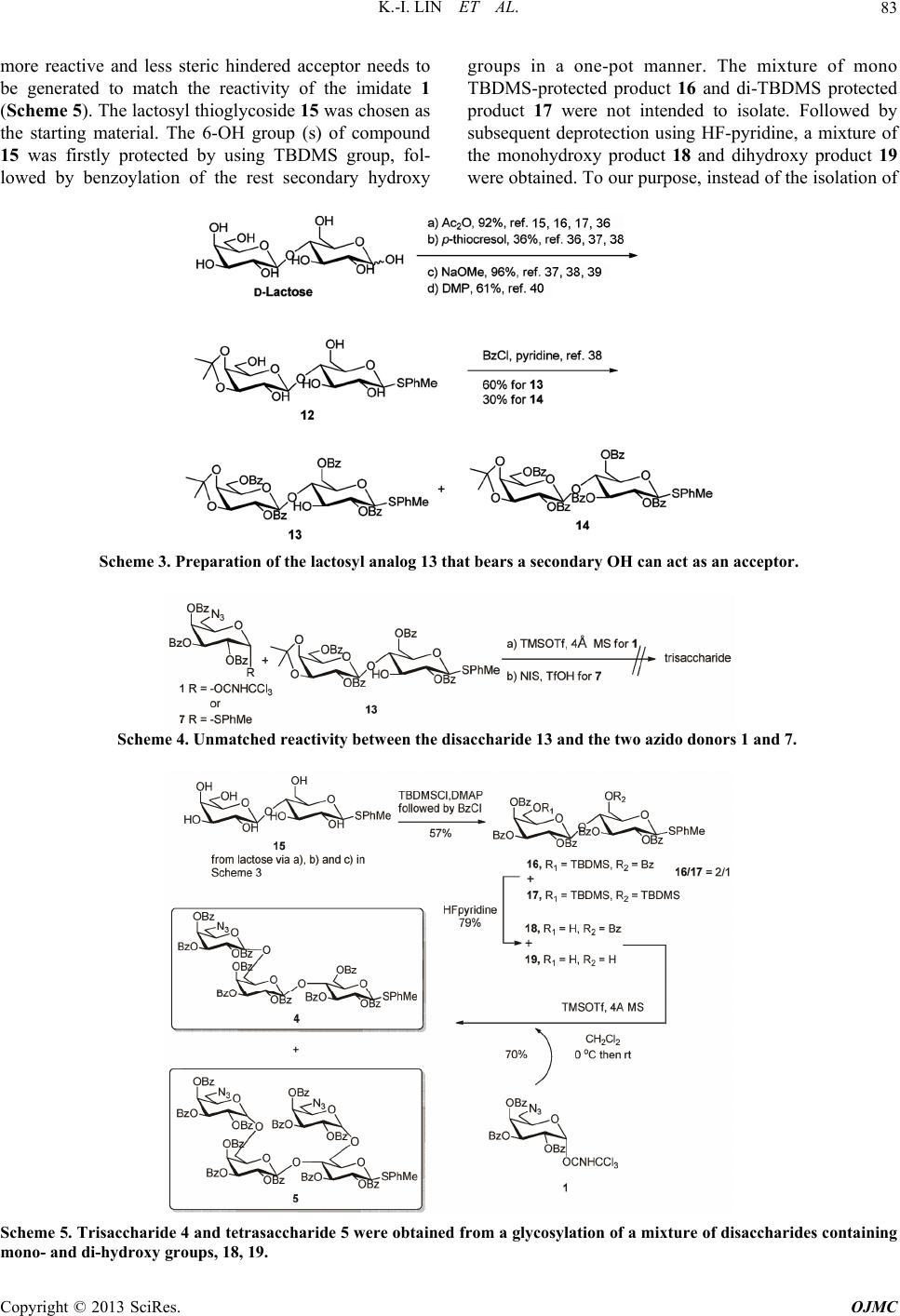 K.-I. LIN ET AL. 83 more reactive and less steric hindered acceptor needs to be generated to match the reactivity of the imidate 1 (Scheme 5). The lactosyl thioglycoside 15 was chosen as the starting material. The 6-OH group (s) of compound 15 was firstly protected by using TBDMS group, fol- lowed by benzoylation of the rest secondary hydroxy groups in a one-pot manner. The mixture of mono TBDMS-protected product 16 and di-TBDMS protected product 17 were not intended to isolate. Followed by subsequent deprotection using HF-pyridine, a mixture of the monohydroxy product 18 and dihydroxy product 19 were obtained. To our purpose, instead of the isolation of Scheme 3. Preparation of the lactosyl analog 13 that bears a secondary OH can act as an acceptor. Scheme 4. Unmatched reactivity between the disaccharide 13 and the two azido donors 1 and 7. Scheme 5. Trisaccharide 4 and tetrasaccharide 5 were obtained from a glycosylation of a mixture of disaccharides containing mono- and di-hydroxy groups, 18, 19. Copyright © 2013 SciRes. OJMC  K.-I. LIN ET AL. 84 the two acceptors, the mixture as a whole was glyco- sylated with the imidate 1. The desired tri- and tetra- saccharides could then be prepared and the subsequent isolation using HPLC proceeded uneventfully. The stereochemistry of glycosidic bonds of mono- saccharide 2, disaccharide 3 and trisaccharide 4 was identified as β-conformation as evidenced from the cou- pling constant ranging from J = 8.0 to 10.0 Hz by 1H- NMR spectroscopy. The data matched the literature [30- 34]. 4. Conclusion In brief, the current glycosylation strategy by using 6- azido galactosyl imidate 1 as a donor and the 6-OH bear- ing saccharides as acceptors were capable of generating the azido-bearing oligosaccharides for subsequent elabo- ration to glycoconjugates. When obtaining the conjugates, the amino groups will be reduced. The subsequent amide conjugation and the bioactivity screening could then be forwarded. 5. Acknowledgements We are grateful to the National Science Council of Tai- wan, Chang-Bing Show Chwan Memorial Hospital, CGMH_NTHU Joint Research, and Chang-Gung Medi- cal Research Project for providing financial support through grant numbers NSC-100-2113-M-007-003, NSC- 101-2113-M-007-010, NSC-97-2314-B-182A-020-MY3, NSC-97-2314-B-182A-020-MY3, CGTH96N2342E1, CMRPG3B0531, CMRPG390661, CMRPG390931, and CMRPG3B0361. REFERENCES [1] P. J. Brennan, M. Brigl and M. B. Brenner, “Invariant Na- tural Killer T Cells: An Innate Activation Scheme Linked to Diverse Effector Functions,” Nature Reviews Immu- nology, Vol. 13, No. 2, 2013, pp. 107-117. doi:10.1038/nri3369 [2] M. J. Smyth, N. Y. Crowe, Y. Hayakawa, K. Takeda, H. Yagita and D. Godfrey, “NKT Cells—Conductors of Tumor Immunity?” Current Opinion in Immunology, Vol. 14, No. 2, 2002, pp. 165-171. doi:10.1016/S0952-7915(02)00316-3 [3] L. Zhang, F. Sun, Y. X. Li, X. Sun, X. M. Liu, Y. S. Huang, L. H. Zhang, X. S. Ye and J. Xiao, “Rapid Syn- thesis of Iminosugar Derivatives for Cell-Based in Situ Screening: Discovery of ‘Hit’ Compounds with Antican- cer Activity,” ChemMedChem, Vol. 2, No. 11, 2007, pp. 1594-1597. doi:10.1002/cmdc.200700120 [4] G. T. Le, G. Abbenante, B. Becker, M. Grathwohl, J. Hal- liday, G. Tometzki, J. Zuegg and W. Meutermans, “Mo- lecular Diversity through Sugar Scaffolds,” Drug Dis- covery Today, Vol. 8, No. 15, 2003, pp. 701-709. doi:10.1016/S1359-6446(03)02751-X [5] B. Elchert, J. Li, J. H. Wang, Y. Hui, R. Rai, R. Ptak, P. Ward, J. Y. Takemoto, M. Bensaci and C. W. T. Chang, “Application of the Synthetic Aminosugars for Glycodi- versification: Synthesis and Antimicrobial Studies of Py- ranmycin,” The Journal of Organic Chemistry, Vol. 69, No. 5, 2004, pp. 1513-1523. doi:10.1021/jo035290r [6] R. Liang, L. Yan, J. Loebach, M. Ge, Y. Uozumi, K. Sekanina, N. Horan, J. Gildersleeve, C. Thompson, A. Smith, K. Biswas, W. C. Still and D. Kahne, “Parallel Synthesis and Screening of a Solid Phase Carbohydrate Library,” Science, Vol. 274, No. 5292, 1996, pp. 1520- 1522. doi:10.1126/science.274.5292.1520 [7] D. L. Boger, J. Desharnais and K. Capps, “Solution-Phase Combinatorial Libraries: Modulating Cellular Signaling by Targeting Protein-Protein or Protein-DNA Interac- tions,” Angewandte Chemie International Edition, Vol. 42, No. 35, 2003, pp. 4138-4176. doi:10.1002/anie.200300574 [8] R. A. Houghten, “Parallel Array and Mixture-Based Syn- thetic Combinatorial Chemistry: Tools for the Next Mil- lennium,” Annual Review of Pharmacology and Toxicol- ogy, Vol. 40, 2000, pp. 273-282. doi:10.1146/annurev.pharmtox.40.1.273 [9] L.-W. Chiang, K. Pei, S.-W. Chen, H.-L. Huang, K.-J. Lin, T.-C. Yen and C.-S. Yu, “Combining a Solution- Phase Derived Library with In-Situ Cellular Bioassay: Prompt Screening of Amide-Forming Minilibraries Using MTT Assay,” Chemical and Pharmaceutical Bulletin, Vol. 57, No. 7, 2009, pp.714-718. doi:10.1248/cpb.57.714 [10] K.-I Lin, C.-H. Yang, C.-W. Huang, J.-Y. Jian, Y.-C. Huang and C.-S. Yu, “Synthesis and Structure-Activity Relationships of Fenbufen Amide Analogs,” Molecules, Vol. 15, No. 12, 2010, pp. 8796-8803. doi:10.3390/molecules15128796 [11] Y.-H. Su, L.-W. Chiang, K.-C. Jeng, H.-L. Huang, J. Chen, W. Z. Lin, C.-W. Huang and C.-S. Yu, “Solution- Phase Parallel Synthesis and Screening of Anti-Tumor Activities from Fenbufen and Ethacrynic Acid Libraries,” Bioorganic & Medicinal Chemistry Letters, Vol. 21, No. 5, 2011, pp. 1320-1324. doi:10.1016/j.bmcl.2011.01.068 [12] Y.-C. Huang, L.-W. Chiang, K.-S. Chang, W.-C. Su, Y.-H. Lin, K.-C. Jeng, K.-I. Lin, K.-Y. Liao, H.-L. Huang and C.-S. Yu, “Synthesis of Amino Cores of Galactosyl Ceramide Analogs for Developing INKT-Cell Inducers,” Molecules, Vol. 17, No. 3, 2012, pp. 3058-3081. [13] H.-L. Huang, C.-N. Yeh, K.-W. Chang, J. Chen, K.-J. Lin, L.-W. Chiang, K.-C. Jeng, W.-T. Wang, K.-H. Lim, C. G. Chen, K.-I. Lin, Y.-C. Huang, W.-J. Lin, T.-C. Yen and C.-S. Yu, “Synthesis and Evaluation of [18F]Fluorobutyl Ethacrynic Amide: A Potential PET Tracer for Studying Glutathione Transferase,” Bioorganic & Medicinal Che- mistry Letters, Vol . 22, No. 13, 2012, pp. 3998-4003. [14] H.-L. Huang, C.-N. Yeh, W.-Y. Lee, Y.-C. Huang, K.-W. Chang, K.-J. Lin, S.-F. Tien, W.-C. Su, C.-H. Yang, J.-T. Chen, W.-J. Lin, S.-S. Fan and C.-S. Yu, “[123I]Iodooctyl Fenbufen Amide as a SPECT Tracer for Imaging Tumors that Over-Express COX Enzymes,” Biomaterials, Vol. 34, No. 13, 2013, pp. 3355-3365. Copyright © 2013 SciRes. OJMC  K.-I. LIN ET AL. 85 [15] O. Plettenburg, V. Bodmer-Narkevitch and C. H. Wong, “Synthesis of Alpha-Galactosyl Ceramide, a Potent Im- munostimulatory Agent,” The Journal of Organic Chem- istry, Vol. 67, No. 13, 2002, pp. 4559-4564. doi:10.1021/jo0201530 [16] C. S. Yu, H. Y. Wang, L. W. Chiang and K. Pei, “Syn- thesis of the Rhamnosyl Trisaccharide Repeating Unit to Mimic the Antigen Determinant of Pseudomonas Syrin- gae Lipopolysaccharide,” Synthesis, No. 9, 2007, pp. 1412-1420. doi:10.1055/s-2007-965995 [17] C. S. Yu, K. Niikura, C. C. Lin and C. H. Wong, “The Thioglycoside and Glycosyl Phosphite of 5-Azido Sialic Acid: Excellent Donors for the Alpha-Glycosylation of Primary Hydroxy Groups,” Angewandte Chemie Interna- tional Edition, Vol. 40, No. 15, 2001, pp. 2900-2903. doi:10.1002/1521-3773(20010803)40:15<2900::AID-AN IE2900>3.0.CO;2-4 [18] Z. Y. Zhang, I. R. Ollmann, X. S. Ye, R. Wischnat, T. Baasov and C. H. Wong, “Programmable One-Pot Oligo- saccharide Synthesis,” Journal of the American Chemical Society, Vol. 121, No. 4, 1999, pp. 734-753. doi:10.1021/ja982232s [19] S. Y. Hsieh, M. D. Jan, L. N. Patkar, C. T. Chen and C. C. Lin, “Synthesis of a Carboxyl Linker Containing P-K Trisaccharide,” Carbohydrate Research, Vol. 340, No. 1, 2005, pp. 49-57. doi:10.1016/j.carres.2004.10.024 [20] A. Patel and T. K. Lindhorst, “Synthesis of ‘Mixed-Type’ Oligosaccharide Mimetics Based on a Carbohydrate Scaf- fold,” European Journal of Organic Chemistry, No. 1, 2002, pp. 79-86. [21] C. S. Yu, C. H. Wu, L. W. Chiang and R. T. Wang, H. Y. Wang, C. H. Yeh and K. I. Lin, “Synthesis of (E)-5- (2-Radioiodovinyl)arabinosyl Uridine Analog for Probing HSV-1 Thymidine Kinase Gene,” Chemistry Letters, Vol. 34, No. 10, 2005, pp. 1390-1391. doi:10.1246/cl.2005.1390 [22] K.-I. Lin, L.-W. Chiang, C.-H. Wu, S.-W. Chen and C.-S. Yu, “Synthesis of 5-Radioiodoarabinosyl Uridine Analog for Probing the HSV-1 Thymidine Kinase Gene,” Journal of the Chinese Chemical Society, Vol. 54, No. 2, 2007, pp. 563-568. [23] C. S. Yu and F. Oberdorfer, “Synthesis of 4-O-Methyl- Protected 5-(2-Hydroxyethy)-2’-Deoxyuridine Deriva- tives and their Nucleophilic Fluorination to 5-(2-Fluoro- ethyl)-2’-Deoxyuridine,” Synthesis, No. 12, 1999, pp. 2057-2064. doi:10.1055/s-1999-3641 [24] C. S. Yu and F. Oberdorfer, “Synthesis of (E)-5-[2-(Tri- N-Butylstannyl)Vinyl] Substituted 2’-Deoxyuridine De- rivatives for Use in Halogenation and Radiohalogenation Reactions,” Synlett, No. 1, 2000, pp. 86-88. [25] C. S. Yu, R. T. Wang, L. W. Chiang and M. H. Lee, “Synthesis of 4’,4’-C-Diaminomethyl Nucleoside Deri- vative As a Building Block for Constructing Libraries via Amide Bond Formation,” Tetrahedron Letters, Vol. 48, No. 17, 2007, pp. 2979-2982. doi:10.1016/j.tetlet.2007.03.002 [26] H. C. Hansen and G. Magnusson, “Synthesis of Selected Aminodeoxy Analogues of Galabiose and Globotriose,” Carbohydrate Research, Vol. 322, No. 3-4, 1999, pp. 166-180. doi:10.1016/S0008-6215(99)00229-3 [27] F. L. Lin, H. van Halbeek and C. R. Bertozzi, Synthesis of Mono- and Dideoxygenated, -Trehalose Analogs,” Carbohydrate Research, Vol. 342, No. 14, 2007, pp. 2014-2030. doi:10.1016/j.carres.2007.05.009 [28] X. Zhu and R. R. Schmidt, “Glycoside Synthesis from 1- Oxygen-Substituted Glycosyl Imidates,” In: A. V. Dem- chenko, Ed., Handbook of Chemical Glycosylation: Ad- vances in Stereoselectivity and Therapeutic Relevance, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2008, pp. 143-185. [29] P. B. Alper, S. C. Hung and C. H. Wong, “Metal Cata- lyzed Diazo Transfer for the Synthesis of Azides from Amines,” Tetrahedron Letters, Vol. 37, No. 34, 1996, pp. 6029-6032. doi:10.1016/0040-4039(96)01307-X [30] A. X. Li and F. Z. Kong, “Syntheses of Beta-(1→6)- Branched Beta-(1→3)-Linked D-Galactans that Exist in the Rhizomes of Atractylodes Lancea DC,” Carbohydrate Research, Vol. 340, No. 12, 2005, pp. 1949-1962. doi:10.1016/j.carres.2005.05.017 [31] Y. G. Gu, M. M. Zhang, F. Yang and G. F. Gu, “A Sim- ple Access to 3,6-Branched Oligosaccharides: Synthesis of a Glycopeptide Derivative that Relates to Lycium Bar- barum L,” Journal of the Chemical Society, Perkin Tran- sactions 1, No. 23, 2001, pp. 3122-3127. [32] A. X. Li and F. Z. Kong, “Syntheses of Arabinogalactans Consisting of Beta-(1→6)-Linked D-Galactopyranosyl Backbone and Alpha-(1→3)-Linked L-Arabinofuranosyl Side Chains,” Carbohydrate Research, Vol. 339, No. 11, 2004, pp. 1847-1856. doi:10.1016/j.carres.2004.05.007 [33] T. Yamamura, N. Hada, A. Kaburaki, K. Yamano and T. Takeda, “Synthetic Studies on Glycosphingolipids from Protostomia Phyla: Total Syntheses of Glycosphingolip- ids from the Parasite, Echinococcus Multilocularis,” Car- bohydrate Research, Vol. 339, No. 17, 2004, pp. 2749- 2759. doi:10.1016/j.carres.2004.09.015 [34] J. Ning, Y. Yi and Z. Yao, “An Efficient Method for the Synthesis of 2,6-Branched Galacto-Oligosaccharides and its Applications to the Synthesis of three Tetrasaccharides and a Hexasaccharide Related to the Arabinogalactans (Ags),” Synlett, No. 14, 2003, 2208-2212. [35] Y. Zeng, Z. Wang, D. Whitfield and X. Huang, “Instal- lation of Electron-Donating Protective Groups, a Strategy for Glycosylating Unreactive Thioglycosyl Acceptors Using the Preactivation-Based Glycosylation Method,” The Journal of Organic Chemistry, Vol. 73, No. 20, 2008, pp. 7952-7962. doi:10.1021/jo801462r [36] C. S. Chao, M. C. Chen, S. C. Lin and K. K. T. Mong, “Versatile Acetylation of Carbohydrate Substrates with Bench-Top Sulfonic Acids and Application to One-Pot Syntheses of Peracetylated Thioglycosides,” Carbohy- drate Research, Vol. 343, No. 5, 2008, pp. 957-964. doi:10.1016/j.carres.2008.01.014 [37] L. Chen, F. F. Liang, M. F. Xu, G. W. Xing and Z. W. Deng, “Synthesis of the Methyl Glycoside of Ganglioside GM(3) Trisaccharide Derivative with N-Acetyl-5-N,4-O- Oxazolidinone Protected P-Toluenethiosialoside,” Acta Chimica Sinica, Vol. 67, No. 12, 2009, pp. 1355-1362. Copyright © 2013 SciRes. OJMC  K.-I. LIN ET AL. Copyright © 2013 SciRes. OJMC 86 [38] C. Y. Liu, H. L. Chen, C. M. Ko and C. T. Chen, “Che- moselective Deacylation of Functionalized Esters Cata- lyzed by Dioxomolybdenum Dichloride,” Tetrahedron, Vol. 67, No. 5, 2011, pp. 872-876. doi:10.1016/j.tet.2010.12.024 [39] M.-C. Yan, Y.-N. Chen, H.-T. Wu, C.-C. Lin, C.-T. Chen and C.-C. Lin, “Removal of Acid-Labile Protecting Groups on Carbohydrates Using Water-Tolerant and Re- coverable Vanadyl Triflate Catalyst,” The Journal of Or- ganic Chemistry, Vol. 72, No. 1, 2007, pp. 299-302. doi:10.1021/jo061881g [40] C. C. Lin, M. D. Jan, S. S. Weng, C. C. Lin and C. T. Chen, “O-Isopropylidenation of Carbohydrates Catalyzed by Vanadyl Triflate,” Carbohydrate Research, Vol. 341, No. 14, 2006, pp. 1948-1953. doi:10.1016/j.carres.2006.04.001 [41] T. Tsukida, M. Yoshida, K. Kurokawa, Y. Nakai, T. Achiha, T. Kiyoi and H. Kondo, “A Highly Practical Syn- thesis of Sulfated Lewis X: One-Pot, Two-Step Glycosy- lation Using ‘Armed/Disarmed’ Coupling and Selective Benzoylation and Sulfation,” The Journal of Organic Chemistry, Vol. 62, No. 20, 1997, pp. 6876-6881. doi:10.1021/jo970076m
|