New Matrix Tablet from Okra Gum: Effects of Method of Preparation and Gum Concentration on Tablet Properties
488
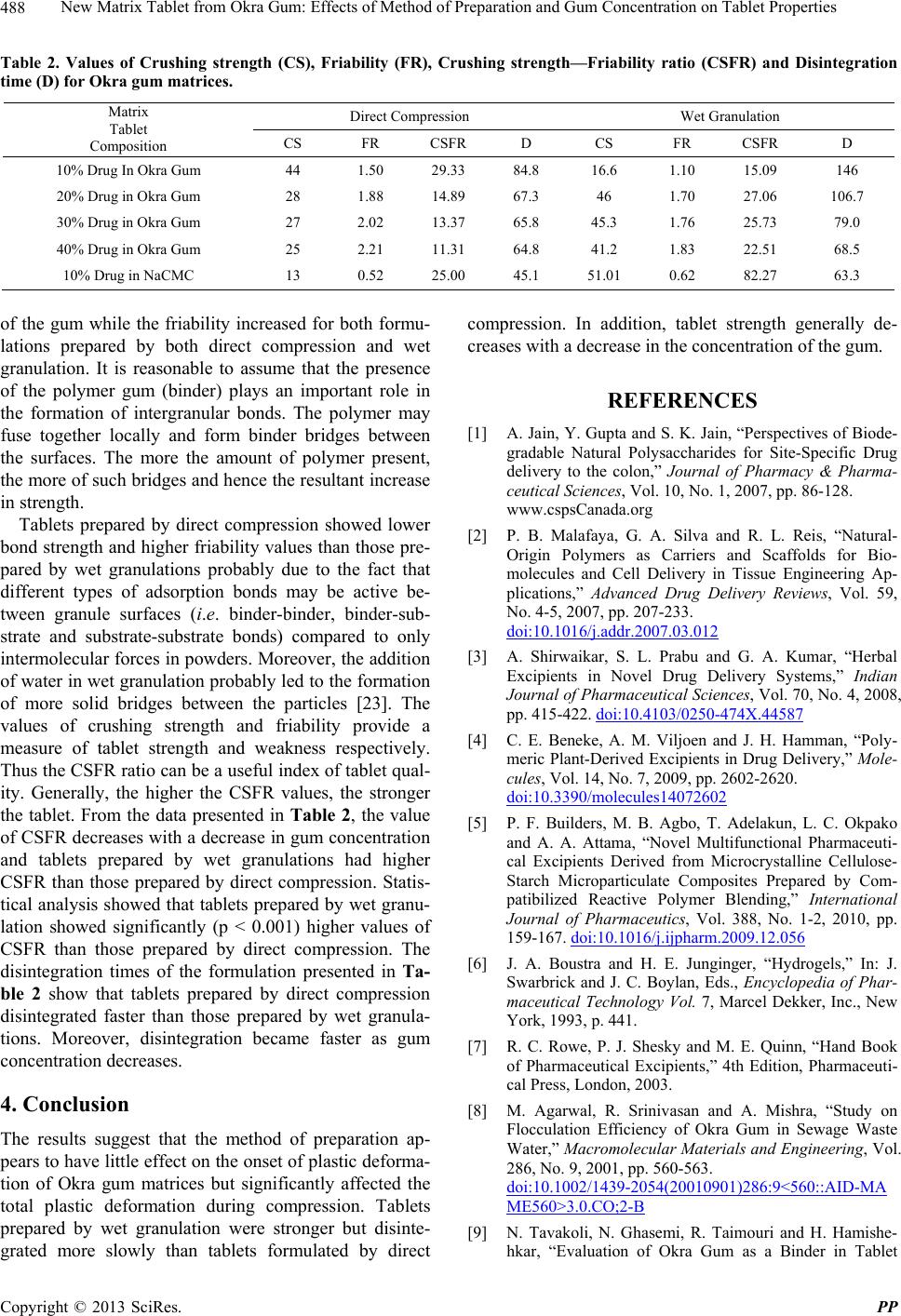
Table 2. Values of Crushing strength (CS), Friability (FR), Crushing strength—Friability ratio (CSFR) and Disintegration
time (D) for Okra gum matrices.
Direct Compression Wet Granulation
Matrix
Tablet
Composition CS FR CSFR D CS FR CSFR D
10% Drug In Okra Gum 44 1.50 29.33 84.8 16.6 1.10 15.09 146
20% Drug in Okra Gum 28 1.88 14.89 67.3 46 1.70 27.06 106.7
30% Drug in Okra Gum 27 2.02 13.37 65.8 45.3 1.76 25.73 79.0
40% Drug in Okra Gum 25 2.21 11.31 64.8 41.2 1.83 22.51 68.5
10% Drug in NaCMC 13 0.52 25.00 45.1 51.01 0.62 82.27 63.3
of the gum while the friability increased for both formu-
lations prepared by both direct compression and wet
granulation. It is reasonable to assume that the presence
of the polymer gum (binder) plays an important role in
the formation of intergranular bonds. The polymer may
fuse together locally and form binder bridges between
the surfaces. The more the amount of polymer present,
the more of such bridges and hence the resultant increase
in strength.
Tablets prepared by direct compression showed lower
bond strength and higher friability values than those pre-
pared by wet granulations probably due to the fact that
different types of adsorption bonds may be active be-
tween granule surfaces (i.e. binder-binder, binder-sub-
strate and substrate-substrate bonds) compared to only
intermolecular forces in powders. Moreover, the addition
of water in wet granulation probably led to the formation
of more solid bridges between the particles [23]. The
values of crushing strength and friability provide a
measure of tablet strength and weakness respectively.
Thus the CSFR ratio can be a useful index of tablet qual-
ity. Generally, the higher the CSFR values, the stronger
the tablet. From the data presented in Table 2, the value
of CSFR decreases with a decrease in gum concentration
and tablets prepared by wet granulations had higher
CSFR than those prepared by direct compression. Statis-
tical analysis showed that tablets prepared by wet granu-
lation showed significantly (p < 0.001) higher values of
CSFR than those prepared by direct compression. The
disintegration times of the formulation presented in Ta-
ble 2 show that tablets prepared by direct compression
disintegrated faster than those prepared by wet granula-
tions. Moreover, disintegration became faster as gum
concentration decreases.
4. Conclusion
The results suggest that the method of preparation ap-
pears to have little effect on the onset of plastic deforma-
tion of Okra gum matrices but significantly affected the
total plastic deformation during compression. Tablets
prepared by wet granulation were stronger but disinte-
grated more slowly than tablets formulated by direct
compression. In addition, tablet strength generally de-
creases with a decrease in the concentration of the gum.
REFERENCES
[1] A. Jain, Y. Gupta and S. K. Jain, “Perspectives of Biode-
gradable Natural Polysaccharides for Site-Specific Drug
delivery to the colon,” Journal of Pharmacy & Pharma-
ceutical Sciences, Vol. 10, No. 1, 2007, pp. 86-128.
www.cspsCanada.org
[2] P. B. Malafaya, G. A. Silva and R. L. Reis, “Natural-
Origin Polymers as Carriers and Scaffolds for Bio-
molecules and Cell Delivery in Tissue Engineering Ap-
plications,” Advanced Drug Delivery Reviews, Vol. 59,
No. 4-5, 2007, pp. 207-233.
doi:10.1016/j.addr.2007.03.012
[3] A. Shirwaikar, S. L. Prabu and G. A. Kumar, “Herbal
Excipients in Novel Drug Delivery Systems,” Indian
Journal of Pharmaceutical Sciences, Vol. 70, No. 4, 2008,
pp. 415-422. doi:10.4103/0250-474X.44587
[4] C. E. Beneke, A. M. Viljoen and J. H. Hamman, “Poly-
meric Plant-Derived Excipients in Drug Delivery,” Mole-
cules, Vol. 14, No. 7, 2009, pp. 2602-2620.
doi:10.3390/molecules14072602
[5] P. F. Builders, M. B. Agbo, T. Adelakun, L. C. Okpako
and A. A. Attama, “Novel Multifunctional Pharmaceuti-
cal Excipients Derived from Microcrystalline Cellulose-
Starch Microparticulate Composites Prepared by Com-
patibilized Reactive Polymer Blending,” International
Journal of Pharmaceutics, Vol. 388, No. 1-2, 2010, pp.
159-167. doi:10.1016/j.ijpharm.2009.12.056
[6] J. A. Boustra and H. E. Junginger, “Hydrogels,” In: J.
Swarbrick and J. C. Boylan, Eds., Encyclopedia of Phar-
maceutical Technology Vol. 7, Marcel Dekker, Inc., New
York, 1993, p. 441.
[7] R. C. Rowe, P. J. Shesky and M. E. Quinn, “Hand Book
of Pharmaceutical Excipients,” 4th Edition, Pharmaceuti-
cal Press, London, 2003.
[8] M. Agarwal, R. Srinivasan and A. Mishra, “Study on
Flocculation Efficiency of Okra Gum in Sewage Waste
Water,” Macromolecular Materials and Engineering, Vol.
286, No. 9, 2001, pp. 560-563.
doi:10.1002/1439-2054(20010901)286:9<560::AID-MA
ME560>3.0.CO;2-B
[9] N. Tavakoli, N. Ghasemi, R. Taimouri and H. Hamishe-
hkar, “Evaluation of Okra Gum as a Binder in Tablet
Copyright © 2013 SciRes. PP