 Journal of Biomaterials and Nanobiotechnology, 2013, 4, 327-333 http://dx.doi.org/10.4236/jbnb.2013.44041 Published Online October 2013 (http://www.scirp.org/journal/jbnb) 327 Synthesis and Structural Characterization of Hydroxyapatite-Wollastonite Biocomposites, Produced by an Alternative Sol-Gel Route Martín A. Encinas-Romero1*, Jesús Peralta-Haley2, Jesús L. Valenzuela-García1, Felipe F. Castillón-Barraza3 1Departamento de Ingeniería Química y Metalurgia, Universidad de Sonora, Hermosillo, México; 2Posgrado en Ciencias de la Ingeniería: Ingeniería Química, Universidad de Sonora, Hermosillo, México; 3Centro de Nanociencias y Nanotecnología, Uni- versidad Nacional Autónoma de México, Ensenada B.C., México. Email: *maencinas@iq.uson.mx Received July 20th, 2013; revised August 18th, 2013; accepted September 3rd, 2013 Copyright © 2013 Martín A. Encinas-Romero et al. This is an open access article distributed under the Creative Commons Attribu- tion License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Hydroxyapatite is a type of calcium phosphate-based material with great interest for biomedical applications, due to the chemical similarity between this material and the mineral part of human bone. However, synthetic hydroxyapatite is essentially brittle; the practice indicates that the use of hydroxyapatite without additives for implant production is not efficient, due to its low strength parameters. In the present work, biocomposites of hydroxyapatite-wollastonite were synthesized by an alternative sol-gel route, using calcium nitrate and ammonium phosphate as precursors of hydroxya- patite, and high purity natural wollastonite was added in ratios of 20, 50 and 80 percent by weight immersed in aqueous medium. Formation of hydroxyapatite occurs at a relatively low temperature of about 350˚C, while the wollastonite re- mains unreacted. After that, these biocomposites were sintered at 1200˚C for 5 h to produce dense materials. The char- acterization techniques demonstrated the presence of hydroxyapatite and wollastonite as unique phases in all products. Keywords: Hydroxyapatite; Wollastonite; Bioceramics; Biocomposites; Sol-Gel 1. Introduction Hydroxyapatite (Ca10(PO4)6(OH)2), is the predominant mineral component of vertebrate bones and dental tissue: teeth and enamel. Its clinical applications are of great importance, because it is the calcium phosphate ceramic chemically more similar to the biological apatite crystals. For this reason, many processing routes have been de- veloped for synthesizing fine hydroxyapatite and sinter- ing the reactive powders to form a dense bioceramic. The most common approaches reported include precipitation, solid state reaction, sol-gel methods, hydrothermal route, emulsion and microemulsion techniques, mechanochemi- cal reactions, and a combination of mechanochemical, hydrothermal, and ultrasonically assisted reactions [1-4]. The sol-gel process is one of the most important methods for the production of biomaterials, its advantages include the use of inexpensive and readily available reagents, and it is an effective method for the preparation of highly pure powder due to the possibility of a strict control of the process parameters. This method offers a molecular mixing of calcium and phosphorus, capable of improving chemical homogeneity. Moreover, the high reactivity of the sol-gel powders allows a reduction of the processing temperature and of any degradation phenomena during sintering [5]. However, the mechanical properties of hydroxyapatite are not good enough to be used as an implant in load bearing situations, like artificial teeth or bones. One ap- proach to solve this problem is to combine it with a suit- able reinforcement phase, producing a biocomposite which provides optimum mechanical properties, by overcoming mechanical limitations. The ideal biocomposite should be the one that who combines their biological and mechani- cal properties, to provide adequate support for the hard tissues [6,7]. Wollastonite (CaSiO3), is a calcium silicate which has *Corresponding author. Copyright © 2013 SciRes. JBNB  Synthesis and Structural Characterization of Hydroxyapatite-Wollastonite Biocomposites, Produced by an Alternative Sol-Gel Route 328 been widely used as a filler to fabricate composites with improved mechanical properties. Moreover, it has also been used as a medical material for artificial bones and dental roots because of its good bioactivity and biocom- patibility. Natural bone is a bioceramic composite made up of small hydroxyapatite crystal particles reinforced by organic collagen fibers. Because of its outstanding me- chanical properties, researchers sought ways of duplicat- ing its mechanical properties [8,9]. Kokubo and col- leagues attempted to prepare a similar composite by the crystallization process, developing a clinically important glass ceramic (A-W glass-ceramic) [8,10]. A-W glass- ceramic is an assembly of small apatite particles, effec- tively reinforced by wollastonite in a glassy matrix. The bending strength, fracture toughness, and Young’s mo- dulus of A-W glass-ceramic are the highest among bio- active glass and glass-ceramics [11]. Many studies have involved the wollastonite phase in natural or synthetic forms to produce different composites in which its pres- ence improved some mechanical properties as well as the bioactivity and porosity of the composites [12-14]. The application of extra-pure natural materials instead of synthetic materials reduces the cost of implant produc- tion. Also, as distinct from synthesized materials, natural wollastonite has a needle-shaped habit, with a ratio be- tween the needle length and their diameter equal to 15 - 20 or more. This will presumably facilitate the produc- tion of an interwoven reinforcing mesh of wollastonite needles in the composite [6]. The aim of the present work was to synthesize and characterize hydroxyapatite-wollastonite biocomposite powders by a sol-gel route, using calcium nitrate and ammonium phosphate as precursors of hydroxyapatite, and high-purity natural wollastonite as a reinforcement element. These powders were processed by sintering to produce dense materials for the evaluation of their struc- tural characteristics, whereas their bioactive and me- chanical properties will be evaluated in a parallel study. 2. Experimental Procedure 2.1. Sol-Gel Synthesis of Hydroxyapatite-Wollastonite Biocomposite Powders The biocomposites of hydroxyapatite-wollastonite were prepared by sol-gel processing from calcium nitrate (Ca(NO3)2·4H2O, Sigma-Aldrich USA) and ammonium phosphate ((NH4)2HPO4, Sigma, Japan) and high-purity natural wollastonite, NYAD M200 (CaSiO3, from NYCO’s Pilares deposit in Hermosillo, Sonora, México), with 98.25% purity. Table 1 shows the chemical analysis of the natural wollastonite used in this study [15]. The amounts of the precursors reagents were chosen in order Table 1. Chemical composition of wollastonite NYAD® M200, produced by Minera NYCO S.A. de C.V. [15]. Chemical composition CaSiO3 Component Typical value (%) CaO 46.25 SiO2 52.00 Fe2O3 0.25 Al2O3 0.40 MnO 0.025 MgO 0.50 TiO2 0.025 K2O 0.15 wt% loss (1000˚C) 0.40 to maintain the Ca/P molar ratio at 1.67 of stoichiometric hydroxyapatite, and the amounts of wollastonite were chosen in order to obtain 20, 50, and 80 wt%. First, to produce about 1.0 g of pure hydroxyapatite powder, 0.1639 mol of calcium nitrate was dissolved in 10 mL of deionized water by ultrasonic stirring during 15 minutes, and then 0.0979 mol of ammonium phosphate was dissolved by ultrasonic stirring during 30 minutes. The mixture of precursors was stirred at room tem- perature by magnetic stirring, while the pH of the mix- ture was controlled between 6 and 7 with liquid ammonia for approximately 2 h, until the gelation was observed. The gel was then dried at 120˚C for 12 h. To produce the hydroxiapatite-wollastonite biocompo- sites, a suitable amount of wollastonite to obtain 20 wt% (0.25 g), 50 wt% (1.0 g), and 80 wt% (4.0 g) was sus- pended, by ultrasonic stirring, in a proper volume of de- ionized water to keep the solid: liquid ratio at 1:2 (w/v) for all experiments. Then the same procedure, as de- scribed previously, was followed until the dried gels mixed with natural wollastonite were obtained. Finally, the dried gels were ground to a fine powder and heat treated in a furnace at 750˚C in air for 3 h; the heating was done at a rate of 10˚C/min. The flow chart in Figure 1 outlines the complete experimental procedure. 2.2. Sintering of Hydroxyapatite-Wollastonite Biocomposites For sintering experiments, the powders were ground us- ing tungsten carbide milling balls in a 50 mL tungsten carbide container with a Fritsch Pulverisette 6 planetary mono mill (Idar-Oberstein, Germany). Cylindrical tablets were produced by uniaxial pressing of powders (0.5 g) into a 10 mm diameter die in a Carver press Hydraulic Unit 3912 (Carver, Wabash, IN). Sintering tests were carried out in a Lindberg/Blue M high temperature fur- Copyright © 2013 SciRes. JBNB 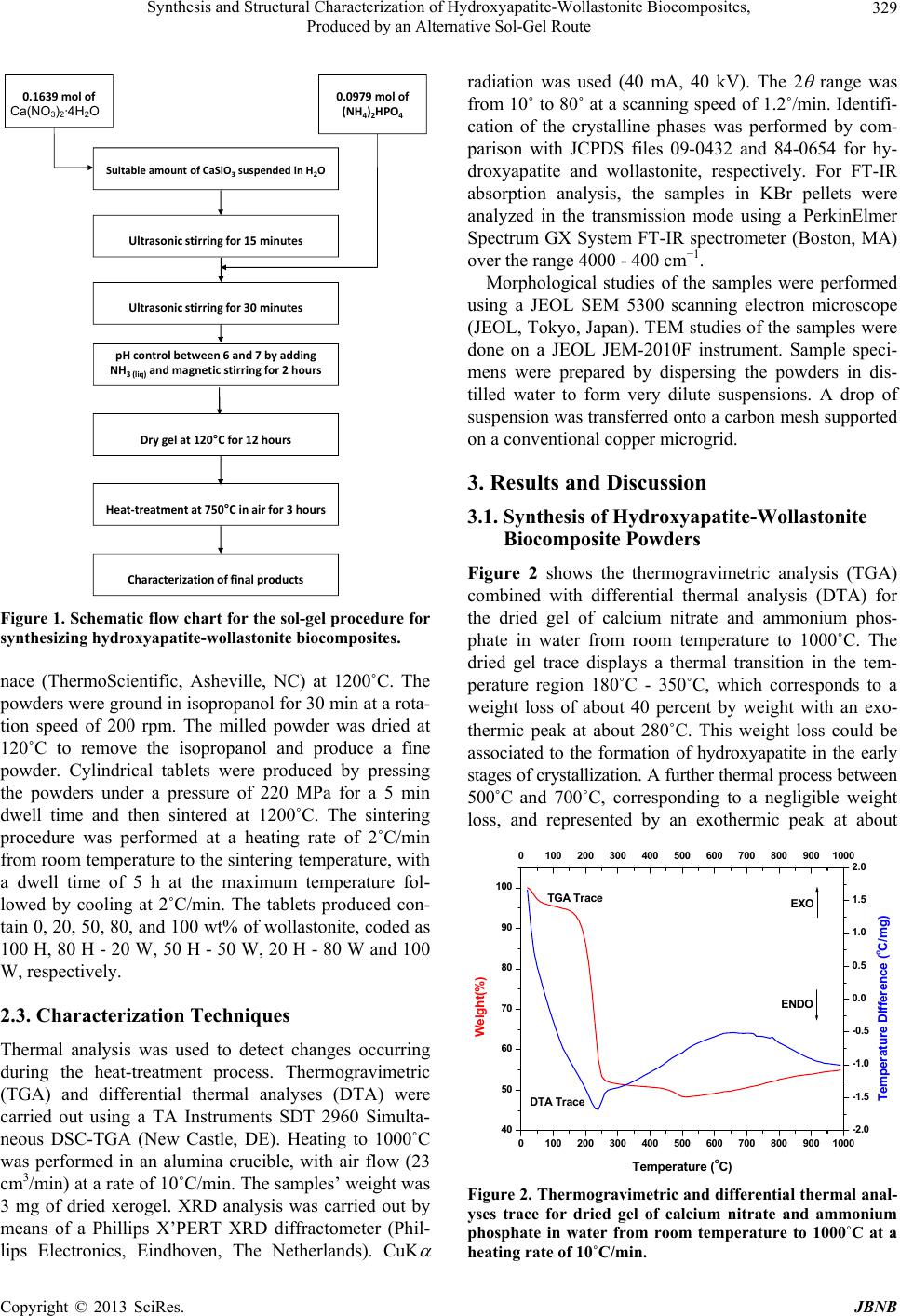 Synthesis and Structural Characterization of Hydroxyapatite-Wollastonite Biocomposites, Produced by an Alternative Sol-Gel Route 329 Ultrasonicstirringfor15minutes Ultrasonicstirringfor30minutes pHcontrolbetween6and7byadding NH 3(liq) andmagn et icstirringfor2hours Drygelat120°Cfor12hours Heat‐treatmentat750°Cinairfor3hours Characterization offinalproducts SuitableamountofCaSiO3suspendedinH2O 0.1639 molof Ca(NO 3 ) 2 .4H 2 O 0.0979molof (NH 4 ) 2 HPO 4 Ca(NO3)2·4H2O Figure 1. Schematic flow chart for the sol-gel procedure for synthesizing hydroxyapatite- w o llastonite biocomposite s. nace (ThermoScientific, Asheville, NC) at 1200˚C. The powders were ground in isopropanol for 30 min at a rota- tion speed of 200 rpm. The milled powder was dried at 120˚C to remove the isopropanol and produce a fine powder. Cylindrical tablets were produced by pressing the powders under a pressure of 220 MPa for a 5 min dwell time and then sintered at 1200˚C. The sintering procedure was performed at a heating rate of 2˚C/min from room temperature to the sintering temperature, with a dwell time of 5 h at the maximum temperature fol- lowed by cooling at 2˚C/min. The tablets produced con- tain 0, 20, 50, 80, and 100 wt% of wollastonite, coded as 100 H, 80 H - 20 W, 50 H - 50 W, 20 H - 80 W and 100 W, respectively. 2.3. Characterization Techniques Thermal analysis was used to detect changes occurring during the heat-treatment process. Thermogravimetric (TGA) and differential thermal analyses (DTA) were carried out using a TA Instruments SDT 2960 Simulta- neous DSC-TGA (New Castle, DE). Heating to 1000˚C was performed in an alumina crucible, with air flow (23 cm3/min) at a rate of 10˚C/min. The samples’ weight was 3 mg of dried xerogel. XRD analysis was carried out by means of a Phillips X’PERT XRD diffractometer (Phil- lips Electronics, Eindhoven, The Netherlands). CuK radiation was used (40 mA, 40 kV). The 2 range was from 10˚ to 80˚ at a scanning speed of 1.2˚/min. Identifi- cation of the crystalline phases was performed by com- parison with JCPDS files 09-0432 and 84-0654 for hy- droxyapatite and wollastonite, respectively. For FT-IR absorption analysis, the samples in KBr pellets were analyzed in the transmission mode using a PerkinElmer Spectrum GX System FT-IR spectrometer (Boston, MA) over the range 4000 - 400 cm−1. Morphological studies of the samples were performed using a JEOL SEM 5300 scanning electron microscope (JEOL, Tokyo, Japan). TEM studies of the samples were done on a JEOL JEM-2010F instrument. Sample speci- mens were prepared by dispersing the powders in dis- tilled water to form very dilute suspensions. A drop of suspension was transferred onto a carbon mesh supported on a conventional copper microgrid. 3. Results and Discussion 3.1. Synthesis of Hydroxyapatite-Wollastonite Biocomposite Powders Figure 2 shows the thermogravimetric analysis (TGA) combined with differential thermal analysis (DTA) for the dried gel of calcium nitrate and ammonium phos- phate in water from room temperature to 1000˚C. The dried gel trace displays a thermal transition in the tem- perature region 180˚C - 350˚C, which corresponds to a weight loss of about 40 percent by weight with an exo- thermic peak at about 280˚C. This weight loss could be associated to the formation of hydroxyapatite in the early stages of crystallization. A further thermal process between 500˚C and 700˚C, corresponding to a negligible weight loss, and represented by an exothermic peak at about 0100 200 300 400 500 600 700 800 9001000 40 50 60 70 80 90 100 0100 200 300 400 500 600 700 800 9001000 ENDO EXO DTA Trace TGA Trace Tem erature oC -2.0 -1.5 -1.0 -0.5 0.0 0.5 1.0 1.5 2.0 Temperature Difference (oC/mg) Weight(%) Figure 2. Thermogravimetric and differential thermal anal- yses trace for dried gel of calcium nitrate and ammonium phosphate in water from room temperature to 1000˚C at a heating rate of 10˚C/min. Copyright © 2013 SciRes. JBNB 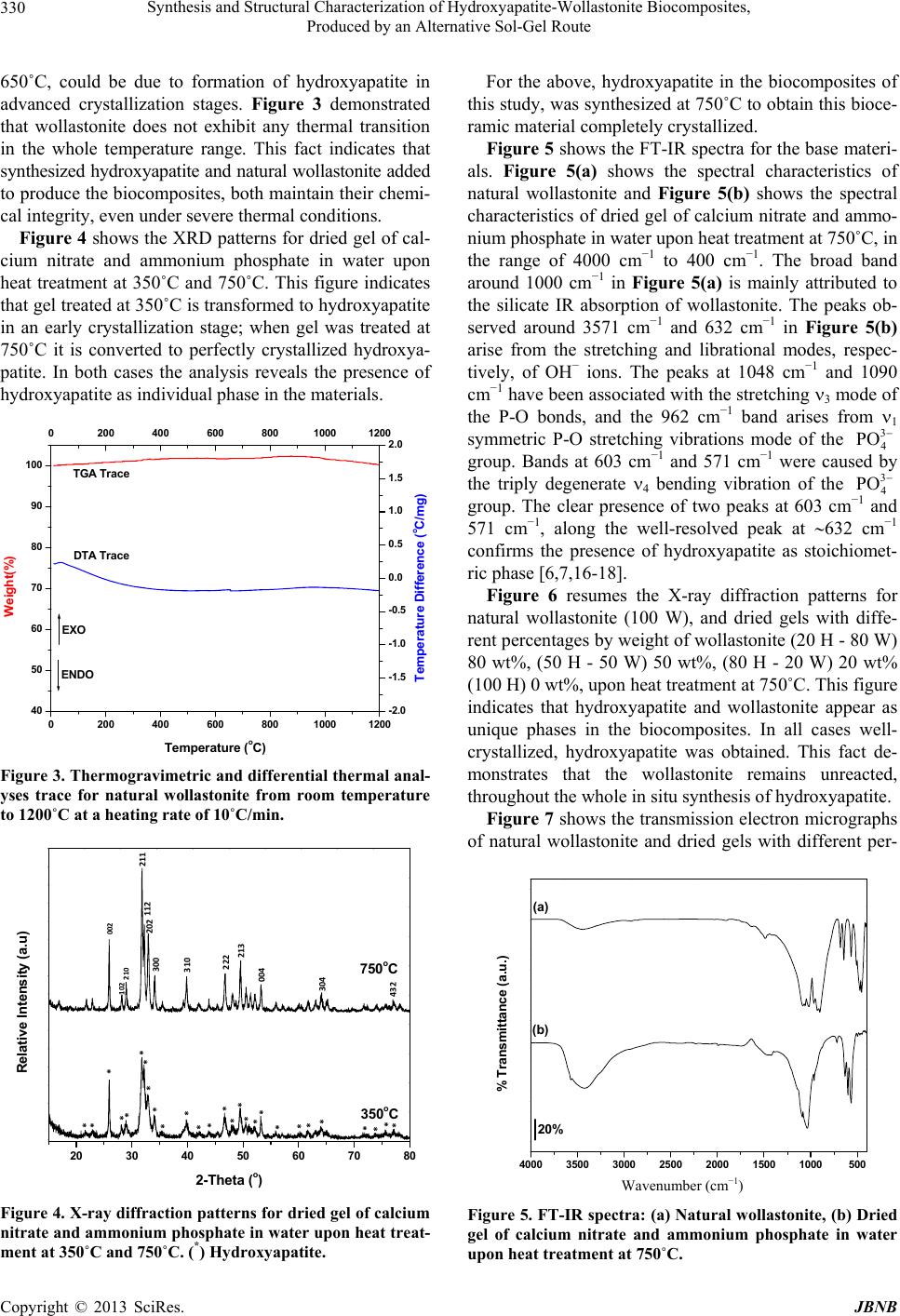 Synthesis and Structural Characterization of Hydroxyapatite-Wollastonite Biocomposites, Produced by an Alternative Sol-Gel Route 330 650˚C, could be due to formation of hydroxyapatite in advanced crystallization stages. Figure 3 demonstrated that wollastonite does not exhibit any thermal transition in the whole temperature range. This fact indicates that synthesized hydroxyapatite and natural wollastonite added to produce the biocomposites, both maintain their chemi- cal integrity, even under severe thermal conditions. Figure 4 shows the XRD patterns for dried gel of cal- cium nitrate and ammonium phosphate in water upon heat treatment at 350˚C and 750˚C. This figure indicates that gel treated at 350˚C is transformed to hydroxyapatite in an early crystallization stage; when gel was treated at 750˚C it is converted to perfectly crystallized hydroxya- patite. In both cases the analysis reveals the presence of hydroxyapatite as individual phase in the materials. 0200 400 600 80010001200 40 50 60 70 80 90 100 0200 400 600 80010001200 DTA T race TGA Trace ENDO EXO Temperature oC Weight(%) -2.0 -1.5 -1.0 -0.5 0.0 0.5 1.0 1.5 2.0 Temperature Difference (oC/mg) Figure 3. Thermogravimetric and differential thermal anal- yses trace for natural wollastonite from room temperature to 1200˚C at a heating rate of 10˚C/min. 20 30 40 50 60 70 80 750oC 350oC Intensit y (a. u) 2-Theta (o) 002 102 210 211 112 300 202 310 222 213 004 304 432 * * * * * * * *** * * * * * * * * * * * * * * * * * Relative Intensity (a.u) Figure 4. X-ray diffraction patterns for dried gel of calcium nitrate and ammonium phosphate in water upon he at treat- ment at 350˚C and 750˚C. (*) Hydroxyapatite. For the above, hydroxyapatite in the biocomposites of this study, was synthesized at 750˚C to obtain this bioce- ramic material completely crystallized. Figure 5 shows the FT-IR spectra for the base materi- als. Figure 5(a) shows the spectral characteristics of natural wollastonite and Figure 5(b) shows the spectral characteristics of dried gel of calcium nitrate and ammo- nium phosphate in water upon heat treatment at 750˚C, in the range of 4000 cm−1 to 400 cm−1. The broad band around 1000 cm−1 in Figure 5(a) is mainly attributed to the silicate IR absorption of wollastonite. The peaks ob- served around 3571 cm−1 and 632 cm−1 in Figure 5(b) arise from the stretching and librational modes, respec- tively, of OH− ions. The peaks at 1048 cm−1 and 1090 cm−1 have been associated with the stretching 3 mode of the P-O bonds, and the 962 cm−1 band arises from 1 symmetric P-O stretching vibrations mode of the 3 4 PO group. Bands at 603 cm−1 and 571 cm−1 were caused by the triply degenerate 4 bending vibration of the 3 4 PO group. The clear presence of two peaks at 603 cm−1 and 571 cm−1, along the well-resolved peak at 632 cm−1 confirms the presence of hydroxyapatite as stoichiomet- ric phase [6,7,16-18]. Figure 6 resumes the X-ray diffraction patterns for natural wollastonite (100 W), and dried gels with diffe- rent percentages by weight of wollastonite (20 H - 80 W) 80 wt%, (50 H - 50 W) 50 wt%, (80 H - 20 W) 20 wt% (100 H) 0 wt%, upon heat treatment at 750˚C. This figure indicates that hydroxyapatite and wollastonite appear as unique phases in the biocomposites. In all cases well- crystallized, hydroxyapatite was obtained. This fact de- monstrates that the wollastonite remains unreacted, throughout the whole in situ synthesis of hydroxyapatite. Figure 7 shows the transmission electron micrographs of natural wollastonite and dried gels with different per- 4000 3500 3000 2500 2000 1500 1000500 (b) (a) 20% % Transmittance (a.u.) Wavenumber (cm−1) Figure 5. FT-IR spectra: (a) Natural wollastonite, (b) Dried gel of calcium nitrate and ammonium phosphate in water upon heat treatment at 750˚C. Copyright © 2013 SciRes. JBNB 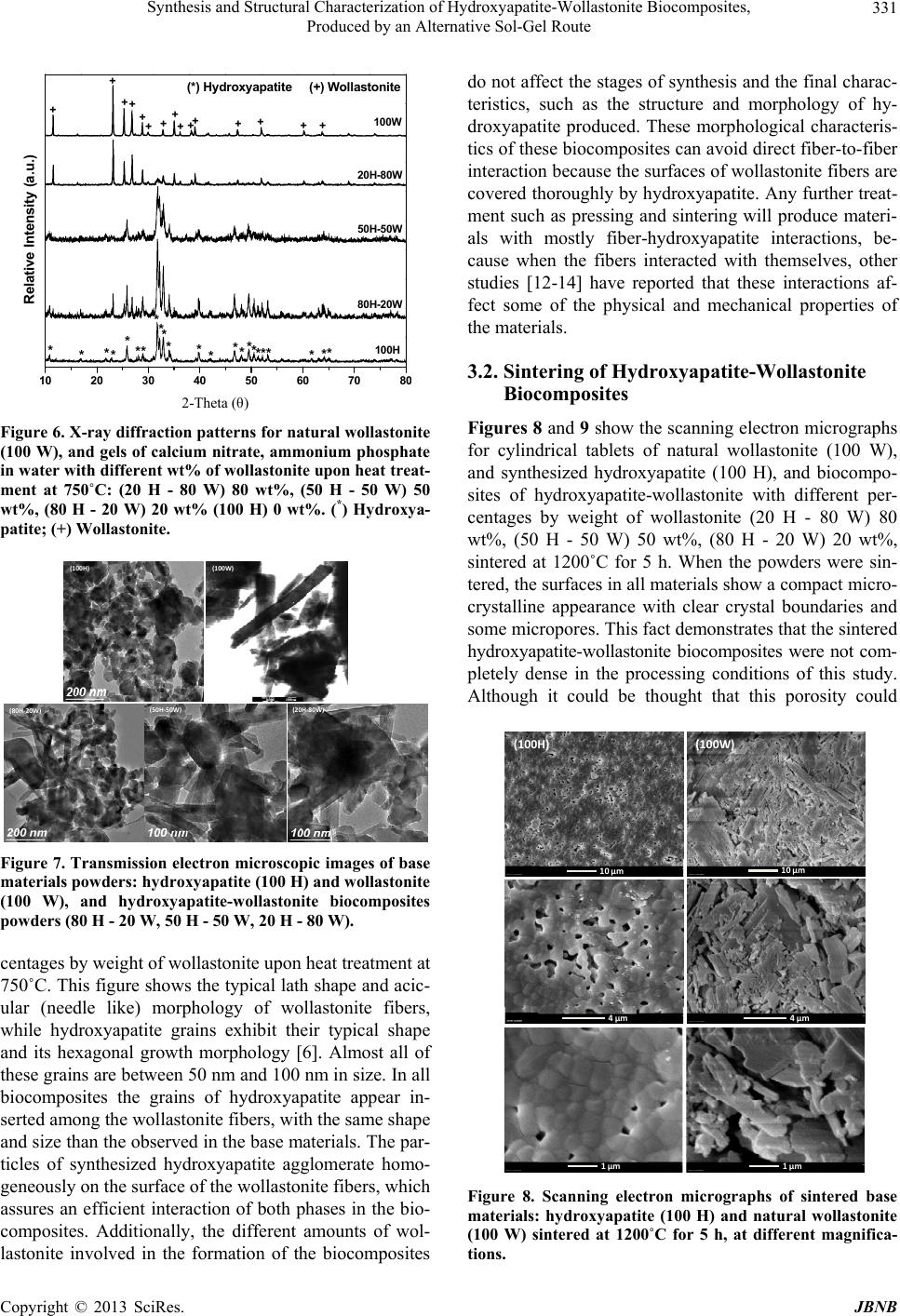 Synthesis and Structural Characterization of Hydroxyapatite-Wollastonite Biocomposites, Produced by an Alternative Sol-Gel Route 331 10 20 30 40 50 6070 8 (*) Hydroxyapatite (+) Wollastonite + + + + + + + + + + + + + + + ** * *** ****** ** * * ** * ** * *100H 80H-20W 50H-50W 20H-80W Relative Inten s ity (a.u.) 100W 2-Theta (θ) Figure 6. X-ray diffraction patterns for natural wollastonite (100 W), and gels of calcium nitrate, ammonium phosphate in water with different wt% of wollastonite upon heat treat- ment at 750˚C: (20 H - 80 W) 80 wt%, (50 H - 50 W) 50 wt%, (80 H - 20 W) 20 wt% (100 H) 0 wt%. (*) Hydroxya- patite; (+) Wollastonite. (100H) (100W) (80H‐20W) (50H‐50W) (20H‐80W) Figure 7. Transmission electron microscopic images of base materials powders: hydroxyapatite (100 H) and wollastonite (100 W), and hydroxyapatite-wollastonite biocomposites powders (80 H - 20 W, 50 H - 50 W, 20 H - 80 W). centages by weight of wollastonite upon heat treatment at 750˚C. This figure shows the typical lath shape and acic- ular (needle like) morphology of wollastonite fibers, while hydroxyapatite grains exhibit their typical shape and its hexagonal growth morphology [6]. Almost all of these grains are between 50 nm and 100 nm in size. In all biocomposites the grains of hydroxyapatite appear in- serted among the wollastonite fibers, with the same shape and size than the observed in the base materials. The par- ticles of synthesized hydroxyapatite agglomerate homo- geneously on the surface of the wollastonite fibers, which assures an efficient interaction of both phases in the bio- composites. Additionally, the different amounts of wol- lastonite involved in the formation of the biocomposites do not affect the stages of synthesis and the final charac- teristics, such as the structure and morphology of hy- droxyapatite produced. These morphological characteris- tics of these biocomposites can avoid direct fiber-to-fiber interaction because the surfaces of wollastonite fibers are covered thoroughly by hydroxyapatite. Any further treat- ment such as pressing and sintering will produce materi- als with mostly fiber-hydroxyapatite interactions, be- cause when the fibers interacted with themselves, other studies [12-14] have reported that these interactions af- fect some of the physical and mechanical properties of the materials. 3.2. Sintering of Hydroxyapatite-Wollastonite Biocomposites Figures 8 and 9 show the scanning electron micrographs for cylindrical tablets of natural wollastonite (100 W), and synthesized hydroxyapatite (100 H), and biocompo- sites of hydroxyapatite-wollastonite with different per- centages by weight of wollastonite (20 H - 80 W) 80 wt%, (50 H - 50 W) 50 wt%, (80 H - 20 W) 20 wt%, sintered at 1200˚C for 5 h. When the powders were sin- tered, the surfaces in all materials show a compact micro- crystalline appearance with clear crystal boundaries and some micropores. This fact demonstrates that the sintered hydroxyapatite-wollastonite biocomposites were not com- pletely dense in the processing conditions of this study. Although it could be thought that this porosity could (100H) (100W) 10µm 10µm 4µm 4µm 1µm 1µm Figure 8. Scanning electron micrographs of sintered base materials: hydroxyapatite (100 H) and natural wollastonite (100 W) sintered at 1200˚C for 5 h, at different magnifica- tions. Copyright © 2013 SciRes. JBNB  Synthesis and Structural Characterization of Hydroxyapatite-Wollastonite Biocomposites, Produced by an Alternative Sol-Gel Route 332 Figure 9. Scanning electron micrographs of sintered hy- droxyapatite-wollastonite biocomposites (80 H - 20 W, 50 H - 50 W, 20 H - 80 W) sintered at 1200˚C for 5 h, at different magnifications. have a negative effect on the mechanical properties, the porosity actually enhances the bioactive properties, be- cause the pores increase the penetration of physiological solutions in the specimens and permit the crystal growth of the bone-like apatite towards the surface, resulting in more efficient bioactive properties [7,19]. Therefore, the appropriate relationship between porosity and mechani- cal behavior in these materials has important implications in bone regeneration. Consequently, in a complementary study the porosity effect will be analyzed, and its rela- tionship with the microstructure and mechanical proper- ties of the materials will be evaluated. 4. Conclusion Hydroxyapatite-wollastonite biocomposites were synthe- sized using an alternative sol-gel route using calcium nitrate and ammonium phosphate as hydroxyapatite pre- cursors, and high purity natural wollastonite as rein- forcement phase in aqueous medium. Formation of hy- droxyapatite in the biocomposites occurs at the relatively low temperature of about 350˚C, in the early stages of crystallization. A further thermal treatment of about 750˚C produces completely crystallized hydroxyapatite, while wollastonite remains unreacted. The particles of hydroxyapatite appear agglomerated on the surface of wollastonite fibers. The hydroxyapatite-wollastonite bio- composite powders sintered at 1200˚C for 5 h, experi- ence no significant decomposition upon this heat treat- ment. The hydroxyapatite and wollastonite remain as single phases in the sintered biocomposites, exhibiting a certain grade of porosity. Although this porosity could have a negative effect on the mechanical properties, it is important for ensuring the efficient integration of these materials with bony tissues, which could be modulated to vary the amounts of base materials in the biocomposites. 5. Acknowledgements The authors are grateful to Francisco Brown Bojórquez and Víctor Emmanuel Alvarez Montaño from DIPyM- UNISON, Israel Gradilla, Eloisa Aparicio, Jesús Antonio Díaz, David Dominguez, Eric Flores Aquino and Jaime Mendoza López from CNyN-UNAM for assistance in the characterization and discussions. This work was partially support by PACAC UNISON-UNAM through project Px-864 and DGAPA-PAPIIT-UNAM through grant IN207511. REFERENCES [1] L. L. Hench, “Bioceramics: From Concept to Clinic,” Journal of the American Ceramic Society, Vol. 74, No. 7, 1991, pp. 1487-1510. http://dx.doi.org/10.1111/j.1151-2916.1991.tb07132.x [2] R. Z. LeGeros, “Calcium Phosphate Materials in Restora- tive Dentistry: A Review,” Advances in Dental Research, Vol. 2, No. 1, 1998, pp. 164-180. [3] R. Z. LeGeros and J. P. LeGeros, “Dense Hydroxyapa- tite,” In: L. L. Hench and J. Wilson, Eds., An Introduction to Bioceramics, World Scientific, Singapore, 1993, pp. 139-180. http://dx.doi.org/10.1142/9789814317351_0009 [4] R. Petit, “The Use of Hydroxyapatite in Orthopaedic Surgery: A Ten-Year Review,” European Journal of Or- thopaedic Surgery & Traumatology, Vol. 9, No. 2, 1999, pp. 71-74. http://dx.doi.org/10.1007/BF01695730 [5] G. Bezzi, G. Celotti, E. Landi, T. M. G. La Torretta, I. Sopyan and A. Tampieri, “A Novel Sol-Gel Technique for Hydroxyapatite Preparation,” Materials Chemistry and Physics, Vol. 78, No. 3, 2003, pp. 816-824. http://dx.doi.org/10.1016/S0254-0584(02)00392-9 [6] M. A. Encinas-Romero, S. Aguayo-Salinas, S. J. Castillo, F. F. Castillón-Barraza and V. M. Castaño, “Synthesis and Characterization of Hydroxyapatite-Wollastonite Com- posite Powders by Sol-Gel Processing,” International Journal of Applied Ceramic Technology, Vol. 5, No. 4, 2008, pp. 401-411. http://dx.doi.org/10.1111/j.1744-7402.2008.02212.x [7] M. A. Encinas-Romero, S. Aguayo-Salinas, J. L. Valen- zuela-García, S. R. Payán and F. F. Castillón-Barraza, “Mechanical and Bioactive Behavior of Hydroxyapatite- Wollastonite Sintered Composites,” International Journal of Applied Ceramic Technology, Vol. 7, No. 2, 2010, pp. 164-167. http://dx.doi.org/10.1111/j.1744-7402.2009.02377.x [8] T. Kokubo, “A/W Glass-Ceramic: Processing and Proper- ties,” In: L. L. Hench and J. Wilson, Eds., An Introduc- tion to Bioceramics, World Scientific, Singapore, 1993, pp. 75-88. http://dx.doi.org/10.1142/9789814317351_0005 Copyright © 2013 SciRes. JBNB  Synthesis and Structural Characterization of Hydroxyapatite-Wollastonite Biocomposites, Produced by an Alternative Sol-Gel Route Copyright © 2013 SciRes. JBNB 333 [9] M. J. Olszta, X. Cheng, S. S. Jee, R. Kumar, Y. Kim, M. J. Kaufman, E. Douglas and L. B. Gower, “Bone Structure and Formation: A New Perspective,” Materials Science and Engineering: R, Vol. 58, No. 3, 2007, pp. 77-116. http://dx.doi.org/10.1016/j.mser.2007.05.001 [10] T. Yamamuro, “A/W Glass-Ceramic: Clinical Aplica- tions,” In: L. L. Hench and J. Wilson, Eds., An Introduc- tion to Bioceramics, World Scientific, Singapore, 1993, pp. 89-103. http://dx.doi.org/10.1142/9789814317351_0006 [11] S. M. Best, A. E. Porter, E. S. Thian and J. Huang, “Bio- ceramics: Past, Present and for the Future,” Journal of the European Ceramic Society, Vol. 28, No. 7, 2008, pp. 1319-1327. http://dx.doi.org/10.1016/j.jeurceramsoc.2007.12.001 [12] Y. E. Greish and P. W. Brown, “Characterization of Wol- lastonite-Reinforced Hap-Ca polycarboxylate Compos- ites,” Journal of Biomedical Materials Research , Vol. 55, No. 4, 2001, pp. 618-628. http://dx.doi.org/10.1002/1097-4636(20010615)55:4<618 ::AID-JBM1056>3.0.CO;2-9 [13] V. V. Shumkova, V. M. Pogrebenkov, A. V. Karlov, V. V. Kozik and V. I. Vereshchagin, “Hydroxyapatite-Wollas- tonite Bioceramics,” Glass Ceramic, Vol. 57, No. 9-10, 2000, pp. 350-353. http://dx.doi.org/10.1023/A:1007198521974 [14] H.-S. Ryu, J. K. Lee, H. Kim and K. S. Hong, “New Type of Bioactive Materials: Hydroxyapatite/ -Wollastonite Composites,” Journal of Materials Research, Vol. 20, No. 5, 2005, pp. 1154-1162. http://dx.doi.org/10.1557/JMR.2005.0144 [15] NYCO Minerals Inc., “Premium Quality Wollastonite NYAD M325,” NYCO IN-299-04-01 Booklet, NYCO Minerals, Willsboro, 2001. [16] M. Markovic and B. O. Fowler, “Preparation and Com- prehensive Characterization of a Calcium Hydroxyapatite. Reference Material,” Journal of Research of the National Institute of Standards and Technology, Vol. 109, No. 6, 2004, pp. 553-568. http://dx.doi.org/10.6028/jres.109.042 [17] P. N. de Aza, F. Guitián and C. Santos, “Vibrational Properties of Calcium Phosphate Compounds. 2. Com- parison between Hydroxyapatite and b-Tricalcium Phos- phate,” Chemistry of Materials, Vol. 9, No. 4, 1997, pp. 916-922. http://dx.doi.org/10.1021/cm9604266 [18] C. Paluszkiewicz, M. Blazewicz, J. Podporska and T. Gumuła, “Nucleation of Hydroxyapatite Layer on Wol- lastonite Material Surface: FTIR Studies,” Vibrational Spectroscopy, Vol. 48, No. 2, 2008, pp. 263-268. http://dx.doi.org/10.1016/j.vibspec.2008.02.020 [19] M. Kawata, H. Uchida, K. Itatani, I. Okada, S. Koda and M. Aizawa, “Development of Porous Ceramics with Well- Controlled Porosities and Pores Size from Apatite Fibers and their Evaluation,” Journal of Materials Science: Ma- terials in Medicine, Vol. 15, No. 7, 1998, pp. 817-823. http://dx.doi.org/10.1023/B:JMSM.0000032823.66093.aa
|