 Open Journal of Nephrology, 2013, 3, 168-180 http://dx.doi.org/10.4236/ojneph.2013.33030 Published Online September 2013 (http://www.scirp.org/journal/ojneph) Cyclosporine-Associated Nephrotoxicity Maria Delia Colombo1,2*, Rena ta Pere go 2, Gilberto Bellia1 1Novartis Farma S.p.A., Origgio (VA), Italy 2Department of Dermatology, Marchesi Hospital, Inzago (MI), Italy Email: *delia.colombo@novartis.com Received July 11, 2013; revised August 9, 2013; accepted August 24, 2013 Copyright © 2013 Maria Delia Colombo et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Cyclosporine (CsA) has revolutionized transplant medicine and is currently one of the most important immunosuppres- sive agents for a wide range of organ transplantations and of autoimmune and inflammatory diseases, such as rheuma- toid arthritis, uveitis, psoriasis, and atopic dermatitis. Renal impairment represents the main limitation to CsA long-term continuous therapy. However, it has been shown that nephrotoxicity is associated with longer treatment duration, larger cumulative doses and higher daily dose of CsA. With low dose regimens (<5 mg/kg/day), stable serum creatinine levels have been observed up to 15 - 20 years after kidney transplantation. Intermittent therapy may offer a good therapeutic strategy to limit long-term renal dysfunction, given the fact that renal structural changes are dose- and time-dependent. The best predictor of permanent renal damage is a persistent increase in serum creatinine level one month after treat- ment withdrawal. In patients with autoimmune diseases, the percentage increase in serum creatinine above baseline value during CsA therapy has been shown to predict CsA-induced nephropathy. Before CsA therapy initiation, patients should undergo a thorough baseline evaluation including laboratory assessments, in particular electrolytes, serum creatinine, and urea levels. Furthermore, patients should be evaluated for factors that might increase the risk of nephro- toxicity, such as obesity, older age, hypertension, concomitant use of nephrotoxic drugs, and pre-existing renal condi- tions. In the present paper, CsA-induced nephropathy will be reviewed in terms of pathophysiology, pathologic and clinical findings, and strategies for prevention and management. Keywords: Cyclosporine; Nephrotoxicity; Immunosuppression; Transplant; Creatinine 1. Introduction Since its discovery in Sandoz laboratories in 1972, cyc- losporine (CsA) has revolutionized transplant medicine. CsA is currently one of the most important immunosup- pressive agents for a wide range of organ transplantations, including kidney, liver, heart, lung, pancreas, and intes- tine [1]. CsA has been found to have many immunologic properties that make it an attractive agent for immuno- suppression: it is found to inhibit both in vitro cell-me- diated lysis as well as lymphocyte sensitization by allo- geneic target cells [2]. Clinically, as summarized by Ha- riharan et al. in 2000, who reviewed 93,000 transplants from 1988 and 1996, CsA obtained one-year graft sur- vival rates in 94% and 88% in living related and de- ceased donor allografts respectively [3]. More recent data from the United Network for Organ Sharing (UNOS) from 1998 to 2007 show one-year adjusted survival rates of 96.6% and 91.6% in living related and deceased donor allografts respectively. In its 40 years of life, CsA was shown to be also an effective treatment option in auto- immune and inflammatory diseases, such as rheuma- toid arthritis, uveitis, psoriasis, and atopic dermatitis [4-8]. The fact CsA was nephrotoxic was discovered early after its initial use, when Calne et al. found a significant and unexpected nephrotoxicity that had not been observed in animal experiments in their first attempt to use CsA following transplantation using a dose of 25 mg/kg [9]. Currently, it is well known that renal damage may be an important side effect of CsA therapy, but it is also known that most persistent renal dysfunction is related to pro- longed therapy, or doses of greater than 5 mg/kg/day, both of which can result in structural renal changes. Fur- thermore, it has been reported that nephrotoxicity is also related to individual susceptibility [10]. In the present paper, CsA-induced nephropathy will be reviewed in terms of pathophysiology, pathologic and clinical find- ings, and strategies for prevention and management. *Corresponding author. C opyright © 2013 SciRes. OJNeph  M. D. Colombo ET AL. 169 2. Risk Factors for CsA Nephrotoxicity 2.1. Systemic Levels of CsA The main issue after renal transplantation is to maintain a reasonable balance between efficacy against rejection and toxicity, especially nephrotoxicity. Klintmalm et al. were the first to demonstrate a relationship between CsA doses, plasma levels, and renal allograft interstitial fibro- sis [11,12]. This association between CsA nephrotoxicity and higher CsA doses (>5 mg/kg/day) was then con- firmed by others [13]. After these findings, it became evident that CsA has a relatively narrow therapeutic win- dow and great care has to be given to keep dosage within preset target ranges [14]. However, maintaining CsA concentrations within pre- set target ranges proved to be difficult due to its high inter-and intraindividual pharmacokinetic variability [15], which was especially observed with the first CsA formu- lation, Sandimmun. This variability appeared to be largely due to significant inter- and intraindividual vari- ability in the expression and function of the metabolizing cytochrome P450 3A isoenzymes, mainly CYP3A4 and CYP3A5, and of the multidrug efflux transporter P- gly- coprotein. This was partly attributed to interactions with other drugs, which cause either inhibition or stimulation of expression or activity of these enzymes and transpor- ter. After development of a new CsA emulsion, Neoral, better absorption, less inter- and intraindividual variabil- ity, earlier stabilization of pharmacokinetics, and dose linearity in CsA exposure were observed compared with the old formulation [16-18]. However, it has been sug- gested that during chronic treatment of transplant recipe- ents, therapeutic drug monitoring of CsA may be useful and in fact, it is now adopted in clinical practice [19]. 2.2. Local Renal Exposure to CsA It has been demonstrated that levels of CsA in the renal tissue are much higher than in blood [20,21]. In addition to the degree of renal CsA exposure, there is also evi- dence that the susceptibility to CsA nephrotoxicity is determined by local renal factors, independent of local CsA levels. These factors include both the age of the recipient and of the transplanted kidney, the latter being independently associated with chronic histologic damage [22]. Second, local renal P-glycoprotein not only could play a role in renal accumulation of CsA, but could also be important for tubular epithelial cell detoxification and protection against apoptotic stress. Third, the use of non- steroidal anti-inflammatory drugs (NSAIDs) has been shown to increase renal susceptibility to acute CsA ne- phrotoxicity, with decreases in renal plasma flow and GFR [23-26]. Finally, genetic polymorphisms in genes involved in the pathogenesis of CsA nephrotoxicity have been associated with the risk for chronic nephrotoxicity [27-37]. Given the above evidences, it could be anticipated that younger patients with native kidneys or recipients of transplanted kidneys from younger donors could be less susceptible to CsA nephrotoxicity. Moreover, great at- tention should be paid to drug interactions: Table 1 lists the drugs that may increase the risk of CsA-associated nephrotoxicity. Finally, determination of a patient’s or donor’s genotype of drug-metabolizing genes or of mol- ecules involved in CsA nephrotoxicity, like TGF-β, could provide a reasonable tool to determine which patients are most susceptible for CsA nephrotoxicity. 2.3. CsA-Independent Risk of Chronic Renal Failure Following Transplantation Ojo et al. conducted a population-based cohort analysis to evaluate the incidence of chronic renal failure and the risk factors for it in 69,321 patients undergone non renal transplantation in the United States between 1990 and 2000 [38]. The 5-year risk varied depending on the type of organ transplanted, ranging from 7% among heart- lung recipients to 21% in recipients of intestine trans- plants. Other risk factors were increasing age, female sex, hypertension, diabetes mellitus, pre-transplantation heap- titis B infection, and postoperative acute renal failure. These data show that after transplantation of non-renal organs patients are at higher risk of chronic renal failure, independently from the post-transplantation immunosup- pressive therapy. 3. Pathophysiology of CsA Nephrotoxicity The etiology of chronic CsA nephrotoxicity has been studied extensively. A combination of CsA-induced hemodynamic changes and direct toxic effects of CsA on tubular epithelial cells is thought to play a role [19]. Renal dysfunction can be functional or structural. Functional impairment, which may begin soon after commencing Table 1. Drugs that may increase the risk of CsA-associated nephrotoxicity [115]. Aminoglycosides Melfalan Diclofenac Amphotericin B Ketoconazole Trimetoprim (with or without sulphametoxazole) Fluoroquinilones Acyclovir Cidofovir Foscarnet Cimetidine, ranitidine Tacrolimus Colquicine NSAIDs Analgesics Contrast media Fibrates Copyright © 2013 SciRes. OJNeph  M. D. Colombo ET AL. 170 treatment, can be subdivided into vascular dysfunction and tubular dysfunction. Vascular dysfunction is caused by vasoconstriction of the afferent glomerular arterioles, leading to increased vascular resistance. This results in decreased renal glomerular filtration rate (GFR) and re- nal blood flow with decreased clearance of creatinine. Tubular dysfunction is characterized by decreased mag- nesium reabsorption, decreased uric acid excretion, de- creased potassium and hydrogen ion secretion, and distal tubular acidosis. Hypomagnesemia, decreased bicarbon- ate concentration, hyperuricemia, and hyperkalemia may also result. There is no loss of urinary concentrating power, as is the case with other nephrotoxins. 3.1. Acute Nephrotoxicity Vasoconstriction of the afferent arterioles was first sug- gested by Murray et al. in 1985, which was proposed to be due to activation of the renal sympathetic nervous system, since a concomitant stimulation of plasma renin activity was demonstrated [39]. These authors also noted a reduction in the rate of decline of renal blood flow in denervated rats. Barros et al. also demonstrated an in- crease in vascular resistance in both afferent and efferent arterioles with a reduction in renal plasma flow and glomerular filtration rate (GFR), an effect that was at- tenuated by the administration of the angiotensin-con- verting enzyme (ACE) inhibitor captopril and the cal- cium channel blocker verapamil [40]. In addition to its activation of the renin-angiotensin system (RAS), CsA has been shown to increase the vasoconstrictor factors endothelin and tromboxane. It also demonstrated to re- duce vasodilator factors, prostacyclin, prostaglandin E2 and nitric oxide (NO) [41,42]. Activation of the RAS by CsA occurs by two mechanisms, a direct effect on jux- taglomerular cells [43] and indirectly through arterial vasoconstriction and reduced renal plasma flow. Another pathogenic mechanism was that observed by Hoecherl et al. who demonstrated a marked reduction of COX-2 ex- pression and of the down- stream production of arachi- donic acid metabolites, and a consequent vasoconstric- tion [44]. The role of the innate immune system has also been implicated in the nephrotoxicity of CsA. Injured tubular epithelial cells may activate toll-like receptors (TLR) and TNF-alfa which, in turn, stimulate secretion of chemoki- nes that initiate phagocytic activity and immune activa- tion [45]. This mechanism may provide a link between innate immunity and the direct effects of CsA on renal tubular cells. 3.2. Chronic Nephrotoxicity Chronic nephrotoxicity is the main drawback of current CsA immunosuppressive regimens [46]. Myers et al. in 1984 were the first demonstrating that high doses of CsA not only induce reversible alterations in renal vascular resistance, but may also be associated with irreversible damage of the renal architecture [46]. In cardiac trans- plant recipients surviving more than 12 months and treated with CsA at very high doses (up to 17 mg/kg/day), which are no longer used even in transplant recipients, they observed significant reduction in GFR, renal plasma flow, and renal blood flow. Biopsies of five CsA-treated patients showed tubulointerstitial injury and focal glomerular sclerosis, which seemed to correlate in inten- sity with the degree of renal impairment47. Further evi- dence of chronic nephrotoxicity related to high dose (>5 mg/kg/die), long-term CsA use (>2 years) was the find- ing of impaired renal function in heart, liver, and lung transplant recipients as well as in patients with autoim- mune diseases [47-49]. More recently, other authors reported much more en- couraging results following long-term use of CsA in kidney transplant recipients. Sandrini et al. reported on renal function in 638 cadaveric kidney transplant patients treated with CsA for up to 15 years. At 15 years, patient and graft survival rates were 82.7% and 56.1% respect- tively, renal function remained stable in 266 patients (46.6%) with preserved serum creatinine values observed even after a 15-year treatment period [50]. Kandaswamy et al studied the impact of continuing CsA-based immu- nosuppression in the second decade after kidney trans- plantation in a total of 1263 patients [10]. They observed that not all transplanted patients on CsA developed pro- gressive renal changes, but conversely in a subset or pa- tients on long term CsA, serum creatinine levels were stable up to 20 years post-transplantation. The authors’ conclusions were that identifying recipients’ predispose- tion to CsA toxicity and individualizing immunosuppres- sive therapy might be important in order to improve long-term kidney function. They also noted that reduce- tion in CsA exposure over time might preserve renal function. 4. Pathologic Findings The hallmark finding in CsA nephrotoxicity is arteriolar hyalinosis, characterized by nodular hyaline deposits in the tunica media of afferent arterioles. Another common finding is interstitial or so-called striped fibrosis. This is hypothesized to be secondary to the above mentioned vasoconstriction effects of CsA with subsequent arterial narrowing. The subsequent tissue ischemia/hypoxia leads to a reperfusion type injury, with the formation of reac- tive oxygen species and free radicals, leading to cellular injury and apoptosis [19,51]. Activation of the RAS is also implied in the patho- genesis of CsA nephrotoxicity, not only for its vasocon- strictive effects but also due to the action of angiotensin Copyright © 2013 SciRes. OJNeph  M. D. Colombo ET AL. 171 II, which has been shown to possibly induce fibrosis [19, 52,53]. Nankivell et al in 2004 gave one of the most important contributions to the documentation of the long-term nephrotoxic effects of CsA and the associated pathologic findings [54]. These authors examined serial kidney bi- opsies, performed at the time of organ implantation, at weeks 1, 2 and 4, at months 3, 6 and 12, and then yearly for 10 years. In total 888 biopsies were obtained in 99 patients. At 10 years these authors observed lesions con- sistent with chronic CsA toxicity in 100% of patients, but it has to be taken into account that immunosuppression protocols in those years were complex, with transplanted patients usually receiving triple therapy, including CsA, prednisone and azathioprine, so that renal damage cannot be attributed to CsA alone [55]. Table 2 summarizes the histological lesions associated with CsA acute and chronic use. However, the differen- tial diagnosis between CsA-related nephrotoxicity and other injury phenomena is very difficult, especially in kidney transplantation. The calcineurin inhibitors nephro- toxicity score proposed by Kambham et al. in 2007 [56] represents a first step in the standardization of the com- posite histological changes induced by CsA, but further validation studies are necessary. In non-renal organ trans- plantation the picture may be clearer and the study of CsA nephrotoxicity in native kidneys seems to be less troublesome [38,57]. 5. CsA-Induced Nephropathy in Patients with Autoimmune Diseases Feutren et al. in 1992 [4] studied the incidence of and the risk factors for CsA-induced nephropathy in patients with various autoimmune diseases. They retrospectively analyzed clinical and renal biopsy data from 192 patients (152 with insulin-dependent diabetes mellitus, 23 with posterior uveitis, 11 with psoriasis, 5 with Sjoegren, 1 with polychondritis), including 63 children of <15 years of age. The duration of CsA therapy ranged from 4 to 39 Table 2. Histological lesions associated with cyclosporine use (modified from Naesens et al. [19]). Acute CsA nephrotoxicity Acute arteriolopathy = renal dysfunction without histological alterations Tubular vacuolization Thrombotic microangiopathy Chronic CsA nephrotoxicity Interstitial fibrosis and tubular atrophy Medial arteriolar hyalinosis Glomerular capsular fibrosis Global glomerulosclerosis Focal segmental glomerulosclerosis Juxtaglomerular apparatus hyperplasia Tubular microcalcification months and in most patients CsA doses were higher than currently recommended (8.2 ± 2.8 mg/kg/day) and were increased during the first months of therapy. Renal biopsies were performed in all patients and CsA-induced nephropathy was defined as the presence of moderate or more severe alterations of the tubulointerstitial space, the glomerular arterioles or both. Forty-one of the 192 pa- tients had evidence of CsA-induced nephropathy: 25 had diabetes, 14 had uveitis, and 1 each had polychondritis and Sjoegren’s syndrome. Interstitial fibrosis with tu- bular atrophy were the predominant morphologic lesions in CsA-induced nephropathy. The percent increase in serum creatinine above baseline values was the best predictor of nephropathy. The impairment of renal function did not seem to be a direct consequence of the morphologic alterations, since it was reversible in most patients after a reduction in the dose or discontinuation of CsA therapy, even when morphologic lesions were present. The dose of CsA, the type of underlying disease, and the patient’s age were additional risk factors for nephropathy. The incidence of nephropathy was lower in children than in adults, probably because the clearance of CsA is greater in children [58]. In the authors’ opinion, the results of this study suggest that CsA-induced ne- phropathy may not be the result of long-term and cumu- lative toxic effects on arterioles and tubules, but rather a consequence of a brief insult brought about by the ad- ministration of excessive doses of CsA (> 10 mg/kg/day), which were rather frequent in early nineties. This ana- lysis suggests that in patients with autoimmune and in- flammatory disease and normal renal function, the likeli- hood of the development of CsA-induced nephropathy can be minimized by using doses ≤ 5 mg/kg/day and avoiding increases in serum creatinine concentrations greater than 30% above the patient’s baseline value by appropriate dose. Several studies have been conducted specifically in psoriasis patients treated with CsA. Nine studies evalu- ated changes in renal structure, assessed in kidney biopsy specimens, together with the variation in GFR, in pa- tients with psoriasis treated with CsA [59,67]. Different grading and scoring systems were used to evaluate CsA nephrotoxicity, making it difficult to combine the results. Biopsy studies included a total of 104 patients receiving CsA for a period ranging from 1 to 10 years at doses commonly ranging between 1.9 and 5 mg/kg/day, with some patients receiving up to 7.5 mg/kg/day. These stu- dies showed slight to moderate interstitial fibrosis after 1 year of CsA in some subjects, and after 3 to 4 years, in- terstitial fibrosis was moderate to severe [59,61-64]. The frequency of glomerular sclerosis in biopsies increased from 12.5% at 3 years to 26% at 10 years [60,66]. Tubu- lar atrophy has also been described. Renal arteriolar ab- normalities, consisting of either necrosis of smooth- Copyright © 2013 SciRes. OJNeph  M. D. Colombo ET AL. 172 muscle cells and nodular protein deposits in the wall of afferent glomerular arterioles or arteriolar intima hya- linosis may also be seen [68]. The percentage of increase in serum creatinine above 30% of baseline was found to be a predictor of structural kidney changes. Moreover, the severity of recurrent acute nephrotoxicity was shown to correlate with chronic histological changes (r = 0.8, p = 0.0003) [64]. Increases in serum creatinine were re- versible 1 month to 10 years [65,66,69] after stopping CsA therapy. It seems that structural kidney damage can be expected in patients in whom serum creatinine does not decrease after cessation of CsA therapy. Young et al. [62] showed that older patients may be more vulnerable to CsA-induced renal injury and that CsA-associated hy- pertension was associated with a greater degree of pro- gressive renal interstitial fibrosis on serial biopsies. Other six studies described the incidence of increases in serum creatinine by more than 30% above the baseline value in psoriasis patients [4,70-74]. Overall, more than 50% of the patients had a significant increase in serum creatinine (>30% of baseline) if treatment was prolonged for ≥2 years. Comparing the incidence of in- creases in serum creatinine >30% in patients receiving continuous or in- termittent CsA treatment no significant difference was found (OR 1.35, p = 0.66). In conclusion, in psoriasis patients nephrotoxicity was associated with longer use, larger cumulative dose, higher daily dose and the occur- rence of acute increases in serum creatinine. Slight to moderate interstitial fibrosis was observed in patients treated for at least 1 - 2 years, while glomerular sclerosis or severe interstitial fibrosis were seen in some cases after 3 years or more. The functional signification and the reversibility of the structural changes have not been fully characterized in the available studies. Regarding rheumatoid arthritis (RA), following the es- timation of potential risk factors for the development of CsA-induced nephropathy in autoimmune diseases4, dos- ing recommendations for the use of CsA in RA were established, stating that the starting dose should be 2.5 - 3.5 mg/kg/day, the maximum daily dose should not ex- ceed 5 mg/kg/day and that the dose should be reduced whenever serum creatinine increases by > 30% [75,76]. The 1994 International Consensus Report on the treat- ment of RA by CsA concluded that CsA-induced neph- ropathy can be avoided when these rules are observed. Subsequently, data from the Kidney Biopsy Registry on 60 first and 14 second renal biopsies performed in RA patients treated with CsA for up to 87 months were re- viewed by Rodriguez et al. in order to describe the bi- opsy findings in all evaluable RA patients, collect infor- mation about the long-term follow-up of renal function and discuss the risk factors for the development of nephrotoxicity [5]. Of the 22 patients who started CsA at dosages <4 mg/kg/day and did not exceed 5 mg/kg/day, none developed CsA-associated nephropathy. Continu- ous assessment of renal function did not show any evi- dence of deterioration over time in patients maintained on long-term, low-dose CsA. A single-center prospective cohort study was con- ducted to assess the long-term renal tolerance of a low- dose CsA treatment in patients affected by sight-threat- ening posterior idiopathic uveitis, having healthy kidneys before CsA therapy [6]. Forty-one patients were included in the study undergoing a mean CsA treatment duration of 44.9 ± 3.6 months and receiving no other nephrotoxic drugs. Mean CsA daily dosage was gradually tapered from 4.3 ± 1.6 mg/kg/day to 1.8 ± 0.9 mg/kg/day over 5 years. Renal effects were evaluated by creatinine clear- ance, GFR and effective renal plasma flow; additionally 11 patients underwent kidney biopsies before and after 2 years of CsA treatment. The authors suggest that in order to obtain the expected benefits to patients with uveitis, CsA should be used at the lowest effective dose, possibly ≤ 3 mg/kg/day, so that CsA-associated nephrotoxicity might be prevented. A large cohort of 285 recently diagnosed type 1 dia- betic patients having received CsA for a mean of 20 months was monitored for 13 years and compared with a parallel group of 100 similar patients treated with insulin alone. The CsA-treated patients showed a transient in- crease in creatininemia during the first 18 months of treatment, associated with a transient increase in renal vascular resistance, both of which disappeared later, with values remaining then normal [77]. The authors conclude that low-dose CsA combined with tight and careful moni- toring should not result in long-term renal dysfunction. 6. Prevention of CsA Nephrotoxicity Despite the above mentioned evidences of CsA-asso- ciated renal toxicity, the exclusion of CsA from the immunosuppressive regimens following organ trans- plantation does not allow to preserve the allograft func- tion, due to the inadequate rejection prophylaxis obtained with other immunosuppressive regimens [78-81]. There- fore, different strategies aimed at prevent- ing renal da- mage during long-term CsA treatment have been deve- loped. For example, the week-end therapy was evaluated in the PREWENT study, which assessed the impact on efficacy and safety of a two consecutive days a week regimen in psoriasis patients, no difference in the inci- dence of adverse effects was observed between placebo and CsA [82]. 6.1. CsA Minimization There is increasing interest in CsA minimization proto- cols, in which the doses of CsA are adjusted to lower target levels, both in de novo immunosuppressive proto- Copyright © 2013 SciRes. OJNeph  M. D. Colombo ET AL. 173 cols both from time of transplantation and for rescue therapy after detection of renal histologic damage or dysfunction [80]. These CsA-sparing regimens appear to be relatively safe [79-81]. By minimizing CsA levels, nephrotoxicity might be partially avoided, but it has be- come clear that the increased risk of allograft rejection could annihilate these positive effects. It will be impor- tant to develop and apply new tools for clinical immu- nologic monitoring in order to avoid that minimization strategies will result in under-immunosuppression with the risk of chronic rejection. 6.2. Calcium Antagonists Because vasoconstriction of the afferent arterioles appears to play a pivotal role in acute and chronic CsA nephrotoxicity, many authors have studied the preventive effects of vasodilatory agents. The calcium antagonist nitrendipine and later lacidipine were shown to prevent the fall in renal plasma flow and GFR associated with CsA administration [83-85]. In a randomized trial in renal transplant recipients, patients treated with the com- bination of CsA and nifedipine had better renal function with the same degree of blood pressure control [86]. A similar effect was shown by lacidipine in another ran- domized trial [87]. Likewise, in heart transplantation, treatment with nife- dipine normalized BP and improved renal function [88]. More recently, another randomized trial with amlodipine confirmed these positive effects of calcium antagonists on CsA nephrotoxicity again in heart transplant reci- pients [89]. In contrast, a long-term follow-up study did not find a protective effect of calcium channel blockers in preventing CsA nephrotoxicity [90], but it has to be pointed out that the type of calcium antagonist is not specified in this study. 6.3. RAS Inhibition Given the pivotal role of RAS activation in the patho- genesis of CsA nephrotoxicity, it can be expected that RAS inhibition will be useful in preventing its deve- lopment. In human studies, ACE inhibitors showed to reduce CsA nephrotoxicity [91] and to improve the cardiovascular alterations observed in renal transplant recipients [92]. On the other hand, results with the angio- tensin-receptor blocker (ARB) losartan are currently contradictory [93,94] and it is therefore not clear whether combination with ARB could effectively slow the pro- gression of CsA-associated renal toxicity. Similarly inconsistent are the results of the comparison between nifedipine and lisinopril [95,96]. 6.4. Other Pharmacological Approaches Vasodilatory prostanoids, like misoprostol, did not pre- vent CsA nephrotoxicity both in transplant recipients and in rheumatoid arthritis patients [97,98]. Despite promis- ing results in rats, the nitric oxide donor L-arginine showed no effect on CsA nephrotoxicity prevention in humans [99]. Other therapeutic approaches are promising, such as anti-TGF-β antibodies [100,101], antioxidants [102-105], statins [106] and magnesium supplementation [107,108], but no human studies are yet available with these agents. 7. Management of CsA Nephrotoxicity A protocol for the management of nephrotoxicity asso- ciated with long term CsA administration has been pro- posed by Griffith et al. in their international consensus statement on CsA in psoriasis clinical practice [7]. Based on the observation that CsA nephropathy is strictly related to drug dose (>5 mg/kg/day) and treatment duration [60,70,109], these authors propose that the risk of renal toxicity during CsA treatment is reduced by the use of intermittent, short courses of the drug. The drug- free days should allow renal recovery and restoration of normal renal function [109]. Renal safety during short course CsA therapy has been shown in studies where only a minority of patients (4%, 17% and 10% - 27%) [72,110-112] experienced an elevation in serum creati- nine, which was typically transient and commonly re- turned to baseline within 4 weeks following dose reduc- tion or treatment cessation [72]. According to Griffiths et al. [7], the chance of devel- oping renal impairment during CsA therapy should be minimized by screening patients at baseline for any risk factors of renal toxicity: hypertension, advanced age, pre-existing renal conditions, and abnormalities in ab- sorption of CsA. Concomitant medications and weight may represent additional risk factors [4]. Most impor- tantly, the dose of CsA should only exceptionally exceed 5 mg/kg/day and the duration should be only as long as is necessary to achieve clearance or near-clearance of the disease. Further management guidelines for monitoring renal safety during CsA therapy proposed by Griffiths et al are summarized in Figure 1. 8. Conclusions Renal impairment represents the main limitation to CsA long-term continuous therapy. However, it has been shown that nephrotoxicity is associated with longer treatment duration, larger cumulative doses and higher daily dose of CsA. Its prevalence with doses ≤5 mg/ kg/day is low. Renal structural changes including slight to moderate interstitial fibrosis were mainly observed in patients treated for ≥2 years consecutively with high dosages, significant lesions such as glomerular sclerosis or severe interstitial fibrosis seen after 3 years or more. Copyright © 2013 SciRes. OJNeph 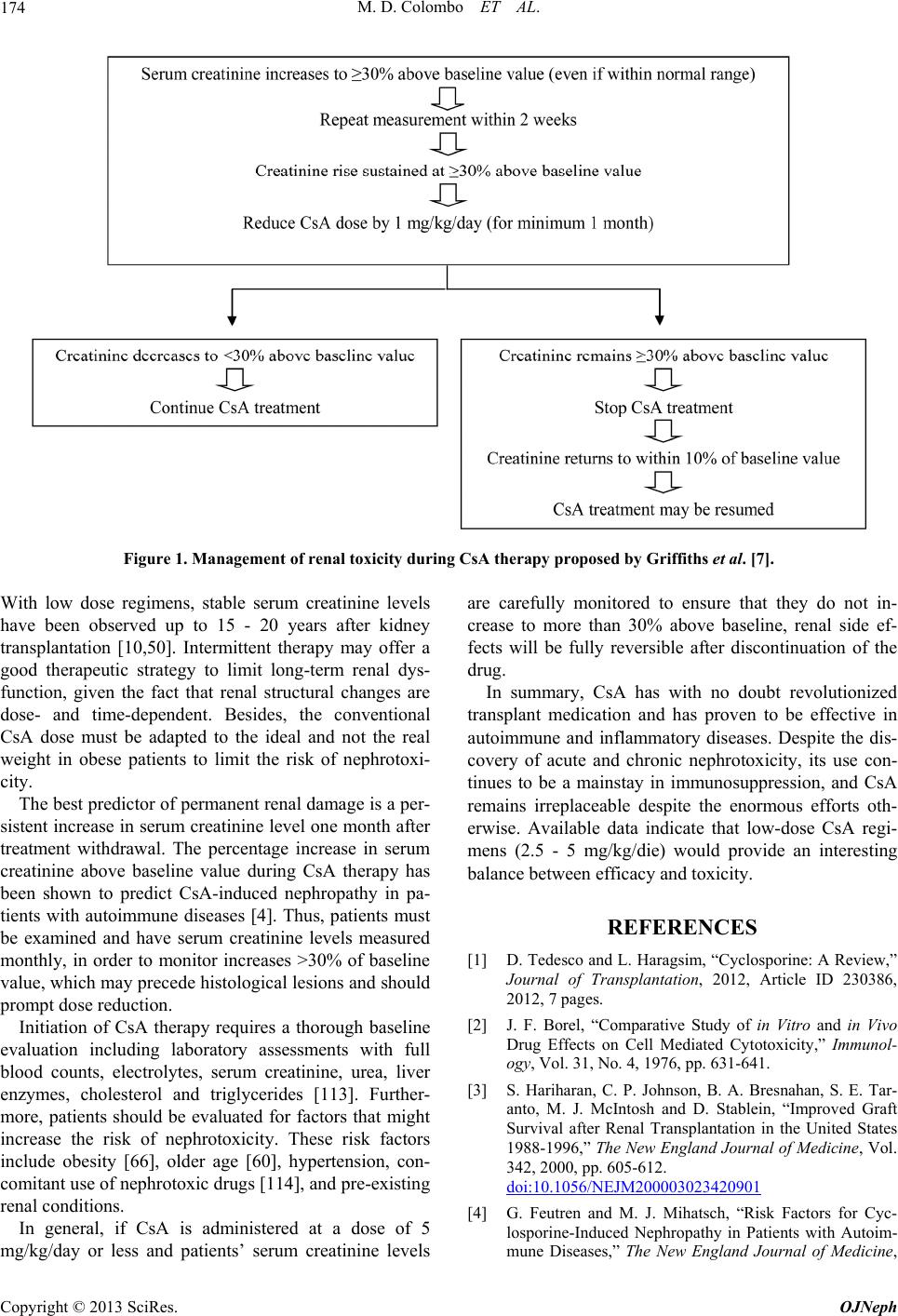 M. D. Colombo ET AL. Copyright © 2013 SciRes. OJNeph 174 Figure 1. Management of renal toxicity during CsA therapy proposed by Griffiths et al. [7]. With low dose regimens, stable serum creatinine levels have been observed up to 15 - 20 years after kidney transplantation [10,50]. Intermittent therapy may offer a good therapeutic strategy to limit long-term renal dys- function, given the fact that renal structural changes are dose- and time-dependent. Besides, the conventional CsA dose must be adapted to the ideal and not the real weight in obese patients to limit the risk of nephrotoxi- city. The best predictor of permanent renal damage is a per- sistent increase in serum creatinine level one month after treatment withdrawal. The percentage increase in serum creatinine above baseline value during CsA therapy has been shown to predict CsA-induced nephropathy in pa- tients with autoimmune diseases [4]. Thus, patients must be examined and have serum creatinine levels measured monthly, in order to monitor increases >30% of baseline value, which may precede histological lesions and should prompt dose reduction. Initiation of CsA therapy requires a thorough baseline evaluation including laboratory assessments with full blood counts, electrolytes, serum creatinine, urea, liver enzymes, cholesterol and triglycerides [113]. Further- more, patients should be evaluated for factors that might increase the risk of nephrotoxicity. These risk factors include obesity [66], older age [60], hypertension, con- comitant use of nephrotoxic drugs [114], and pre-existing renal conditions. In general, if CsA is administered at a dose of 5 mg/kg/day or less and patients’ serum creatinine levels are carefully monitored to ensure that they do not in- crease to more than 30% above baseline, renal side ef- fects will be fully reversible after discontinuation of the drug. In summary, CsA has with no doubt revolutionized transplant medication and has proven to be effective in autoimmune and inflammatory diseases. Despite the dis- covery of acute and chronic nephrotoxicity, its use con- tinues to be a mainstay in immunosuppression, and CsA remains irreplaceable despite the enormous efforts oth- erwise. Available data indicate that low-dose CsA regi- mens (2.5 - 5 mg/kg/die) would provide an interesting balance between efficacy and toxicity. REFERENCES [1] D. Tedesco and L. Haragsim, “Cyclosporine: A Review,” Journal of Transplantation, 2012, Article ID 230386, 2012, 7 pages. [2] J. F. Borel, “Comparative Study of in Vitro and in Vivo Drug Effects on Cell Mediated Cytotoxicity,” Immunol- ogy, Vol. 31, No. 4, 1976, pp. 631-641. [3] S. Hariharan, C. P. Johnson, B. A. Bresnahan, S. E. Tar- anto, M. J. McIntosh and D. Stablein, “Improved Graft Survival after Renal Transplantation in the United States 1988-1996,” The New England Journal of Medicine, Vol. 342, 2000, pp. 605-612. doi:10.1056/NEJM200003023420901 [4] G. Feutren and M. J. Mihatsch, “Risk Factors for Cyc- losporine-Induced Nephropathy in Patients with Autoim- mune Diseases,” The New England Journal of Medicine,  M. D. Colombo ET AL. 175 Vol. 326, 1992, pp. 1654-1660. doi:10.1056/NEJM199206183262502 [5] F. Rodriguez, J. C. Krayenbuehl, W. B. Harrison, O. Forre, B. A. C. Dijkmans, P. Tugwell, et al., “Renal Bi- opsy Findings and Follow Up of Renal Function in Rheu- matoid Arthritis Patients Treated with Cyclosporine A. An Update from the International Kidney Biopsy Regis- ter,” Arthritis & Rheumatism, Vol. 39, No. 9, 1996, pp. 1491-1498. doi:10.1002/art.1780390908 [6] C. Isnard Bagnis, S. Tezenas du Montcel, H. Beaufils, C. Jouanneau, C. Jaudon, P. Macsud, et al., “Long-Term Re- nal Effects of Low-Dose Cyclosporine in Uveitis-Treated Patients: Follow-Up Study,” Journal of the American So- ciety of Nephrology, Vol. 13, No. 12, 2002, pp. 2962- 2968. doi:10.1097/01.ASN.0000034945.61533.26 [7] C. E. M. Griffiths, L. Dubertret, C. N. Ellis, A. Y. Finlay, A. F. Finzi, V. C. Ho, et al., “Cyclosporin in Psoriasis Clinical Practice: An International Consensus Statement,” British Journal of Dermatology, Vol. 150, No. 67, 2004, pp. 11-23. doi:10.1111/j.0366-077X.2004.05949.x [8] J. I. Harper, J. Berth-Jones, R. D. Camp, M. J. Dillon, A. Y. Finlay, C. A. Holden, et al., “Cyclosporin for Atopic Dermatitis in Children,” Dermatology, Vol. 203, No. 1, 2001, pp. 3-6. doi:10.1159/000051694 [9] R. Y. Calne, S. Thiru and D. J. G. White, “Cyclosporin A in Patients Receiving Renal Allografts from Cadaver Do- nors,” Lancet, Vol. 2, No. 8104, 1978, pp. 1323-1327. [10] R. Kandaswamy, A. Humar, V. Casingal, K. J. Gilling- ham, H. Ibrahim and A. J. Matas, “Stable Kidney Func- tion in the Second Decade after Kidney Transplantation While on Cyclosporine-Based Immunosuppression,” Trans- plantation, Vol. 83, No. 6, 2007, pp. 722-726 doi:10.1097/01.tp.0000256179.14038.e2 [11] G. Klintmalm, S. O. Bohman, B. Sundelin and H. Wiczek, “Interstitial Fibrosis in Renal Allografts after 12 to 46 Months of Cyclosporine Treatment: Beneficial Effects of Low Doses in Early Post-Transplantation Period,” Lancet, Vol. 2, No. 8409, 1984, pp. 950-954 doi:10.1016/S0140-6736(84)91166-8 [12] G. Klintmalm, J. Sawe, O. Ringden, B. C. Von Bahr and A. Magnusson, “Cyclosporine Plasma Levels in Renal Transplant Patients. Association with Renal Toxicity and Allograft Rejection,” Transplantation, Vol. 39, No. 2, 1985, pp. 132-137. doi:10.1097/00007890-198502000-00005 [13] F. C. Henny, C. H. Kleinbloesem, A. J. Moolenaar, L. C. Paul, D. D. Breimaer and L. A. van Es, “Pharmacokinet- ics and Nephrotoxicity of Cyclosporine in Renal Trans- plant Recipients,” Transplantation, Vol. 40, No. 3, 1985, pp. 261-265. doi:10.1097/00007890-198509000-00008 [14] R. J. Ptachcinski, G. J. Burckart and R. Venkataramanan, “Cyclopsorine Concentration Determinations for Moni- toring and Pharmacokinetic Studies,” The Journal of Cli- nical Pharmacology, Vol. 26, No. 5, 1986, pp. 358-366. doi:10.1002/j.1552-4604.1986.tb03538.x [15] A. Fahr, “Cyclosporine Clinical Pharmacokinetics,” Cli- nical Pharmacokinetics, Vol. 24, No. 6, 1993, pp. 472- 495. doi:10.2165/00003088-199324060-00004 [16] D. W. Holt, E. A. Mueller, J. M. Kovarik, J. B. van Bree, F. Richard and K. Kutz, “Sandimmun Neoral Pharma- cokinetics: Impact of the New Oral Formulation,” Trans- plant Proceeding, Vol. 27, No. 1, 1995, pp. 1434-1437. [17] M. A. Masri, A. Barbari, A. Stephan, G. Kamel, G. Frem, F. Younan, et al., “Cyclosporine Pharmacokinetics in Sta- ble Renal Transplant Patients: Effect of Formulation San- dimmun versus Consupren versus Neoral,” Transplant Proceeding, Vol. 28, No. 3, 1996, pp. 1318-1320. [18] H. Humbert, “Variability of the Bioavailability of Cyc- losporine: Benefit of the Neoral Formulation,” Therapie, Vol. 52, No. 4, 1997, pp. 353-357. [19] M. Naesens, D. R. J. Kuypers and M. Sarwal, “Calci- neurin Inhibitors Nephrotoxicity,” Clinical Journal of the American Society of Nephrology, Vol. 4, No. 2, 2009, pp. 481-508. [20] P. F. Halloran, L. M. Helms, L. Kung and J. Noujaim, “The Temporal Profile of Calcineurin Inhibition by Cyc- losporine in Vivo,” Transplantation, Vol. 68, No. 9, 1999, pp. 1356-1361.doi:10.1097/00007890-199911150-00023 [21] K. Iwasaki, T. Shiraga, H. Matsuda, Y. Teramura, A. Ka- wamura, T. Hata, et al., “Absorption, Distribution, Me- tabolism and Excretion of Tacrolimus (FK506) in the Rat,” Drug Metabolism and Pharmacokinetics, Vol. 13, No. 3, 1998, pp. 259-265. doi:10.2133/dmpk.13.259 [22] M. Kubo, Y. Kiyohara, I. Kato, Y. Tanizaki, R. Katafuchi, H. Hirakata, et al., “Risk Factors for Renal Glomerular and Vascular Changes in an Autopsy-Based Population Survey: The Hisayama Study,” Kidney International, Vol. 63, 2003, pp. 1508-1515. doi:10.1046/j.1523-1755.2003.00886.x [23] N. D. Sturrock, C. C. Lang and A. D. Struthers, “Indo- methacin and Cyclosporine Together Produce Marked Renal Vasoconstriction in Humans,” Journal of Hyper- tension, Vol. 12, No. 8, 1994, pp. 919-924. doi:10.1097/00004872-199408000-00009 [24] R. D. Altman, G. O. Perez and G. N. Sfakianakis, “Inter- action of Cyclosporine A and Nonsteroidal Anti-Inflam- matory Drugs on Renal Function in Patients with Rheu- matoid Arthritis,” The American Journal of Medicine, Vol. 93, No. 4, 1992, pp. 396-402. doi:10.1016/0002-9343(92)90169-C [25] J. M. Kovarik, P. Kurki, E. Mueller, M. Guerret, E. Markert, R. Alten, et al., “Diclofenac Combined with Cyclosporine in Treatment Refractory Rheumatoid Ar- thritis: Longitudinal Safety Assessment and Evidence of a Pharmacokinetic/Dynamic Interaction,” Journal of Rheu- matology, Vol. 23, No. 12, 1996, pp. 2033-2038. [26] R. M. Soubhia, G. E. Mendes, F. Z. Mendonca, M. A. Baptista, J. P. Cipullo and E. A. Burdmann, “Tacrolimus and Nonsteroidal Anti-Inflammatory Drugs: An Associa- tion to Be Avoided,” American Journal of Nephrology, Vol. 25, 2005, pp. 327-334. doi:10.1159/000086569 [27] C. C. Baan, A. H. Balk, C. T. Holweg, I. C. van Rie- msdijk, L. P. Maat, P. J. Vantrimpont, et al., “Renal Fail- ure after Clinical Heart Transplantation Is Associated with the TGF-beta 1 Codon 10 Gene Polymorphism,” Jour- nal of Heart Lung Transplant, Vol. 19, No. 9, 2000, pp. 866-872. doi:10.1016/S1053-2498(00)00155-8 [28] J. van de Wetering, C. H. Weimar, A. H. Balk, J. I. Copyright © 2013 SciRes. OJNeph  M. D. Colombo ET AL. 176 Roodnat, C. T. Holweg, C. C. Baan, et al., “The Impact of Transforming Growth Factor-Beta1 Gene Polymor- phism on End-Stage Renal Failure after Heart Transplan- tation,” Transplantation, Vol. 82, No. 12, 2006, pp. 1744- 1748. doi:10.1097/01.tp.0000250360.78553.5e [29] S. Di Filippo, A. Zeevi, K. K. McDade, G. J. Boyle, S. A. Miller, S. K. Gandhi and S. A. Webber, “Impact of TGFbeta1 Gene Polymorphisms on Late Renal Function in Pediatric Heart Transplantation,” Human Immunology, Vol. 66, No. 2, 2005, pp. 133-139. doi:10.1016/j.humimm.2004.09.018 [30] J. R. Jonsson, C. Hong, D. M. Purdie, C. Hawley, N. Isbel, M. Butler, et al., “Role of Cytokine Gene Polymorphisms in Acute Rejection and Renal Impairment after Liver Transplantation,” Liver Transplant, Vol. 7, No. 3, 2001, pp. 255-263. doi:10.1053/jlts.2001.22450 [31] J. Mytilineos, G. Laux and G. Opelz, “Relevance of IL10, TGF-beta1, TNFalpha, and IL4Ralpha Gene Polymor- phisms in Kidney Transplantation: A Collaborative Trans- plant Study Report,” American Journal of Transplanta- tion, Vol. 4, No. 10, 2004, pp. 1684-1690. doi:10.1111/j.1600-6143.2004.00561.x [32] B. Rigat, C. Hubert, F. Henc-Gelas, F. Cambien, P. Cor- vol and F. Soubrier, “An Insertion/Deletion Polymor- phism in the Angiotensin I-Converting Enzyme Gene Ac- counting for Half the Variance of Serum Enzyme Lev- els,” Journal of Clinical Investigation, Vol. 86, No. 4, 1990, pp. 1343-1346. doi:10.1172/JCI114844 [33] J. Beige, S. Scherer, A. Weber, S. Engeli, G. Offermann, G. Opelz, A. Distle and A. M. Sharma, “Angiotensin- Converting Enzyme Genotype and Renal Allograft Sur- vival,” Journal of the American Society of Nephrology, Vol. 8, No. 8, 1997, pp. 1319-1323. [34] J. Broekroelofs, C. A. Stegeman, G. Navis, A. M. Teg- zess, Z. D. De and P. E. De Jong, “Risk Factors for Long-Term Renal Survival after Renal Transplantation: A Role for Angiotensin-Converting Enzyme (Insertion/De- letion) Polymorphism?” Journal of the American Society of Nephrology, Vol. 9, No. 11, 1998, pp. 2075-2081. [35] S. Barocci, F. Ginevri, U. Valente, F. Torre, R. Gusmano and A. Nocera, “Correlation between Angiotensin-Con- verting Enzyme Gene Insertion/Deletion Polymorphism and Kidney Graft Long-Term Outcome in Pediatric Re- cipients: A Single-Center Analysis,” Transplantation, Vol. 67, No. 4, 1999, pp. 534-538. doi:10.1097/00007890-199902270-00008 [36] M. B. Juckett, E. P. Cohen, C. A. Keever-Taylor, Y. Zheng, C. A. Lawton, J. E. Moulder and J. Klein, “Loss of Renal Function Following Bone Marrow Transplanta- tion: An Analysis of Angiotensin Converting Enzyme D/I Polymorphism and Other Clinical Risk Factors,” Bone Marrow Transplant, Vol. 27, No. 4, 2001, pp. 451-456. doi:10.1038/sj.bmt.1702797 [37] R. Abdi, T. B. Tran, R. Zee, B. M. Brenner and E. L. Milford, “Angiotensin Gene Polymorphism as a Deter- minant of Post-Transplantation Renal Dysfunction and Hypertension,” Transplantation, Vol. 72, No. 4, 2001, pp. 726-729. doi:10.1097/00007890-200108270-00028 [38] A. O. Ojo, P. J. Held, F. K. Port, R. A. Wolfe, A. B. Leichtman, E. W. Young, et al., “Chronic Renal Failure after Transplantation of Non-Renal Organ,” New England Journal of Medicine, Vol. 349, 2003, pp. 931-940. doi:10.1056/NEJMoa021744 [39] B. M. Murray, M. S. Paller and T. F. Ferris, “Effect of Cyclosporine Administration on Renal Hemodynamics in Conscious Rats,” Kidney International, Vol. 28, 1985, pp. 767-774. doi:10.1038/ki.1985.196 [40] E. J. G. Barros, M. A. Boim and H. Ajzen, “Glomerular Hemodynamics and Hormonal Participation on Cyc- losporine Nephrotoxicity,” Kidney International, Vol. 32, 1987, pp. 19-25. doi:10.1038/ki.1987.166 [41] S. C. Textor, J. C. Burnett, J. C. Romero Jr., V. J. Can- zanello, S. J. Taler, R. Wiesner, et al., “Urinary Endo- thelin and Renal Vasoconstriction with Cyclosporine or FK506 after Liver Transplantation,” Kidney International, Vol. 47, 1995, pp. 1426-1433. doi:10.1038/ki.1995.200 [42] S. Hortelano, M. Castilla, A. M. Torres, A. Tejedor and L. Bosc´a, “Potentiation by Nitric Oxide of Cyclosporin A and FK506-Induced Apoptosis in Renal Proximal Tubule Cells,” Journal of the American Society of Nephrology, Vol. 11, No. 12, 2000, pp. 2315-2323. [43] A. Kurtz, R. Della Bruna and K. Kuhn, “Cyclosporine A Enhances Renin Secretion and Production in Isolated Jux- taglomerular Cells,” Kidney International, Vol. 33, 1988, pp. 947-953. doi:10.1038/ki.1988.92 [44] K. Hoecherl, F. Dreher, H. Vitzthum, J. Koehler and A. Kurtz, “Cyclosporin A Suppresses Cyclooxygenase-2 Expression in the Rat Kidney,” Journal of the American Society of Nephrology, Vol. 13, No. 10, 2002, pp. 2427- 2436. doi:10.1097/01.ASN.0000031702.86799.B9 [45] S. W. Lim, C. Li, K. O. Ahn, et al., “Cyclosporine-In- duced Renal Injury Induces Toll-Like Receptor and Maturation of Dendritic Cells,” Transplantation, Vol. 80, No. 5, 2005, pp. 691-699. doi:10.1097/01.tp.0000173594.69089.a0 [46] W. M. Bennet, A. De Mattos, M. M. Meyer, T. Andoh and J. M. Barry, “Chronic Cyclosporine Nephropathy: The Achilles’ Heel of Immunosuppressive Therapy,” Kid- ney International, Vol. 50, 1996, pp. 1089-1100. doi:10.1038/ki.1996.415 [47] B. D. Myers, J. Ross and L. Newton, “Cyclosporine- Associated Chronic Nephropathy,” New England Journal of Medicine, Vol. 311, No. 11, 1984, pp. 699-705. [48] M. E. Falkenhain, F. G. Cosio and D. D. Sedmak, “Pro- gressive Histologic Injury in Kidneys from Heart and Liver Transplant Recipients Receiving Cyclosporine,” Transplantation, Vol. 62, No. 3, 1996, pp. 364-370. doi:10.1097/00007890-199608150-00011 [49] J. S. Zaltzman, Y. Pei, J. Maurer, A. Patterson and D. C. Cattran, “Cyclosporine Nephrotoxicity in Lung Trans- plant Recipients,” Transplantation, Vol. 54, No. 5, 1992, pp. 875-878. doi:10.1097/00007890-199211000-00021 [50] S. Sandrini, G. Setti, N. Bossini, R. Zubani, S. Cassamali, P. Maiorca, et al., “Experience with Cyclosporine,” Transplant Proceedings, Vol. 36, Suppl. 2S, 2004, pp. 152S-157S. doi:10.1016/j.transproceed.2003.12.036 Copyright © 2013 SciRes. OJNeph  M. D. Colombo ET AL. 177 [51] D. Diederich, J. Skopec, A. Diederich and F.-X. Dai, “Cyclosporine Produces Endothelial Dysfunction by In- creased Production of Superoxide,” Hypertension, Vol. 23, 1994, pp. 957-961. doi:10.1161/01.HYP.23.6.957 [52] R. Christiane and W. Gunter, “Renin-Angiotensin-Aldos- terone System and Progression of Renal Disease,” Jour- nal of the American Society of Nephrology, Vol. 17, No. 11, 2006, pp. 2985-2991. doi:10.1681/ASN.2006040356 [53] G. Wolf, “Renal Injury Due to Renin-Angiotensin-Aldos- terone System Activation of the Transforming Growth Factor-β Pathway,” Kidney International, Vol. 70, No. 11, 2006, pp. 1914-1919. [54] B. J. Nankivell, R. J. Borrows, C. L. S. Fung, P. J. O’Connell, J. R. Chapman and R. D. M. Allen, “Cal- cineurin Inhibitor Nephrotoxicity: Longitudinal Assess- ment by Protocol Histology,” Transplantation, Vol. 78, No. 4, 2004, pp. 557-565. doi:10.1097/01.TP.0000128636.70499.6E [55] E. M. Johnson, D. M. Canafax, K. J. Gillingham, A. Hu- mar, K. Pandian, S. R. Kerr, et al., “Effect of Early Cyc- losporine Levels on Kidney Allograft Rejection,” Clinical Transplantation, Vol. 11, No. 6, 1997, pp. 552-557. [56] N. Kambham, S. Nagarajan, S. Shah, L. Li, O. Salvatierra and M. M. Sarwal, “A Novel, Semiquantitative, Clini- cally Correlated Calcineurin Inhibitor Toxicity Score for Renal Allograft Biopsies,” Clinical Journal of the Ameri- can Society of Nephrology, Vol. 2, No. 1, 2007, pp. 135- 142. doi:10.2215/CJN.01320406 [57] A. Greenberg, J. W. Egel, M. E. Thompson, R. L. Hardesty, B. P. Griffith, H. T. Bahnson, et al., “Early and Late Forms of Cyclosporine Nephrotoxicity: Studies in Cardiac Transplant Recipients,” American Journal of Kidney Diseases, Vol. 9, No. 1, 1987, pp. 12-22. [58] P. F. Hoyer, G. Offner, K. Wonigeit, J. Brodehl and R. Pichelmayr, “Dosage of Cyclosporin A in Children with Renal Transplant,” Clinical Nephrology, Vol. 22, No. 2, 1984, pp. 68-71. [59] H. Zachariae, K. Kragballe, H. E. Hansen, N. Marcussen and S. Olsen, “Renal Biopsy Findings in Long-Term Cyc- losporin Treatment of Psoriasis,” The British Journal of Dermatology, Vol. 136, No. 4, 1997, pp. 531-535. [60] N. J. Lowe, J. M. Wieder, A. Rosenbach, K. Johnson, R. Kunkel, C. Bainbridge, et al., “Long-Term Low-Dose Cyclosporine Therapy for Severe Psoriasis: Effects on Renal Function and Structure,” Journal of the American Academy of Dermatology, Vol. 35, No. 5, 1996, pp. 710- 719. doi:10.1016/S0190-9622(96)90726-4 [61] J. M. Messana, K. J. Johnson and M. J. Mihatsch, “Renal Structure and Function Effects after Low Dose Cyc- losporine in Psoriasis Patients: A Preliminary Report,” Clinical Nephrology, Vol. 43, No. 3, 1995, pp. 150-153. [62] E. W. Young, C. N. Ellis, J. M. Messana, K. J. Johnson, A. B. Leichtman, M. J. Mihatsch, et al., “A Prospective Study of Renal Structure and Function in Psoriasis Pa- tients Treated with Cyclosporine,” Kidney International, Vol. 46, 1994, pp. 1216-1222. doi:10.1038/ki.1994.387 [63] E. Svarstad, S. Helland, T. Morken, L. Bostad, A. Myk- ing, B. M. Iversen and J. Ofstad, “Renal Effects of Main- tenance Low-Dose Cyclosporin A Treatment in Psoria- sis,” Nephrology, Dialysis, Transplantation, Vol. 9, No. 10, 1994, pp. 1462-1467. [64] Y. Pei, J. W. Scholey, A. Katz, R. Schachter, G. F. Murphy and D. Cattran, “Chronic Nephrotoxicity in Pso- riatic Patients Treated with Low-Dose Cyclosporine,” American Journal of Kidney Diseases, Vol. 23, No. 4, 1994, pp. 528-536. [65] A. V. Powles, T. Cook, B. Hulme, B. S. Baker, H. M. Lewis, E. Thomas, et al., “Renal Function and Biopsy Findings after 5 Years’ Treatment with Low-Dose Cyc- losporin for Psoriasis,” The British Journal of Dermato- logy, Vol. 128, No. 2, 1993, pp. 159-165. doi:10.1111/j.1365-2133.1993.tb15145.x [66] A. V. Powles, C. M. Hardman, W. M. Porter, T. Cook, B. Hulme and L. Fry, “Renal Function after 10 Years’ Treatment with Cyclosporin for Psoriasis,” The British Journal of Dermatology, Vol. 138, No. 3, 1998, pp. 443- 439. doi:10.1046/j.1365-2133.1998.02122.x [67] H. Zachariae, H. E. Hansen, K. Kragballe and S. Olsen, “Morphologic Renal Changes during Cyclosporine Treat- ment of Psoriasis. Studies on Pretreatment and Post- Treatment Kidney Biopsy Specimens,” Journal of the American Academy of Dermatology, Vol. 26, No. 3, 1992, pp. 415-419. [68] M. J. Mihatsch, G. Thiel and B. Ryffel, “Morphology of Cyclosporine Nephropathy,” Progress in Allergy, Vol. 38, 1986, pp. 447-465. [69] U. Mrowietz, L. Faerber, H. H. Henneicke-von Zepelin, H. Bachmann, D. Welzel and E. Christophers, “Long- Term Maintenance Therapy with Cyclosporine and Post- Treatment Survey in Severe Psoriasis: Results of a Mul- ticenter Study. German Multicenter Study,” Journal of the American Academy of Dermatology, Vol. 33, No. 3, 1995, pp. 470-475. [70] C. Laburte, R. Grossman, J. Abi-Rached, K. H. Abey- wickrama and L. Dubertret, “Efficacy and Safety of Oral Cyclosporin A (CyA; Sandimmun) for Long-Term Treat- ment of Chronic Severe Plaque Psoriasis,” British Jour- nal of Dermatology, Vol. 130, No. 3, 1994, pp. 366-375. doi:10.1111/j.1365-2133.1994.tb02935.x [71] J. Shupack, E. Abel, E. Bauer, M. Brown, L. Drake, R. Freinkel, et al., “Cyclosporine as Maintenance Therapy in Patients with Severe Psoriasis,” Journal of the American Academy of Dermatology, Vol. 36, No. 3, 1997, pp. 423- 432. [72] V. C. Ho, C. E. Griffiths, G. Albrecht, F. Vanaclocha, G. León-Dorantes, N. Atakan, et al., “Intermittent Short Courses of Cyclosporin (NeoralR) for Psoriasis Unre- sponsive to Topical Therapy: A 1-Year Multicentre, Randomized Study. The PISCES Study Group,” British Journal of Dermatology, Vol. 141, No. 2, 1999, pp. 283- 291. doi:10.1046/j.1365-2133.1999.02977.x [73] P. Gisondi, M. Del Giglio, V. Di Francesco, et al., “Weight Loss Improves the Response of Obese Patients with Moderate-to-Severe Chronic Plaque Psoriasis to Low-Dose Cyclosporine Therapy: A Randomized, Con- trolled, Investigator-Blinded Clinical Trial,” The Ameri- can Journal of Clinical Nutrition, Vol. 88, No. 5, 2008, Copyright © 2013 SciRes. OJNeph  M. D. Colombo ET AL. 178 pp. 1242-1247. [74] J. F. Honeyman, L. Sànchez and P. Valdés, “Low-Dose Cyclosporine A Improves Severe Disabling Psoriasis in Latin America. Latin American Multicenter Study,” In- ternational Journal of Dermatology, Vol. 34, No. 8, 1995, pp. 583-588. doi:10.1111/j.1365-4362.1995.tb02961.x [75] G. S. Panayi and P. Tugwell, “An International Consen- sus Report: The Use of Cyclosporin A in Rheumatoid Arthritis,” British Journal of Rheumatology, Vol. 32, Suppl. 1, 1993, pp. 1-3. [76] G. S. Panayi and P. Tugwell, “The Use of Cyclosporin A in Rheumatoid Arthritis: Conclusions of an International Review,” British Journal of Rheumatology, Vol. 33, No. 10, 1994, pp. 967-969. doi:10.1093/rheumatology/33.10.967 [77] R. Assan, F. Blanchet, G. Feutren, J. Timsit, E. Larger, C. Boitard, et al., “Normal Renal Function 8 to 13 Years af- ter Cyclosporin A Therapy in 285 Diabetic Patients,” Diabetes/Metabolism Research and Reviews, Vol. 18, No. 6, 2002, pp. 464-472. doi:10.1002/dmrr.325 [78] H. Ekberg, H. Tedesco-Silva, A. Demirbas, S. Vitko, B. Nashan, A. Gurkan, et al., “Reduced Exposure to Cal- cineurin Inhibitors in Renal Transplantation,” New Eng- land Journal of Medicine, Vol. 357, 2007, pp. 2562-2575. doi:10.1056/NEJMoa067411 [79] H. Ekberg, J. Grinyo, B. Nashan, Y. Vanrenterghem, F. Vincenti, A. Voulgari, et al., “Cyclosporine Sparing with Mycophenolate Mofetil, Daclizumab and Corticosteroids in Renal Allograft Recipients: The CAESAR Study,” American Journal of Transplantation, Vol. 7, No. 3, 2007, pp. 560-570. doi:10.1111/j.1600-6143.2006.01645.x [80] T. R. Srinivas and H. U. Meier-Kriesche, “Minimizing Immunosuppression, an Alternative Approach to Reduc- ing Side Effects: Objectives and Interim Result,” Clinical Journal of the American Society Nephrology, Vol. 3, Suppl. 2, 2008, pp. S101-S116. doi:10.2215/CJN.03510807 [81] S. M. Flechner, J. Kobashigawa and G. Klintmalm, “Cal- cineurin Inhibitor-Sparing Regimens in Solid Organ Transplantation: Focus on Improving Renal Function and Nephrotoxicity,” Clinical Transplantation, Vol. 22, No. 1, 2008, pp. 1-15. [82] D. Colombo, N. Cassano, G. Altomare, A. Giannetti and G. A. Vena, “Psoriasis Relapse Evaluation with Week- End Cyclosporine A Treatment: Results of a Randomized, Double-Blind, Multicenter Study,” International Journal of Immunopathology and Pharmacology, Vol. 23, No. 4, 2010, pp. 1143-1152. [83] P. Ruggenenti, N. Perico, L. Mosconi, F. Gaspari, A. Benigni, C. S. Amuchastegui, et al., “Calcium Channel Blockers Protect Transplant Patients from Cyclosporine- Induced Daily Renal Hypoperfusion,” Kidney Interna- tional, Vol. 43, 1993, pp. 706-711. doi:10.1038/ki.1993.101 [84] K. H. Rahn, M. Barenbrock, F. Fritschka, A. Heinecke, J. Lippert, K. Schroeder, et al., “Effect of Nitrendipine on Renal Function in Renal-Transplant Patients Treated with Cyclosporin: A Randomised Trial,” The Lancet, Vol. 354, No. 9188, 1999, pp. 1415-1420. doi:10.1016/S0140-6736(99)08421-4 [85] B. J. Nankivell, J. R. Chapman, G. Bonovas and S. Grue- newals, “Oral Cyclosporine But Not Tacrolimus Reduces Renal Transplant Blood Flow,” Transplantation, Vol. 77, No. 9, 2004, pp. 1457-1459. doi:10.1097/01.TP.0000121196.71904.E0 [86] J. M. Morales, E. Rodriguez-Paternina, A. Araque, A. Andres, E. Hernandez, L. M. Ruilope and J. L. Rodicio, “Long-Term Protective Effect of a Calcium Antagonist on Renal Function in Hypertensive Renal Transplant Pa- tients on Cyclosporine Therapy: A 5-Year Prospective Randomized Study,” Transplant Proceedings, Vol. 26, No. 5, 1994, pp. 2598-2599. [87] D. R. Kuypers, H. H. Neumayer, L. Fritsche, K. Budde, J. Rodicio, Y. Vanrenterghem (Lacidipine Study Group), “Calcium Channel Blockade and Preservation of Renal Graft Function in Cyclosporine-Treated Recipients: A Prospective Randomized Placebo-Controlled 2-Year Study,” Transplantation, Vol. 78, No. 8, 2004, pp. 1204- 1211. doi:10.1097/01.TP.0000137793.23371.42 [88] L. Legault, R. I. Ogilvie, C. J. Cardella and F. H. Leenen, “Calcium Antagonists in Heart Transplant Recipients: Effects on Cardiac and Renal Function and Cyclosporine Pharmacokinetics,” The Canadian Journal of Cardiology, Vol. 9, No. 5, 1993, pp. 398-404. [89] F. H. Leenen, E. Coletta and R. A. Davies, “Prevention of Renal Dysfunction and Hypertension by Amlodipine after Heart Transplant,” American Journal of Cardiology, Vol. 100, No. 3, 2007, pp. 531-535. doi:10.1016/j.amjcard.2007.03.058 [90] B. Lindelow, C. H. Bergh, H. Herlitz and F. Waagstein, “Predictors and Evolution of Renal Function during 9 Years Following Heart Transplantation,” Journal of the American Society of Nephrology, Vol. 11, No. 5, 2000, pp. 951-957. [91] T. P. Hannedouche, S. Natov, C. Boitard, B. Lacour and J. P. Grunfeld, “Angiotensin Converting Enzyme Inhibition and Chronic Cyclosporine-Induced Renal Dysfunction in Type 1 Diabetes,” Nephrology Dialysis Transplantation, Vol. 11, No. 4, 1996, pp. 673-678. doi:10.1093/oxfordjournals.ndt.a027358 [92] M. Hausberg, M. Kosch, H. Hohage, B. Suwelack, M. Barenbrock, K. Kisters and K. H. Rahn, “Antihyperten- sive Treatment in Renal Transplant Patients—Is There a Role for ACE Inhibitors?” Annals of Transplantation, Vol. 6, No. 4, 2001, pp. 31-37. [93] J. M. Campistol, P. Inigo, W. Jimenez, S. Lario, P. H. Clesca, F. Oppenheimer and F. Rivera, “Losartan De- creases Plasma Levels of TGF-Beta1 in Transplant Pa- tients with Chronic Allograft Nephropathy,” Kidney In- ternational, Vol. 56, 1999, pp. 714-719. doi:10.1046/j.1523-1755.1999.00597.x [94] P. Inigo, J. M. Campistol, S. Lario, C. Piera, B. Campos, M. Bescos, et al., “Effects of Losartan and Amlodipine on Intrarenal Hemodynamics and TGF-Beta(1) Plasma Lev- els in a Crossover Trial in Renal Transplant Recipients,” Journal of the American Society of Nephrology, Vol. 12, No. 4, 2001, pp. 822-827. [95] G. Mourad, J. Ribstein and A. Mimran, “Converting- Copyright © 2013 SciRes. OJNeph  M. D. Colombo ET AL. 179 Enzyme Inhibitor Versus Calcium Antagonist in Cyc- losporine-Treated Renal Transplants,” Kidney Interna- tional, Vol. 43, 1993, pp. 419-425. doi:10.1038/ki.1993.61 [96] K. Midtvedt, A. Hartmann, A. Foss, P. Fauchald, K. P. Nordal, K. Rootwelt and H. Holdaas, “Sustained Im- provement of Renal Graft Function for Two Years in Hypertensive Renal Transplant Recipients Treated with Nifedipine as Compared to Lisinopril,” Transplantation, Vol. 72, No. 11, 2001, pp. 1787-1792. doi:10.1097/00007890-200112150-00013 [97] M. Moran, M. F. Mozes, M. Maddux, S. Veremis, C. Bartkus, B. Ketel, et al., “Prevention of Acute Graft Re- jection by the Prostaglandin E1 Analogue Misoprostol in Renal-Transplant Recipients Treated with Cyclosporine and Prednisone,” New England Journal of Medicine, Vol. 322, 1990, pp. 1183-1188. doi:10.1056/NEJM199004263221703 [98] M. Boers, W. G. Bensen, D. Ludwin, C. H. Goldsmith and P. Tugwell, “Cyclosporine Nephrotoxicity in Rheu- matoid Arthritis: No Effect of Short Term Misoprostol Treatment,” The Journal of Rheumatology, Vol. 19, No. 4, 1992, pp. 534-537. [99] X. Z. Zhang, G. Ardissino, L. Ghio, A. S. Tirelli, V. Dacco, D, Colombo, et al., “L-Arginine Supplementation in Young Renal Allograft Recipients with Chronic Trans- plant Dysfunction,” Clinical Nephrology, Vol. 55, No. 6, 2001, pp. 453-439. [100] H. Ling, X. Li, S. Jha, W. Wan, L. Karetskaya, B. Pratt and S. Ledbetter, “Therapeutic Role of TGF-Beta-Neu- tralizing Antibody in Mouse Cyclosporin A Nephropathy: Morphologic Improvement Associated with Functional Preservation,” Journal of the American Society of Ne- phrology, Vol. 14, No. 2, 2003, 377-388. doi:10.1097/01.ASN.0000042168.43665.9B [101] M. Islam, J. F. Burke Jr., T. A. McGowan, Y. Zhu, S. R. Dunn, P. McCue, et al., “Effect of Anti-Transforming Growth Factor-Beta Antibodies in Cyclosporine-Induced Renal Dysfunction,” Kidney International, Vol. 59, 2001, pp. 498-506. doi:10.1046/j.1523-1755.2001.059002498.x [102] M. Tariq, C. Morais, S. Sobki, M. Al Sulaiman and A. Al Khader, “N-Acetylcysteine Attenuates Cyclosporin-In- duced Nephrotoxicity in Rats,” Nephrology Dialysis Transplantation, Vol. 14, No. 4, 1999, pp. 923-929. doi:10.1093/ndt/14.4.923 [103] P. Barany, P. Stenvinkel, A. Ottosson-Seeberger, A. Alvestrand, J. Morrow, J. J. Roberts and A. K. Sala- hudeen, “Effect of 6 Weeks of Vitamin E Administration on Renal Haemodynamic Alterations Following a Single Dose of Neoral in Healthy Volunteers,” Nephrology Di- alysis Transplantation, Vol. 16, No. 3, 2001, pp. 580-584. doi:10.1093/ndt/16.3.580 [104] J. K. Jenkins, H. Huang, K. Ndebele and A. K. Sala- hudeen, “Vitamin E Inhibits Renal mRNA Expression of COX II, HO I, TGF-Beta, and Osteopontin in the Rat Model of Cyclosporine Nephrotoxicity,” Transplantation, Vol. 71, No. 2, 2001, pp. 331-334. doi:10.1097/00007890-200101270-00028 [105] C. Lessio, S. F. de Assuncao, M. A. Gloria, A. B. Di Tommaso, M. M. Gori, G. S. Di Marco, et al., “Cyc- losporine A and NAC on the Inducible Nitric Oxide Syn- thase Expression and Nitric Oxide Synthesis in Rat Renal Artery Cultured Cells,” Kidney International, Vol. 68, 2005, pp. 2508-2516. doi:10.1111/j.1523-1755.2005.00726.x [106] C. Li, B. K. Sun, S. W. Lim, J. C. Song, S. W. Kang, Y. S. Kim, et al., “Combined Effects of Losartan and Pravas- tatin on Interstitial Inflammation and Fibrosis in Chronic Cyclosporine-Induced Nephropathy,” Transplantation, Vol. 79, No. 11, 2005, pp. 1522-1529. [107] A. K. Pere, L. Lindgren, P. Tuomainen, L. Krogerus, P. Rauhala, J. Laakso, et al., “Dietary Potassium and Mag- nesium Supplementation in Cyclosporine-Induced Hy- pertension and Nephrotoxicity,” Kidney International, Vol. 58, 2000, pp. 2462-2472. doi:10.1046/j.1523-1755.2000.00429.x [108] T. Asai, T. Nakatani, S. Tamada, N. Kuwabara, S. Ya- manaka, K. Tashiro, et al., “Activation of Transcription Factors AP-1 and NF-kappaB in Chronic Cyclosporine A Nephrotoxicity: Role in Beneficial Effects of Magnesium Supplementation,” Transplantation, Vol. 75, No. 7, 2003, pp. 1040-1044. doi:10.1097/01.TP.0000057242.96219.AF [109] M. J. Mihatsch, D. Belghiti and S. O. Bohman, “Kidney Biopsies in Control of Cyclosporine A Treated Psoriatic Patients,” British Journal of Dermatology, Vol. 122, Suppl. 22, 1990, pp. 95-100. doi:10.1111/j.1365-2133.1990.tb02887.x [110] L. Faerber, M. Braeutigam, G. Weidinger, U. Mrowietz, E. Christophers, H. J. Schulze, et al., “Cyclosporine in Severe Psoriasis: Results of a Meta-Analysis,” American Journal of Clinical Dermatology, Vol. 2, No. 1, 2001, pp. 41-47. doi:10.2165/00128071-200102010-00007 [111] J. Berth-Jones, C. A. Henderson, C. S. Munro, S. Rogers, R. J. Chalmers, M. J. Boffa, et al., “Treatment of Psoria- sis with Intermittent Short-Course Cyclosporine (Neo- ral®). A Multicentre Study,” British Journal of Derma- tology, Vol. 136, No. 4, 1997, pp. 527-530. [112] V. C. Y. Ho, C. E. M. Griffiths, J. Berth-Jones, K. A. Papp, F. Vanaclocha, E. Dauden, et al., “Intermittent Short Courses of Cyclosporine Microemulsion for the Long-Term Management of Psoriasis: A 2-Year Cohort Study,” Journal of the American Academy of Dermato- logy, Vol. 44, No. 4, 2001, pp. 643-651. doi:10.1067/mjd.2001.112400 [113] D. Pathirana, A. D. Ormerod, P. Saiag, C. Smith, P. I. Spuls, A. Nast, et al., “European S3-Guidelines on the Systemic Treatment of Psoriasis Vulgaris,” Journal of the European Academy of Dermatology and Venereology, Vol. 23, No. 2, 2009, pp. 1-70. doi:10.1111/j.1468-3083.2009.03389.x [114] G. Feutren, K. Abeywickrama, D. Friend and B. Von Graffenried, “Renal Function and Blood Pressure in Pso- riatic Patients Treated with Cyclosporine A,” British Journal of Dermatology, Vol. 122, No. 36, 1990, pp. 57-69. doi:10.1111/j.1365-2133.1990.tb02883.x Copyright © 2013 SciRes. OJNeph  M. D. Colombo ET AL. Copyright © 2013 SciRes. OJNeph 180 [115] H. I. Katz, “Potential Drug Interactions with Cyc- losporine,” International Journal of Dermatology, Vol. 36, No. 1, 1997, pp. 18-24. doi:10.1046/j.1365-4362.36.s1.5.x
|