 Surgical Science, 2013, 4, 393-400 http://dx.doi.org/10.4236/ss.2013.49077 Published Online September 2013 (http://www.scirp.org/journal/ss) Surgical Treatment of Liver Metastases from Gastric Cancer Daniel Vasilev Kostov1, Georgi Leonidov Kobakov2, Daniel Veselov Yankov1 1Department of Surgery, Naval Hospital of Varna, Varna, Bulgaria 2Clinic of Surgery, Specialized Hospital for Oncologic Diseases of Varna, Varna, Bulgaria Email: danielkostov@abv.bg Received June 28, 2013; revised July 30, 2013; accepted August 7, 2013 Copyright © 2013 Daniel Vasilev Kostov et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Hepatectomy for gastric metastases remains controversial. We aimed at assessing the surgical results, clinicopathological features of gastric cancer liver metastases (GCLM) and prognostic factors. Methods: The outcome of 28 consecutive patients with synchronous (n = 24) or metachronous (n = 4) GCLM was retrospectively analyzed. Curatively, initial hepatectomies such as segmentectomy and hemihepatectomy or non-anatomical limited liver resec- tion less extensive than segmentectomy followed complete primary gastric cancer (GC) resections. Results: Median survival time was 16 months (range, 5 - 66 months). The actuarial overall 12-, 36-, and 60-month survival rates after hepatectomy were 67.8% (n = 19), 39.2% (n = 11), and 28.5% (n = 8), respectively. In multivariate analysis, absent GC serosal invasion-hazard ratio (HR) 1; 95% confidence interval (CI) 1.2 - 9.9; P = 0.020; solitary LM-HR 1; 95% CI 1.6 - 16.0; P = 0.005, and curative liver resection with negative resection margin (R0)-HR 1, 95% CI 2.2 - 18.0; P = 0.001 were independent prognostic factors. Conclusions: Surgery of GCLM is a good indication in well-selected patients with an absent serosal invasion of primary tumour, single GCLM and attainment of R0 liver resection. For most GCLM patients, however, there are no other therapeutic modalities. Thus systemic chemotherapy remains the best hope for a longer patient’s survival and an improved individual quality of life. Keywords: Gastric Cancer; Liver Metastases; Hepatectomy; Survival; Prognostic Factors; Univariate Analysis; Multivariate Analysis 1. Introduction Liver is a common site of metastases of GC. However, their treatment has not been well-defined yet. Gastric adenocarcinoma is the fourth most common cancer worldwide and accounts for 1.5% of all new diagnoses and 5.2% of all cancer deaths [1,2]. Early tumour detec- tion, standardized surgical treatment including lymph node dissection and appropriate adjuvant therapy im- prove the survival of primary GC patients. At the time of diagnosis, 35% of the cases present with evidence of distant metastases and 4% - 14% do with metastatic liver disease [3,4]. Patients with isolated metastases are un- usual and account for 0.5% of the cases in a comprehen- sive series [5]. The benefits of surgical approach to me- tastatic GC remain debatable and have been rarely stud- ied [6]. GCLM convey a very poor prognosis. Most me- tastases will be diffused, and chemotherapy is then the optimal treatment modality. Therefore, proper determi- nation of selection criteria for hepatic resection and pre- conditions for long-term survival after hepatectomy is crucial. Several authors report limited experiences of sur- gical resection of GCLM in selected patients, with five- year survival rates of 0% - 38% [7,8]. Complete removal of the primary GC and the LM appears to be the only option for possibly curing the disease, although the bene- fits of metastasectomy are poorly defined. Accordingly, we herewith review retrospectively 28 patients who have undergone liver resection for GCLM in order to clarify the surgical outcome and clinico- pathological features of favourable prognosis in such patients. 2. Methods Between 1992 and 2006, 756 GC patients were surgically treated in the Department of Surgery, Naval Hospital of Varna, Bulgaria. Of these, 88 patients (11.6%) presented with synchronous LM and 52 ones (6.8%) developed metachronous LM after resection of the primary GC. C opyright © 2013 SciRes. SS  D. V. KOSTOV ET AL. 394 Synchronous LM was defined by detection before and during surgery or within 3 months of primary tumour resection. GCLM were diagnosed through endoscopy and biopsy, echography, computer axial tomography (CAT) and intraoperative echography. The following criteria were actually accepted for GCLM resection: 1) good control and complete resection of the primary tumour and lymph nodes involvement in synchronous disease; 2) no signs at preoperative workup of disseminated diseases, hilar lymph nodes metastases, peritoneal dissemination, or extrahepatic metastases; 3) complete LM resection (no residual tumour at macroscopical examination) [9]. Ac- cording to these criteria, twenty-eight out of a total of 140 patients having undergone initial hepatectomy were selected for this study. Disease free was defined and determined in accor- dance with clinical, laboratory, endoscopic and imaging diagnostic methods as following-up ranged from 12 to 122 months (median of 26 months). Postoperatively, all the patients were followed-up every six months clinically, biochemically, echographically, endoscopically and by means of CAT or positron emission tomography (PET) if needed up to the end of the 60th postoperative month. Follow-up ranged from 12 to 122 months (median of 26 months). The following preoperative demographic and clinical information was obtained from the patient re- cords: age, gender, histological differentiation, serosal invasion, extent of lymph node metastases, lymphatic and venous invasion of the primary GC, size and number of LM, lobar distribution, vascular invasion within LM, time of LM appearance (synchronous or metachronous), fibrous pseudocapsule between LM and liver paren- chyma, peritumoural lymphocytes surrounding LM, CEA level before hepatectomy, type of hepatic resection, sur- gical margin and completeness of the resection. In each patient, intraoperative liver ultrasound was performed to assess the extent and number of hepatic lesions and their relationships to intrahepatic vascular and biliary struc- tures. Surgical procedures were classified either as ana- tomical liver resection (segmentectomy and hemihea- tectomy), or as non-anatomical limited liver resection (for all the resections less extensive than segmentectomy). One-, two- and three-month mortality rates were ana- lyzed. The rest 112 patients with GC with concomitant LM were excluded as candidates for potential R0 because of the present multiple bilateral metastases. They underwent systemic or hepatic artery infusion chemotherapy plus local-regional radiation. Actuarial overall survival rates since the date of the liver resection onwards were calculated using the Kap- lan-Meier method. Differences were compared between subgroups according to univariate analysis in the result- ing distributions using the log-rank test. Based on these results, a multivariate stepwise Cox regression analysis was performed to identify factors that were independ- ently associated with prognosis. Statistical significance was defined as a P-value below 0.05. Any statistical analyses were accomplished using SPSS 9.0 package (SPSS Inc., Chicago, IL, USA). 3. Results Of these 28 patients, there were 19 males and 9 females at a mean age of 68 years (range, 51 - 81 years). Curative resection of the GC with lymph node dissection up to the second echelon (D2) was performed in all the cases. Se- rosal invasion was absent in 10 (35.7%) and present in 18 (64.3%) patients. Lymph node grouping was based on Japanese classification on cancer typing. GC with lymph node involvement was established in 21 (75%) patients. A well-to poorly differentiated adenocarcinoma in the primary gastric location was pathologically proven in all the patients. The number of metastatic nodules was one in 19 (67.8%) patients, two in three (10.7%) ones, three in three (10.7%) ones, and four to seven in three (10.7%) ones. GCLM median size was 37 mm (range, 6 - 105 mm). With regard to pathological features in GCLM peritumoural lesion, fibrous pseudocapsule between the tumour and hepatic parenchyma was seen in 16 (57.1%) patients where lymphocytic infiltration was graded as weak in 21 (75%) patients. Synchronous hepatic resec- tion and gastrectomy was performed in 24 (85.7%) pa- tients. The mean interval between gastric and hepatic resection in the remaining 4 (14.2%) patients with meta- chronous metastases was 19 months (range, 6 - 22 months). Anatomical resection was carried out in 19 (67.8%; major 11; minor 8) patients and limited non- anatomical minor resection in 9 (32.1%) ones. In 25 (89.2%) patients the resection was classified as curative (R0), whereas microscopically positive margins were identified in three (10.7%) patients. Adjuvant systemic chemotherapy was administered to all the patients. No patient died within three months of surgery. The overall morbidity rate was 25% (seven patients). Com- plications comprised transient liver failure, bile leakage, biloma, intaabdominal abscessus, pleural effusion, and wound infection. The results of the statistical analysis of prognostic factors are presented in Table 1. The absent serosal inva- sion of primary tumour (P = 0.031) and of lymph node involvement (P = 0.042), the present solitary LM (P = 0.032) and peritumoural fibrous pseudocapsule (P = 0.021) as well as the R0 liver resection (P = 0.003) were significant determinants for a favourable prognosis after hepatic resection at univariate analysis while remaining factors associated with the primary lesion, LM and sur- gery were not. Median survival time was 16 months (range, 5 - 66 months). Five patients remain alive and Copyright © 2013 SciRes. SS 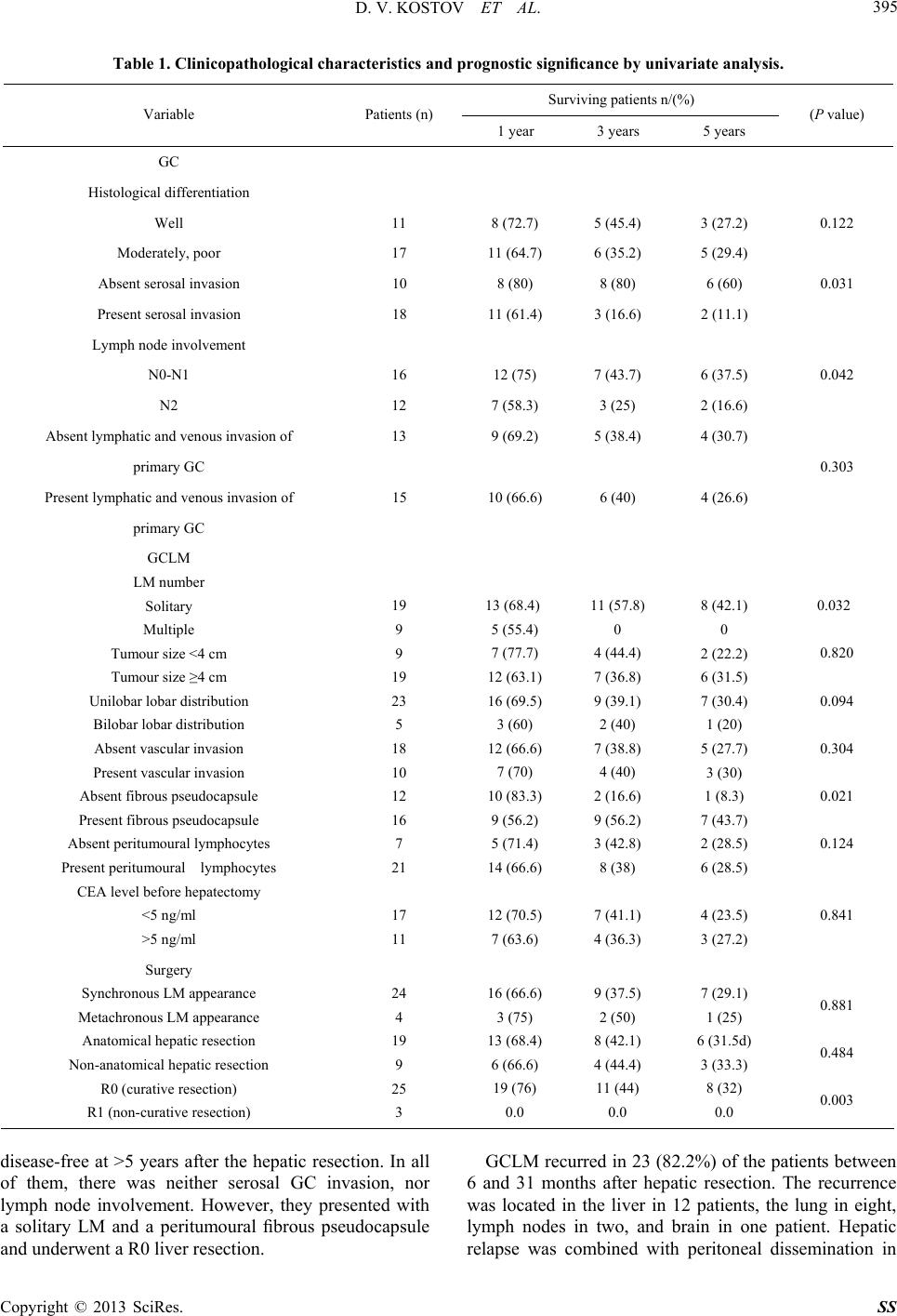 D. V. KOSTOV ET AL. Copyright © 2013 SciRes. SS 395 Table 1. Clinicopathological characteristics and prognostic significance by univariate analysis. Surviving patients n/(%) Variable Patients (n) 1 year 3 years 5 years (P value) GC Histological differentiation Well 11 8 (72.7) 5 (45.4) 3 (27.2) 0.122 Moderately, poor 17 11 (64.7) 6 (35.2) 5 (29.4) Absent serosal invasion 10 8 (80) 8 (80) 6 (60) 0.031 Present serosal invasion 18 11 (61.4) 3 (16.6) 2 (11.1) Lymph node involvement N0-N1 16 12 (75) 7 (43.7) 6 (37.5) 0.042 N2 12 7 (58.3) 3 (25) 2 (16.6) Absent lymphatic and venous invasion of 13 9 (69.2) 5 (38.4) 4 (30.7) primary GC 0.303 Present lymphatic and venous invasion of 15 10 (66.6) 6 (40) 4 (26.6) primary GC GCLM LM number Solitary Multiple Tumour size <4 cm Tumour size ≥4 cm Unilobar lobar distribution Bilobar lobar distribution Absent vascular invasion Present vascular invasion Absent fibrous pseudocapsule Present fibrous pseudocapsule Absent peritumoural lymphocytes Present peritumoural lymphocytes CEA level before hepatectomy <5 ng/ml >5 ng/ml 19 9 9 19 23 5 18 10 12 16 7 21 17 11 13 (68.4) 5 (55.4) 7 (77.7) 12 (63.1) 16 (69.5) 3 (60) 12 (66.6) 7 (70) 10 (83.3) 9 (56.2) 5 (71.4) 14 (66.6) 12 (70.5) 7 (63.6) 11 (57.8) 0 4 (44.4) 7 (36.8) 9 (39.1) 2 (40) 7 (38.8) 4 (40) 2 (16.6) 9 (56.2) 3 (42.8) 8 (38) 7 (41.1) 4 (36.3) 8 (42.1) 0 2 (22.2) 6 (31.5) 7 (30.4) 1 (20) 5 (27.7) 3 (30) 1 (8.3) 7 (43.7) 2 (28.5) 6 (28.5) 4 (23.5) 3 (27.2) 0.032 0.820 0.094 0.304 0.021 0.124 0.841 Surgery Synchronous LM appearance Metachronous LM appearance Anatomical hepatic resection Non-anatomical hepatic resection R0 (curative resection) R1 (non-curative resection) 24 4 19 9 25 3 16 (66.6) 3 (75) 13 (68.4) 6 (66.6) 19 (76) 0.0 9 (37.5) 2 (50) 8 (42.1) 4 (44.4) 11 (44) 0.0 7 (29.1) 1 (25) 6 (31.5d) 3 (33.3) 8 (32) 0.0 0.881 0.484 0.003 disease-free at >5 years after the hepatic resection. In all of them, there was neither serosal GC invasion, nor lymph node involvement. However, they presented with a solitary LM and a peritumoural fibrous pseudocapsule and underwent a R0 liver resection. GCLM recurred in 23 (82.2%) of the patients between 6 and 31 months after hepatic resection. The recurrence was located in the liver in 12 patients, the lung in eight, lymph nodes in two, and brain in one patient. Hepatic relapse was combined with peritoneal dissemination in 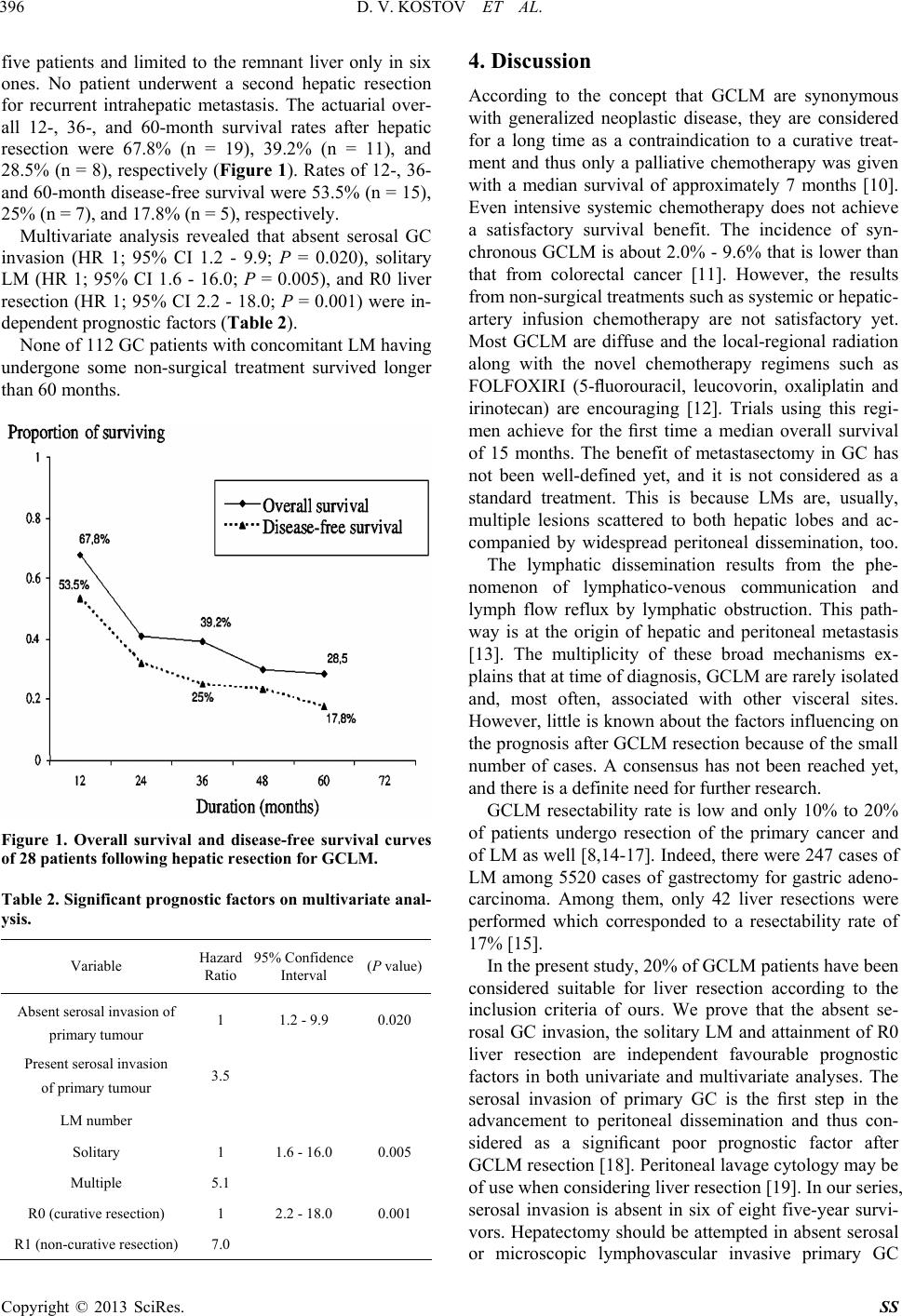 D. V. KOSTOV ET AL. 396 five patients and limited to the remnant liver only in six ones. No patient underwent a second hepatic resection for recurrent intrahepatic metastasis. The actuarial over- all 12-, 36-, and 60-month survival rates after hepatic resection were 67.8% (n = 19), 39.2% (n = 11), and 28.5% (n = 8), respectively (Figure 1). Rates of 12-, 36- and 60-month disease-free survival were 53.5% (n = 15), 25% (n = 7), and 17.8% (n = 5), respectively. Multivariate analysis revealed that absent serosal GC invasion (HR 1; 95% CI 1.2 - 9.9; P = 0.020), solitary LM (HR 1; 95% CI 1.6 - 16.0; P = 0.005), and R0 liver resection (HR 1; 95% CI 2.2 - 18.0; P = 0.001) were in- dependent prognostic factors (Table 2). None of 112 GC patients with concomitant LM having undergone some non-surgical treatment survived longer than 60 months. Figure 1. Overall survival and disease-free survival curves of 28 patients following hepatic resection for GCLM. Table 2. Significant prognostic factors on multivariate anal- ysis. Variable Hazard Ratio 95% Confidence Interval (P value) Absent serosal invasion of primary tumour 1 1.2 - 9.9 0.020 Present serosal invasion of primary tumour 3.5 LM number Solitary 1 1.6 - 16.0 0.005 Multiple 5.1 R0 (curative resection) 1 2.2 - 18.0 0.001 R1 (non-curative resection) 7.0 4. Discussion According to the concept that GCLM are synonymous with generalized neoplastic disease, they are considered for a long time as a contraindication to a curative treat- ment and thus only a palliative chemotherapy was given with a median survival of approximately 7 months [10]. Even intensive systemic chemotherapy does not achieve a satisfactory survival benefit. The incidence of syn- chronous GCLM is about 2.0% - 9.6% that is lower than that from colorectal cancer [11]. However, the results from non-surgical treatments such as systemic or hepatic- artery infusion chemotherapy are not satisfactory yet. Most GCLM are diffuse and the local-regional radiation along with the novel chemotherapy regimens such as FOLFOXIRI (5-fluorouracil, leucovorin, oxaliplatin and irinotecan) are encouraging [12]. Trials using this regi- men achieve for the first time a median overall survival of 15 months. The benefit of metastasectomy in GC has not been well-defined yet, and it is not considered as a standard treatment. This is because LMs are, usually, multiple lesions scattered to both hepatic lobes and ac- companied by widespread peritoneal dissemination, too. The lymphatic dissemination results from the phe- nomenon of lymphatico-venous communication and lymph flow reflux by lymphatic obstruction. This path- way is at the origin of hepatic and peritoneal metastasis [13]. The multiplicity of these broad mechanisms ex- plains that at time of diagnosis, GCLM are rarely isolated and, most often, associated with other visceral sites. However, little is known about the factors influencing on the prognosis after GCLM resection because of the small number of cases. A consensus has not been reached yet, and there is a definite need for further research. GCLM resectability rate is low and only 10% to 20% of patients undergo resection of the primary cancer and of LM as well [8,14-17]. Indeed, there were 247 cases of LM among 5520 cases of gastrectomy for gastric adeno- carcinoma. Among them, only 42 liver resections were performed which corresponded to a resectability rate of 17% [15]. In the present study, 20% of GCLM patients have been considered suitable for liver resection according to the inclusion criteria of ours. We prove that the absent se- rosal GC invasion, the solitary LM and attainment of R0 liver resection are independent favourable prognostic factors in both univariate and multivariate analyses. The serosal invasion of primary GC is the first step in the advancement to peritoneal dissemination and thus con- sidered as a significant poor prognostic factor after GCLM resection [18]. Peritoneal lavage cytology may be of use when considering liver resection [19]. In our series, serosal invasion is absent in six of eight five-year survi- vors. Hepatectomy should be attempted in absent serosal or microscopic lymphovascular invasive primary GC Copyright © 2013 SciRes. SS  D. V. KOSTOV ET AL. 397 involvement [18,20]. There is an insignificant difference in terms of serosal invasion or lymph node GCLM be- tween surviving and non-surviving patients [14,21,22]. A marginal significance of primary tumour serosal invasion is proved [15]. Nobody of our patients presenting with multiple GCLM has survived longer than three years, whereas 42.1% of those with a solitary GCLM have done at least five years. We also demonstrate a limited effect of surgical resec- tion in the patients with multiple GCLM. Survival rates of 56% for single GCLM but no ones for multiple ones after hepatectomy have been reported [14]. The impor- tance of GCLM number as a prognostic factor is sug- gested elsewhere, too [17,23,24]. A single GCLM and absent peritoneal dissemination are significantly independent prognostic factors for such patients with synchronous GCLM [11]. The attainment of R0 liver resection is the third independent prognostic factor to consider for the indications of hepatic resection of GCLM. There is a significant survival difference ac- cording to the tumour-free hepatic resection margin [22]. Long-term survivors with more than three GCLM have been reported [25] enabling the conclusion that if R0 can be achieved, hepatic resection should not be abandoned even in patients with multiple GCLM. A positive resec- tion margin should be considered a powerful determinant of poor outcome [7]. The results concerning the surgical margin of GCLM are conflicting as some authors accept that anatomical hepatic resection with a surgical margin of at least 10 mm is an important factor influencing survival [7,22,26], while other investigators report that both the resection margin and the type of liver surgery do not affect sur- vival rates [14,16,24]. No relationship between the extent of hepatic resection and prognosis has been established yet [5,27]. Micrometastases around the macroscopic tu- mour are more frequent in GCLM than in LM from co- lorectal cancer thus suggesting the need for wider surgi- cal resection margins. Approximately two thirds to three quarters of patients develop liver recurrence [15,16]. Such a high recurrence rate within two years after hepatic resection suggests the presence of intrahepatic microme- tastases around LM at the time of surgery [27]. These poor results motivate the search for the best candidates for liver resection. A generous surgical margin may not be essential for R0 of LM, although positive surgical margins should be avoided. Recurrent tumours are more frequently distri- buted in both lobes than in the resected lobe suggesting that liver recurrence is more probably derived from mul- tiple metastatic foci from the primary disease than from intrahepatic remetastases of the liver lesion. There is no apparent value of surgery if residual disease remains, whether it is involvement of resection margins, other distant LM, or peritoneal carcinosis [28]. The time of GCLM resection represents another con- troversial issue. Synchronous disease is a known prog- nostic factor representative of aggressive biology. Some authors report poor results in terms of survival after sur- gery for synchronous metastases [14,26,29]. The syn- chronous character of GCLM is considered the only in- dependent factor of poor prognosis [25]. Recently, a five- year survival rate of 60% after surgical resection of syn- chronous GCLM is reported [30]. The extent of hepatic involvement and macroscopic peritoneal dissemination detected at surgical exploration represent the factors in- fluencing upon survival in patients with synchronous GCLM [30]. According to other authors, the size of me- tastases smaller than 5 cm relates to better survival [8, 29,31]. In the present study, a fibrous pseudocapsule around GCLM and the status of lymph node involvement are closely associated with patient’s survival at univariate analysis only. In 16 patients, there is a thick fibrous pseudocapsule with more than 8 layers of collagen bun- dles between the tumour and hepatic parenchyma. Pseu- docapsule formation should be considered as a protective immunoinflammatory reaction against the metastastic nodule reflecting the host defence reaction that creates a wall to stop tumour diffusion [14]. The presence of a fibrous pseudocapsule may be a valuable prognostic fac- tor reflecting the aforementioned reaction in GCLM pa- tients [29]. Faithful lymph node dissection up to the second echelon contributes to a better chance of cure for the patients with lymph node metastases and GCLM. There is an insignificant difference concerning the depth of invasion or lymph node GCLM between the surviving and non-surviving patients [14,22]. D2 or greater exten- ded lymphadenectomy is conventionally performed for advanced GC with good outcomes and relatively low- frequent lymph node recurrences [32,33]. Patients with positive paraaortic GCLM present with poor survival rates following liver resection [15]. Long-term survival in patients with both paraaortic lymph nodes and LM is a seldom finding [32]. Therefore, we consider that liver resection for GCLM is not indicated in patients with paraaortic lymph node metastasis. Our study fails to establish any significant association between survival and the rest prognostic factors related to primary GC, LM and surgery in the patients who have undergone hepatectomy for GCLM. Literature data about the clinicopathological features in GCLM patients are contradictory and difficult to interpret. Tumour localiza- tion in primary GC is a marginally predictive negative factor for the overall patients’ survival [34]. GC tumour size larger than 6 cm is associated with a poor prognosis after GCLM resection [11,23]. A disease-free interval between gastric and hepatic resection of >1 year is a Copyright © 2013 SciRes. SS  D. V. KOSTOV ET AL. 398 significant survival advantage as a result of the slow- growing nature of this tumour [29]. The aggregation of lymphocytes enclosing the metastatic tumour is a factor of good prognosis [29]. The unilobular liver nodule dis- tribution is a good predictor as most unilobular tumours are solitary [26]. Unilobar LM distribution is also associ- ated with a better survival after resection, especially, if their size is less than 4 cm [16]. Primary tumour differ- entiation grade is a negative predictor of outcome [6]. Bilobar tumours result in a worse patient’s outcome than unilobar ones [34]. According to other authors, however, these prognostic factors are not signifycant and there are some controversies in this respect [14,15,22]. No primary tumour-related or LM-related factor of prognostic value has been identified [35,36]. Concerning the extrahepatic disease associated to GCLM, this situation constitutes a poor prognosis with significantly lower median survival compared to cases in which resection of isolated GCLM is performed (7 vs. 23 months, respectively) [7,37]. The comparison of the results from different studies demonstrates that one-year survival rates range from 36% to 90%, three-year ones-from 22% to 57%, and five-year ones from 0% to 43% [15,34,37]. The median survival rate ranges from 8 to 25 months [12,14,18,29]. In individual studies, the overall one-, three-, and five- year survival rates after hepatic resection are 80%, 60%, and 60%, respectively, with median survival time rang- ing from 31 to 34 months [15,16]. In our study, the me- dian survival time is 16 months and the actuarial overall one-, three-, and five-year survival rates after hepatic resection are 67.8%, 39.2%, and 28.5%, respectively. These parameters seem acceptable as they have been achieved by means of a strict selection of the patients suitable for hepatectomy. Radio-frequency ablation is another important strategy for GCLM treatment. This technique can be employed either as alternative of, or in association to hepatectomy. It could be the approach of choice in case of poor general conditions contraindicating surgery. Currently, systemic chemotherapy is considered the standard of care, how- ever, long-term survival using chemotherapy alone is rare. Although recent advancements with newer regimens for this disease lead to modest improvements, long-term survival beyond five years is seldom [38]. A five-year survival rate of 75% in a subgroup of eight patients sub- mitted to radical surgery followed by hepatic-artery infu- sion chemotherapy has been reported [28]. Recurrent disease develops, most commonly, in the liver after he- patic resection in approximately 70% of the patients [6,15]. Recurrence rates after liver resection in GCLM could potentially decrease with the addition of these new regimens such as adjuvant therapies [12]. Recently, postoperative adjuvant chemotherapy advances in GCLM as arterial chemotherapy statistically significantly pre- vents hepatic recurrence [12,15,39,40]. Liver surgery rather than hepatic artery infusion could significantly prolong the survival period for patients with synchronous GCLM [28]. GCLM management is not consensual. If systemic chemotherapy is an irrefutable indication for GCLM associated with other extrahepatic metastases, the attitude for isolated LM is still widely debated. Although the level of evidence is low, the results of surgery in this situation in terms of five-year survival and median sur- vival are superior to those obtained with systemic che- motherapy alone. This outcome is even more evident when surgery is indicated for single LM of less than 5 cm in size. 5. Conclusion Nowadays the best treatment option for GCLM is inten- sively discussed. Surgery is a good indication in properly selected patients with an absent serosal invasion of pri- mary tumour, single LM and attainment of R0 liver re- section. However, this is not the most common condition. For the remaining majority of GCLM, there is no suffi- cient scientific evidence to indicate other therapeutic modalities such as radio-frequency ablation, hepatic-ar- tery infusion chemotherapy and palliative gastrectomy. In this case, systemic chemotherapy remains the best hope for a longer survival and an improved quality of life. REFERENCES [1] A. Jemal, R. Siegel, E. Ward, Y. Hao, J. Xu and M. J. Thun, “Cancer Statistics 2009,” CA: A Cancer Journal for Clinicians, Vol. 59, No. 4, 2009, pp. 225-249. doi:10.3322/caac.20006 [2] J. L. Dikken, C. J. van de Velde, D. G. Coit, M. A. Shah, M. Verheij and A. Cats, “Treatment of Resectable Gastric Cancer,” Therapeutic Advances in Gastroenterology, Vol. 5, No. 1, 2012, pp. 49-69. [3] A. Shin, J. Kim and S. Park, “Gastric Cancer Epidemio- logy in Korea,” Journal of Gastric Cancer, Vol. 11, No. 3, 2011, pp. 135-140. doi:10.5230/jgc.2011.11.3.135 [4] B. Schlansky and A. Sonnenberg, “Epidemiology of Non- cardia Gastric Adenocarcinoma in the United States,” American Journal of Gastroenterology, Vol. 106, No. 11, 2011, pp. 1978-1985. doi:10.1038/ajg.2011.213 [5] E. Linhares, M. Monteiro, R. Kesley, C. E. Santos, O. S. Filho and J. H. Simoes, “Major Hepatectomy for Isolated Metastases from Gastric Adenocarcinoma,” HPB (Oxford), Vol. 5, No. 4, 2003, pp. 235-237. [6] K Shirabe, S Wakiyama, T Gion, M Watanabe, M Miya- zaki, K Yoshinaga, M. Tokunaga and T. Nagaie, “Hepatic Resection for the Treatment of Liver Metastases in Gas- tric Carcinoma: Review of the Literature,” HPB (Oxford), Vol. 8, No. 2, 2006, pp. 89-92. doi:10.1080/13651820500472168 Copyright © 2013 SciRes. SS  D. V. KOSTOV ET AL. 399 [7] Thelen, S. Jonas, C. Benckert, E. Lopez-Hänninen, U. Neumann, B. Rudolph, G. Schumacher and P. Neuhaus, “Liver Resection for Metastatic Gastric Cancer,” Euro- pean Journal of Surgical Oncology, Vol. 34, No. 12, 2008, pp. 1328-1334. [8] Y. Sakamoto, S. Ohyama, J. Yamamoto, K. Yamada, M. Seki, K. Ohta, N. Kokudo, T. Yamaguchi, T. Muto and M. Makuuchi, “Surgical Resection of Liver Metastases of Gastric Cancer: An Analysis of a 17-Year Experience with 22 Patients,” Surgery, Vol. 133, No. 5, 2003, pp. 507- 511. doi:10.1067/msy.2003.147 [9] H. Jerraya, A. Saidani, M. Khalfallah, I. Bouasker, R. Nouira and C. Dziri, “Management of Liver Metastases from Gastric Carcinoma: Where Is the Evidence?” La Tunisie Médicale, Vol. 91, No. 1, 2013, pp. 1-5. [10] J. A. Ajani, “Evolving Chemotherapy for Advanced Gas- tric Cancer,” On cologi st, Vol. 10, Suppl. 3, 2005, pp. S49- S58. doi:10.1634/theoncologist.10-90003-49 [11] Y. N. Wang, K. T. Shen, J. Q. Ling, X. D. Gao, Y. Y. Hou, X. F. Wang, J. Qin, Y. H. Sun and X. Y. Qin, “Pro- gnostic Analysis of Combined Curative Resection of the Stomach and Liver Lesions in 30 Gastric Cancer Patients with Synchronous Liver Metastases,” BMC Surgery, Vol. 12, No. 1, 2012, p. 20. [12] S. P. Kerkar, C. D. Kemp and I. Avital, “Liver Resections in Metastatic Gastric Cancer,” HPB (Oxford), Vol. 12, No. 9, 2010, pp. 589-596. doi:10.1111/j.1477-2574.2010.00224.x [13] K. Kumagai, K. Shimizu, N. Yokoyama, S. Aida, T. Ta- naka and K. Yamagata, “Gastrointestinal Cancer Metas- tasis and Lymphatic Advancement,” Surgery Today, Vol. 40, 2010, pp. 301-306. doi:10.1007/s00595-009-4142-2 [14] K. Okano, T. Maeba, K. Ishimura, Y. Karasawa, F. Goda, H. Wakabayashi, H. Usuki and H. Maeta, “Hepatic Re- section for Metastatic Tumors from Gastric Cancer,” An- nals of Surgery, Vol. 235, No. 1, 2002, pp. 86-91. doi:10.1097/00000658-200201000-00011 [15] R. Koga, J. Yamamoto, S. Ohyama, A. Saiura, M. Seki, Y. Seto and T. Yamaguchi, “Liver Resection for Metastatic Gastric Cancer: Experience with 42 Patients Including Eight Long-Term Survivors,” Japanese Journal of Clini- cal Oncology, Vol. 37, No. 11, 2007, pp. 836-842. doi:10.1093/jjco/hym113 [16] Y. Sakamoto, T. Sano, K. Shimada, M. Esaki, M. Saka, T. Fukagawa, H. Usuki and H. Maeta, “Favorable Indica- tions for Hepatectomy in Patients with Liver Metastases from Gastric Cancer,” Journal of Surgical Oncology, Vol. 95, No. 7, 2007, pp. 534-539. doi:10.1002/jso.20739 [17] F. Romano, M. Garancini, F. Uggeri, L. Degrate, L. Nes- poli, L. Gianotti, A. Nespoli and F. Uggeri, “Surgical Treat- ment of Liver Metastases of Gastric Cancer: State of the Art,” World Journal of Surgical Oncology, Vol. 10, 2012, p. 157. doi:10.1186/1477-7819-10-157 [18] T. Ochiai, M. Sasako, S. Mizuno, T. Kinoshita, T. Taka- yama, T. Kosuge, S. Yamazaki and K. Maruyama, “Hepa- tic Resection for Metastatic Tumours from Gastric Cancer: Analysis of Prognostic Factors,” British Journal of Sur- gery, Vol. 81, No. 8, 1994, pp. 1175-1178. doi:10.1002/bjs.1800810832 [19] Y. Kodera, H. Nakanishi, S. Ito, Y. Yamamura, Y. Kane- mitsu, Y. Shimizu, T. Hirai, K. Yasui, T. Kato and M. Tatematsu, “Quantitative Detection of Disseminated Can- cer Cells in the Greater Omentum of Gastric Carcinoma Patients with Real-time RT-PCR: A Comparison with Peritoneal Lavage Cytology,” Gastric Cancer, Vol. 5, No. 2, 2002, pp. 69-76. doi:10.1007/s101200200012 [20] Z. Morise, A. Sugioka, S. Hoshimoto, T. Kato, M. Ikeda, I. Uyama, A. Horiguchi and S. Miyakawa, “The Role of Hepatectomy for Patients with Liver Metastases of Gas- tric Cancer,” Hepatogastroenterology, Vol. 55, No. 85, 2008, pp. 1238-1241. [21] D. Elias, A. Cavalcanti de Albuquerque, P. Eggenspieler, B. Plaud, M. Ducreux, M. Spielmann, C. Theodore, S. Bonvalot and P. Lasser, “Resection of Liver Metastases from a Noncolorectal Primary: Indications and Results Based on 147 Monocentric Patients,” Journal of the Ame- rican College of Surgeons, Vol. 187, No. 5, 1998, pp. 487-493. doi:10.1016/S1072-7515(98)00225-7 [22] M. Miyazaki, H. Itoh, K. Nakagawa, S. Ambiru, H. Shi- mizu, A. Togawa, M. Shiobara, M. Ohtsuka, K. Sasada, Y. Shimizu, S. Yoshioka, N. Nakajima, T. Suwa and F. Kimura, “Hepatic Resection of Liver Metastases from Gastric Carcinoma,” American Journal of Gastro-En- terology, Vol. 92, No. 3, 1997, pp. 490-493. [23] H. Tsujimoto, T. Ichikura, S. Ono, H. Sugasawa, S. Hira- ki, N. Sakamoto, Y. Yaguchi, K. Hatsuse, J. Yamamoto and K. Hase, “Outcomes for Patients Following Hepatic Resection of Metastatic Tumors from Gastric Cancer,” Hepatology International, Vol. 4, No. 1, 2010, pp. 406- 413. doi:10.1007/s12072-009-9161-y [24] S. H. Cheon, S. Y. Rha, H. C. Jeung, C. K. Im, S. H. Kim, H. R. Kim, J. B. Ahn, J. K. Roh, S. H. Noh and H. C. Chung, “Survival Benefit of Combined Curative Resec- tion of the Stomach (D2 Resection) and Liver in Gastric Cancer Patients with Liver Metastases,” Annals of On- cology, Vol. 19, No. 6, 2008, pp. 1146-1153. doi:10.1093/annonc/mdn026 [25] A. Saiura, N. Umekita, S. Inoue, T. Maeshiro, S. Miya- moto, Y. Matsui, M. Asakage and M. Kitamura, “Clini- copathological Features and Outcome of Hepatic Resec- tion for Liver Metastases from Gastric Cancer,” Hepato- gastroenterology, Vol. 49, No. 46, 2002, pp. 1062-1065. [26] S. Ambiru, M. Miyazaki, H. Ito, K. Nakagawa, H. Shi- mizu, H. Yoshidome, Y. Shimizu and N. Nakajima, “Be- nefits and Limits of Hepatic Resection for Gastric Metas- tases,” American Journal of Surgery, Vol. 181, No. 3, 2001, pp. 279-283. doi:10.1016/S0002-9610(01)00567-0 [27] H. R. Roh, K. S. Suh, H. J. Lee, H. K. Yang, K. J. Choe and K. U. Lee, “Outcome of Hepatic Resection for Meta- static Gastric Cancer,” The American Surgeon, Vol. 71, No. 2, 2005, pp. 95-99. [28] K. Ueda, M. Iwahashi, M. Nakamori, M. Nakamura, T. Naka, K. Ishida, T. Ojima and H. Yamaue, “Analysis of the Prognostic Factors and Evaluation of Surgical Treat- ment for Synchronous Liver Metastases from Gastric Cancer,” Langenbeck’s Archives of Surgery, Vol. 394, No. 4, 2009, pp. 647-653. Copyright © 2013 SciRes. SS  D. V. KOSTOV ET AL. Copyright © 2013 SciRes. SS 400 doi:10.1007/s00423-008-0311-9 [29] J. Zacherl, M. Zacherl, C. Scheuba, R. Steininger, E. Wenzl, F. Mühlbacher, R. Jakesz and F. Längle, “Analysis of Hepatic Resection of Metastases Originating from Gastric Adenocarcinoma,” Journal of Gastrointestinal Surgery, Vol. 6, No. 5, 2002, pp. 682-689. doi:10.1016/S1091-255X(01)00075-0 [30] T. Nomura, Y. Kamio, N. Takasu, T. Moriya, A. Take- shita, M. Mizutani, O. Hachiya, I. Hirai and W. Kimura, “Intrahepatic Micrometastases Around Liver Metastases from Gastric Cancer,” Journal of Hepato-Biliary-Pan- creatic Surgery, Vol. 16, No. 4, 2009, pp. 493-501. doi:10.1007/s00534-009-0081-y [31] K. Fujii, S. Fujioka, K. Kato, Y. Machici, Y. Kutsuna, A. Ishikawa, J. Takamizawa, K. Ko, K. Yoshida and Y. Ni- mura, “Resection of Liver Metastases from Gastric Ade- nocarcinoma,” Hepatogastroenterology, Vol. 48, No. 38, 2001, pp. 368-371. [32] M. Garancini, F. Uggeri, L. Degrate, L. Nespoli, L. Gia- notti, A. Nespoli, F. Uggeri and F. Romano, “Surgical Treatment of Liver Metastases of Gastric Cancer: Is Local Treatment in a Systemic Disease Worthwhile?” HPB (Oxford), Vol. 14, No. 3, 2012, pp. 209-215. [33] Y. Maehara, S. Hasuda, T. Koga, E. Tokunaga, Y. Kakeji and K. Sugimachi, “Postoperative Outcome and Sites of Recurrence in Patients Following Curative Resection of Gastric Cancer,” British Journal of Surgery, Vol. 87, No. 3, 2000, pp. 353-357. doi:10.1046/j.1365-2168.2000.01358.x [34] C. H. Yoo, S. H. Noh, D. W. Shin, S. H. Choi and J. S. Min, “Recurrence Following Curative Resection for Gas- tric Carcinoma,” British Journal of Surgery, Vol. 87, No. 2, 2000, pp. 236-242. doi:10.1046/j.1365-2168.2000.01360.x [35] S. H. Cheon, S. Y. Rha, H. C. Jeung, C. K. Im, S. H. Kim, H. R. Kim, J. B. Ahn, J. K. Roh, S. H. Noh and H. C. Chung, “Survival Benefit of Combined Curative Resec- tion of the Stomach and Liver in Gastric Cancer Patients with Liver Metastases,” Annals of Oncology, Vol. 19, No. 6, 2008, pp. 1153-1158. [36] H. Makino, C. Kunisaki, Y. Izumisawa, M. Tokuhisa, T. Oshima, Y. Nagano, S. Fujii, J. Kimura, R. Takagawa, T. Kosaka, H. A. Ono, H. Akiyama, K. Tanaka and I. Endo, “Indication for Hepatic Resection in the Treatment of Liver Metastasis from Gastric Cancer,” Anticancer Re- search, Vol. 30, No. 6, 2010, pp. 2367-2376. [37] R. Adam, L. Chiche, T. Aloia, D. Elias, R. Salmon, M. Rivoire, D. Jaeck, J. Saric, Y. P. Le Treut, J. Belghiti, G. Mantion and G. Mentha; Association Française de Chi- rurgie, “Hepatic Resection for Noncolorectal Nonendo- crine Liver Metastases. Analysis of 1452 Patients and Development of a Prognostic Model,” Annals of Surgery, Vol. 244, No. 4, 2006, pp. 524-535. doi:10.1097/01.sla.0000239036.46827.5f [38] T. Miyazawa, K. Ebe, N. Koide and N. Fujita, “Complete Response of Liver Metastasis of Gastric Cancer Treated by s-1 Chemoradiotherapy: a Case Report,” Case Reports in Oncological Medicine, 2012; 2012:368428. doi: doi:10.1155/2012/368428 [39] D. L. Page, I. D. Flemming, A. Fritz, C. M. Balch, D. G. Haller and M. Morrow, “American Joint Committee on Cancer, “Cancer Staging Manual,” 6th Edition, Springer, New York, 2002. [40] T. J. Vogl, T. Gruber-Rouh, K. Eichler, N. E. Nour-Eldin, J. Trojan, S. Zangos and N. N. Naguib, “Repetitive Tran- sarterial Chemoembolization (TACE) of Liver Metastases from Gastric Cancer: Local Control and Survival Re- sults,” European Journal of Radiology, Vol. 82, No. 2, 2013, pp. 163-258.
|