Paper Menu >>
Journal Menu >>
 p f J ournal o f B i doi:10.4236/jb n Copyright © 2 Heati n Parti c Badia Fasla Macromolecul a Email: redaben m Received Nove m ABSTRA C This paper d e These sphere s non. The sing l p roportional t reach a finite is treated as a f erent space d coverage of m ing the borde r highlight the i medical team. Keywords: H y 1. Introdu c Thermal ther a variety of t u heated papyr u tions [1]. No w one of the ma j The method i soft matter k n tion absorbin g b in and mela n therapy aims as an alternat i the advantag e rapid recover y involving a c quire mathe m of the system Energy deliv e meters like l a Thermal effe c of tissues, th e as well as on sue damage c a i omaterials a n n b.2011.21007 P 2 011 SciRes. ng of B c les: E f , Assia Rac h a r Research Lab o m ouna@yahoo. m ber 4th, 2010; C T e als with hyp e s are heated w l e sphere pro b t o the sphere s range when t r a convolution p d istribution f u m edium heatin g r s of the treat e i mpacts of the y per Thermal T c tion a py has been u u mors. Many u s to treat br e w adays, laser j or tools of t r e s based on th e n owing that bi o g agents such n in with diffe r at eliminating i ve to convent i e s of being no n y [3-6]. It is ombination o f m atical models behavior at d e red by radiati a ser power, be a c ts depend o n e ir optical an d biological de n a n be evaluate n d Nanobiotec h P ublished Onlin B iolo gi f fects o h ida Senoud i o ratory, Depart m com revised Novem b e r thermal the r w ith a laser be a b lem gives a s u s urface and in v r aveling into t h p roduct of the u nctions of na n g while a Ga u e d region. Lo r obtained res u T herapy, Gol d u sed for a lon g years ago, E e ast cancer a n thermal thera p e ating a variet y e interaction o o logical tissu e as water, pro r ent absorptio n g cancerous tis ional surgical n invasive, si m however a c o f distinct phe n to improve o u d ifferent stage s on to tissue d e a m diameter a n the absorpti o d thermo- p hy s n aturation. T h d knowing th e h nolo gy , 201 1 e January 2011 i cal Ti o f Par t i , Abdelhak B m ent of Physics b er 23rd, 2010; a r apy of tumo r a m in the nea r u rface temper a v erse diffusivi t h e s urroundin g sphere densit y n o particles a u ssian distribu t r entzian distri b u lts in develop i d Nano Sphere s g time to treat E gyptians us e n d others infe p y is becomi n y of diseases [ 2 o f radiation wi t e s contain radi teins, hemogl o n spectra. Las sues by heati n treatments wi t m ple allowing o mplex proce n omena that r u r understandi n s of the proce s e pends on pa r a nd waveleng t o n cross secti o s ical paramete h e extend of t i e time and spa c 1 , 2, 49-54 (http://www.Sc i ssues b t icle S B oussaid, M , Faculty of Sci e a ccepted Dece m r s in biologic a r infrared ran g a ture rise foll o t y of the surr o g medium wit h y distribution f a re con s idere d t ion predicts a b ution was als i ng strategies f s , Laser, Heat a e d c- n g 2 ]. t h a- o - er n g t h a ss e- n g s s. a- t h. o n rs i s- c e temper a p heno m leadin g fast up o The dispers damag e needs t of hea t transfe r tions o from h e resona n the ne a genera t larger t ver, bi o good t r sibility tumors for sol v spheri c exami n sphere i RP.org/journal / by Go l ize an d M ustapha Be n e nces Universit y m ber 1st, 2010. a l tissues usin g g e based on s u o wing a unive r o unding mediu m h a certain tim f unction and t d . A uniform s a n important d o considered f f or hyper ther m Transfe r a ture depend e m enon and e n g to cell’s deat h o n further hea t present pape r ion of gold n e s to healthy o design strat e t ing sources a r problem in s p o f application s e ated gold na n n ce phenome n a r infrared and t es photon c a t han standard p o logical tissu e r ansparency in to reach dee p deep inside t h v ing the time c al coordinate n ed first to obt a surface and i m / jbnb) l d Na n d Dist r n mouna, Re d y Abou Bekr B e g dispersions u rface plasmo n r sal form with m . The tempe r e delay. The m t he particle te m s phere distrib u d rop of tempe r f or compariso n m al therapy i n e nce. For e x n dothelial da m h within seco n t ing. r deals with n ano spheres. tissues surro u e gies that incl u a nd a precise p ace and time s . [7-9] Ther m n o spheres is d n on where gol release heat [ 1 a pture several p hoto therma l e s are charac t the near infr a p regions via f h e body. A m and space he s. The singl e a in the rate of m mediate sur r n o r ibuti o d a Benmou n e lkaid, Tlemcen , of gold nano n resonance p a characteris t r ature front is f m any particles p m perature pro f u tion provide s r ature when a p n . An effort is n a joint effort x ample, vaso d m ages appear n ds. Damages s laser therapy In order to m u nding the ta r u de a good dis t resolution of under practic a m al ablation r d ue to surface d absorbs rad 1 0-13]. This r e orders of m l dyes [14,15] . t erized by a r a red providing f iber optics fo r m odel is prese n at transfer pr o e particle pr o temperature r i r oundings. Th e JBNB 49 o n n a , Algeria spheres. henome- t ic time τ f ound to p roblem f ile. Dif- s a good p proach- made to with the d ilatation at 45°C s peed up using a m inimize r get, one t ribution the heat a l condi- r esulting plasmon iation in e sonance agnitude . Moreo- elatively the pos- r remote n ted here o blem in o blem is i se at the e case of  Heating of Biological Tissues by Gold Nano Particles: Effects of Particle Size and Distribution Copyright © 2011 SciRes. JBNB 50 a large number of nano spheres is then examined based on selected particle distributions. 2. The Single Particle Problem Conducting electrons of gold nano spheres exhibit a re- sonance with the incident electromagnetic field and os- cillate producing heat. Resonance at a specific frequency of the applied field is called plasmon resonance. Optimi- zation of process efficiency and enhancement of thermal conversion require an adequate choice of the laser wave- length consistent with the particle shape and size.[13,14] In this section heating by a single gold sphere of radius a is considered. The sphere is exposed to a laser beam of wavelength , intensity I0 (W/m2) and assumed to gener- ate a power proportional to the absorption cross section abs, Pabs=I0 abs (W). This cross section is different from the geometric expression (i.e ., 4a2) and can be eva- luated depending on dielectric properties of the medium. When an electromagnetic radiation of wavelength im- pinges into the sphere, its intensity splits into absorption and scattering. The absorption power which is more re- levant for our purpose depends on the cross section abs. According to the electro-dynamic theory of dielectric media, absorption cross section is related to the polariza- bility of the sphere [16, 17] by abs = [( ½] Im , and the polarizability can be deduced from the Clau- sius-Mossotti [18,19] relationship as a3( – m)/( + 2 m) where is the sphere dielectric permittivity and m that of the biological medium in which it is em- bedded (i.e., the physiological liquid for example). The permittivity is a complex quantity with real and imagi- nary parts that can be evaluated in terms of gold parame- ters and surrounding medium using Lorentz-Drude mod- els. [20,21] It is not our attention to dwell more on this question which is nevertheless important to complete our understanding of the problem. A more detailed investiga- tion along these lines is under progress and we hope to report on some results in the near future essentially for confronting the conversion efficiencies with those of other metals such as iron, nickel, silver or hybrid spheres containing polymers [22]. The problem now consists of solving the following sets of equations 12 2 1 gg abs g g TT P r trr rfor r < a (1) 12 2 10 TT r trr r for r a (2) Subscript g refers to gold while T is the temperature in the surrounding medium; λ’s and ’s are thermal diffu- sivities (m2/s) and conductivities (W·m-1·K-1), respec- tively. The focus is made on the resolution of Equation (2) since T(r,t) is the relevant temperature. In addition, the solution gives access to Ts at the sphere surface which is sufficient to characterize the heating source. The solution must satisfy the following initial and boundary conditions: Initial condition 0 (, 0)Trt T (3) Boundary conditions 0 (,) Trt T (4) 2 4 abs ra P T ra (5) T0 is the initial temperature prior to laser exposure and remains that of healthy tissue away from the infected region. Equation 5 expresses the heat flux from the sphere. To solve equation 2 subject to the indicated initial and boundary conditions, we first make a change of variable from T(r,t) to u(r,t) = r[T(r,t) – T0] then Laplace transform the resulting equation. Resolution of the equation in u(r,t) is straight forward. The final step is to go back to time domain and noting that the inverse Laplace transform of exp() ()akssas (k > 0) is tabulated in [23] as 2 2exp( )(2) erfck takaterfcatk t. The result is 10 1 1max 11 1 ,, 2 exp 2 TrtTrt T ra erfc at a Trra ra terfc t aat (6) where 1max 4 abs P Ta; 2 a; p c (7) and cP represent the sphere density and specific heat, respectively; erfc is the complementary error function 2 21 t X erfcXdt eerfX (8) The subscript 1 is used to distinguish single particle from many particle temperature fields to be discussed in the next section; τ is the characteristic time of sphere photo heating. The procedure used to solve this problem is similar to that reported in [24] where the coupled equa- tions for r < a and r > a were solved. Note that Equation (6) is similar to the result of Kablinski et al. [10]. The characteristic time is proportional to the sphere surface and inverse thermal diffusivity of the surrounding medium meaning that it lasts longer to heat bigger spheres embedded in poorly diffusing media. Moreover, T1max = Pabs/4 a is proportional to Pabs and inversely propor- tional to sphere’s radius and tissue’s thermal conductivity. 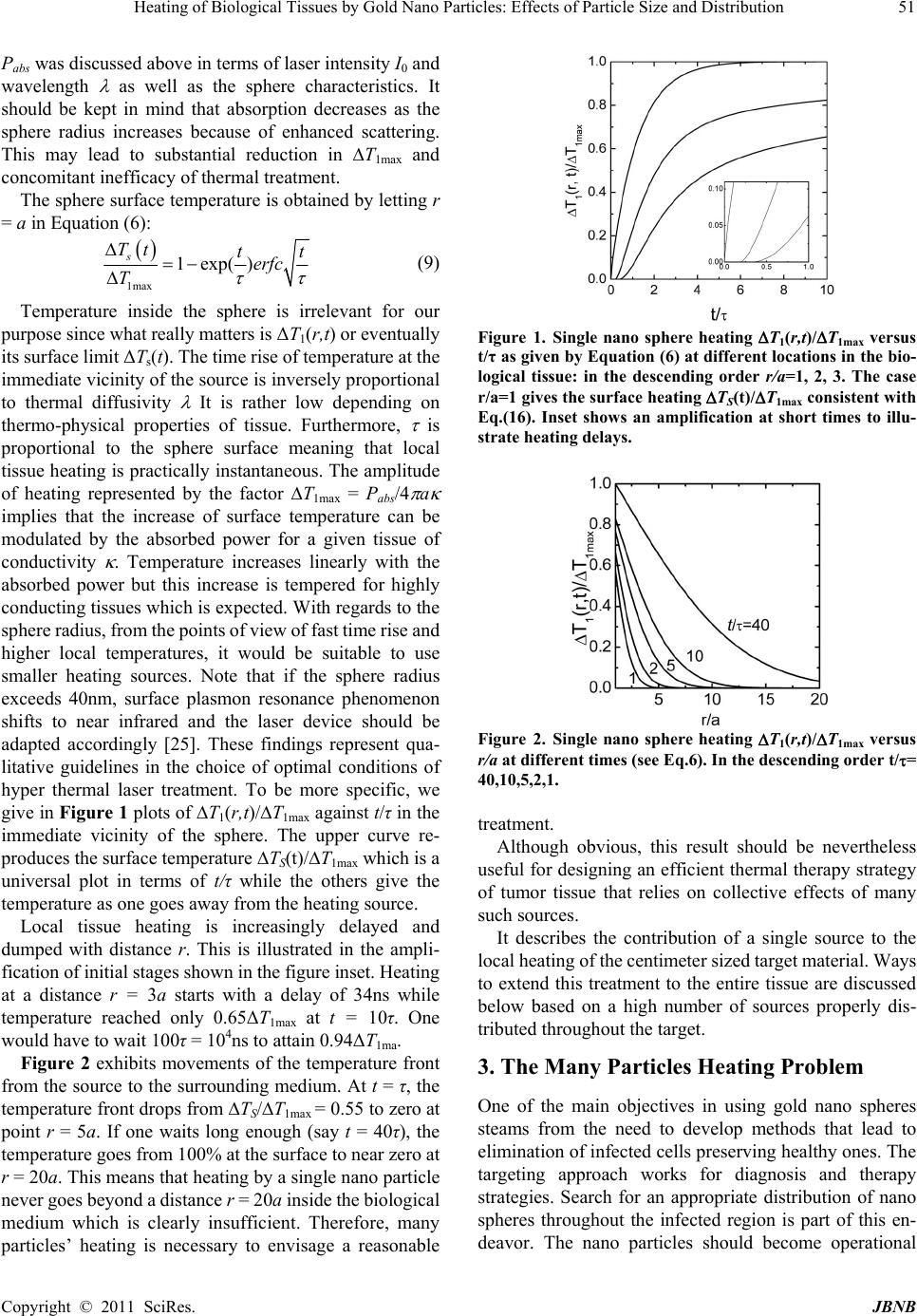 Heating of Biological Tissues by Gold Nano Particles: Effects of Particle Size and Distribution Copyright © 2011 SciRes. JBNB 51 Pabs was discussed above in terms of laser intensity I0 and wavelength as well as the sphere characteristics. It should be kept in mind that absorption decreases as the sphere radius increases because of enhanced scattering. This may lead to substantial reduction in T1max and concomitant inefficacy of thermal treatment. The sphere surface temperature is obtained by letting r = a in Equation (6): 1max 1exp() s Tt tt erfc T (9) Temperature inside the sphere is irrelevant for our purpose since what really matters is ΔT1(r,t) or eventually its surface limit ΔTs(t). The time rise of temperature at the immediate vicinity of the source is inversely proportional to thermal diffusivity It is rather low depending on thermo-physical properties of tissue. Furthermore, is proportional to the sphere surface meaning that local tissue heating is practically instantaneous. The amplitude of heating represented by the factor T1max = Pabs/4 a implies that the increase of surface temperature can be modulated by the absorbed power for a given tissue of conductivity . Temperature increases linearly with the absorbed power but this increase is tempered for highly conducting tissues which is expected. With regards to the sphere radius, from the points of view of fast time rise and higher local temperatures, it would be suitable to use smaller heating sources. Note that if the sphere radius exceeds 40nm, surface plasmon resonance phenomenon shifts to near infrared and the laser device should be adapted accordingly [25]. These findings represent qua- litative guidelines in the choice of optimal conditions of hyper thermal laser treatment. To be more specific, we give in Figure 1 plots of T1(r,t)/T1max against t/τ in the immediate vicinity of the sphere. The upper curve re- produces the surface temperature TS(t)/T1max which is a universal plot in terms of t/τ while the others give the temperature as one goes away from the heating source. Local tissue heating is increasingly delayed and dumped with distance r. This is illustrated in the ampli- fication of initial stages shown in the figure inset. Heating at a distance r = 3a starts with a delay of 34ns while temperature reached only 0.65ΔT1max at t = 10τ. One would have to wait 100τ = 104ns to attain 0.94ΔT1ma. Figure 2 exhibits movements of the temperature front from the source to the surrounding medium. At t = τ, the temperature front drops from TS/T1max = 0.55 to zero at point r = 5a. If one waits long enough (say t = 40τ), the temperature goes from 100% at the surface to near zero at r = 20a. This means that heating by a single nano particle never goes beyond a distance r = 20a inside the biological medium which is clearly insufficient. Therefore, many particles’ heating is necessary to envisage a reasonable Figure 1. Single nano sphere heating T1(r,t)/T1max versus t/τ as given by Equation (6) at different locations in the bio- logical tissue: in the descending order r/a=1, 2, 3. The case r/a=1 gives the surface heating TS(t)/T1max consistent with Eq.(16). Inset shows an amplification at short times to illu- strate heating delays. Figure 2. Single nano sphere heating T1(r,t)/T1max versus r/a at different times (see Eq.6). In the descending order t/= 40,10,5,2,1. treatment. Although obvious, this result should be nevertheless useful for designing an efficient thermal therapy strategy of tumor tissue that relies on collective effects of many such sources. It describes the contribution of a single source to the local heating of the centimeter sized target material. Ways to extend this treatment to the entire tissue are discussed below based on a high number of sources properly dis- tributed throughout the target. 3. The Many Particles Heating Problem One of the main objectives in using gold nano spheres steams from the need to develop methods that lead to elimination of infected cells preserving healthy ones. The targeting approach works for diagnosis and therapy strategies. Search for an appropriate distribution of nano spheres throughout the infected region is part of this en- deavor. The nano particles should become operational 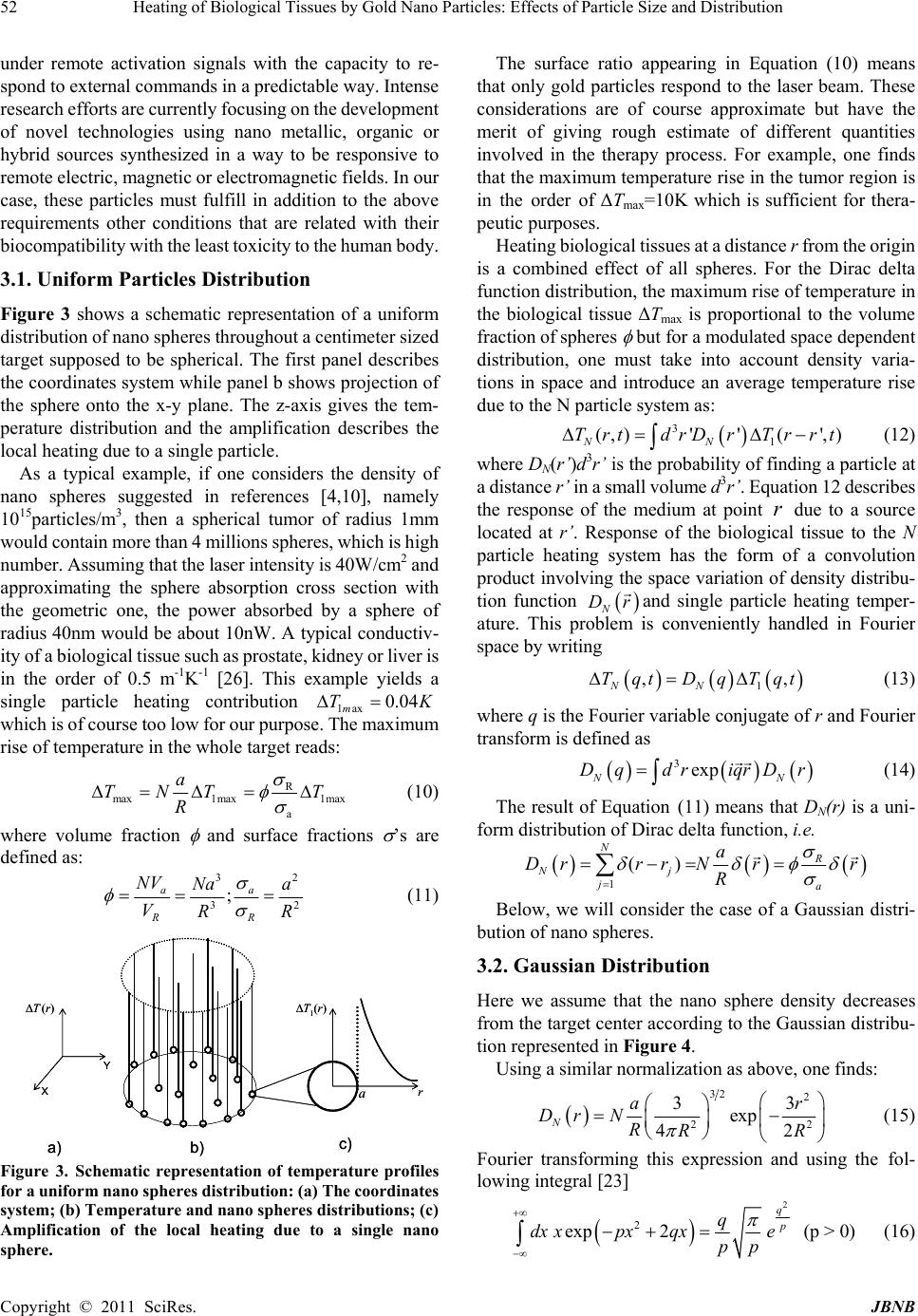 Heating of Biological Tissues by Gold Nano Particles: Effects of Particle Size and Distribution Copyright © 2011 SciRes. JBNB 52 under remote activation signals with the capacity to re- spond to external commands in a predictable way. Intense research efforts are currently focusing on the development of novel technologies using nano metallic, organic or hybrid sources synthesized in a way to be responsive to remote electric, magnetic or electromagnetic fields. In our case, these particles must fulfill in addition to the above requirements other conditions that are related with their biocompatibility with the least toxicity to the human body. 3.1. Uniform Particles Distribution Figure 3 shows a schematic representation of a uniform distribution of nano spheres throughout a centimeter sized target supposed to be spherical. The first panel describes the coordinates system while panel b shows projection of the sphere onto the x-y plane. The z-axis gives the tem- perature distribution and the amplification describes the local heating due to a single particle. As a typical example, if one considers the density of nano spheres suggested in references [4,10], namely 1015particles/m3, then a spherical tumor of radius 1mm would contain more than 4 millions spheres, which is high number. Assuming that the laser intensity is 40W/cm2 and approximating the sphere absorption cross section with the geometric one, the power absorbed by a sphere of radius 40nm would be about 10nW. A typical conductiv- ity of a biological tissue such as prostate, kidney or liver is in the order of 0.5 m-1K-1 [26]. This example yields a single particle heating contribution 1ax 0.04 m TK which is of course too low for our purpose. The maximum rise of temperature in the whole target reads: R max 1max1max a a TNT T R (10) where volume fraction and surface fractions ’s are defined as: 32 32 ; aa RR NV Na a VRR (11) Figure 3. Schematic representation of temperature profiles for a uniform nano spheres distribution: (a) The coordinates system; (b) Temperature and nano spheres distributions; (c) Amplification of the local heating due to a single nano sphere. The surface ratio appearing in Equation (10) means that only gold particles respond to the laser beam. These considerations are of course approximate but have the merit of giving rough estimate of different quantities involved in the therapy process. For example, one finds that the maximum temperature rise in the tumor region is in the order of ΔTma x =10K which is sufficient for thera- peutic purposes. Heating biological tissues at a distance r from the origin is a combined effect of all spheres. For the Dirac delta function distribution, the maximum rise of temperature in the biological tissue ΔTmax is proportional to the volume fraction of spheres but for a modulated space dependent distribution, one must take into account density varia- tions in space and introduce an average temperature rise due to the N particle system as: 3 1 (,)'' ( ',) NN TrtdrD rTrrt (12) where DN(r’)d3r’ is the probability of finding a particle at a distance r’ in a small volume d3r’. Equation 12 describes the response of the medium at point r due to a source located at r’. Response of the biological tissue to the N particle heating system has the form of a convolution product involving the space variation of density distribu- tion function N Dr and single particle heating temper- ature. This problem is conveniently handled in Fourier space by writing 1 ,, NN Tqt DqTqt (13) where q is the Fourier variable conjugate of r and Fourier transform is defined as 3exp NN D qdriqrDr (14) The result of Equation (11) means that DN(r) is a uni- form distribution of Dirac delta function, i.e. 1 () N R Nj ja a Drrr Nrr R Below, we will consider the case of a Gaussian distri- bution of nano spheres. 3.2. Gaussian Distribution Here we assume that the nano sphere density decreases from the target center according to the Gaussian distribu- tion represented in Figure 4. Using a similar normalization as above, one finds: 32 2 22 33 exp 42 N ar Dr N RRR (15) Fourier transforming this expression and using the fol- lowing integral [23] 2 2 exp 2 q p q dx xpxqxe pp (p > 0) (16) r a T 1 (r) X Y T(r) a) b)c) r a T 1 (r) X Y T(r) a) b)c)  Heating of Biological Tissues by Gold Nano Particles: Effects of Particle Size and Distribution Copyright © 2011 SciRes. JBNB 53 Figure 4. Nano sphere distribution function DN in terms of r/R where R is the radius of heated tissue. One finds 22 exp 6 N Naq R Dq R (17) Hence, the temperature rise in the presence of N spheres becomes 22 6 1 ,, qR N Na Tqte Tqt R (18) The Gaussian factor exp(–q2R2/6) representing the temperature drop with qR is shown in Figure 5. At qR=1 for example, this drop is quite substantial since. 1 exp 0.02778 6 Another density distribution of nano spheres that can be considered for comparison would be 2 1 4 r R N Na e Dr Rr R which is also represented in Figure 4. Fourier trans- forming yields a Lorentzian distribution in terms of qR 22 1 1 N Na Dq RqR We mention this form because it is often encountered in soft matter physics and Brownian motion of colloidal dispersions [27,28] although its relevance to therapeutic applications could be subject to doubt. In this case, the total rise of temperature would be 1 22 1 ,, 1 N Na Tqt Tqt RqR where the factor (1+q2R2)-1 represents a space modulated drop of temperature reminiscent of a Lorentzian distribu- tion (see Figure 5). It shows that at tumor’s border, the temperature drops by nearly a factor 2 which is expected since the density of nano particles is lower in this region. Throughout these discussions, Fourier transform of Figure 5. Uniform (DN(q) = 1), Gaussian and Lorentzian distribution functions in Fourier space DN(q) versus qR. ΔT1(r,t) was not needed since this function could be ana- lyzed directly in r-space. 4. Discussion and Conclusion Hyper thermal therapy using laser heated nano sized gold spheres is analyzed by solving the heat transfer problem. It is found that the sphere temperature rises with a cha- racteristic time that is proportional to its surface and in- versely proportional to heat diffusivity of the surrounding biological medium. Heat fronts move from the particle to the medium with increasing delays as one goes away from the surface. A simple numerical example shows that heat generated by a single particle is too low to produce any noticeable effect on the surrounding tissue but the combined effects of a large number of particles is suffi- cient for thermal ablation. Models are used for the particle density distribution function D(r) and a mean temperature rise defined as a convolution product of the distribution and the single particle temperature front. The function D(r-r’) is defined as the probability density of finding a particle at point r’ knowing that there is one at point r. For a uniform distribution of spheres, the probability distri- bution would be a sum of Dirac delta functions and the problem reduces to the single particle contribution T1(r,t) within a constant factor. But if the density of spheres is space modulated and decreases as one goes away from the target center due to some specific long range inter particle interactions, then the probability drops substantially. We have examined the case of Gaussian and Lorentzian dis- tributions. In the latter case, the probability distribution decays according to a long 1/r tail damped exponentially as e r R Dr r where is normalization constant. A colloidal dispersion of charged particles is an example of this distribution. In the case of a Gaussian distribution, one has 2 2 32 2 2 2 3 r R Dr e R  Heating of Biological Tissues by Gold Nano Particles: Effects of Particle Size and Distribution Copyright © 2011 SciRes. JBNB 54 an example of which would be spheres with a harmonic potential interaction. For a uniform distribution, temper- ature raised proportionally to T1(q) within a constant factor defined by the volume fraction of spheres times their fractional area. For Lorentzian and Gaussian distri- butions, the temperature was also proportional to T1 modulated by a space dependent factor reminiscent of the drift in the concentration of spheres as the border of the target region was approached. Lorentzian distribution predicted a moderate drop of temperature rise compared to the Gaussian case where a much stronger exponential drop was obtained. It is worthwhile to note that biological systems such as proteins (10-100 nm), cells (10-100 mm) and bacteria (1-10 mm) are characterized by a broad range of sizes. Use of nano spheres of diameter between 10 and 40 nm in hyper thermal therapy seems to be adequate and efficient at the proteins level. It should be kept in mind that foreign particles can react with the host medium selectively de- pending on a variety of conditions related with the nature of particles and biological tissue. Hydrophobic and hy- drophilic particles react differently and can be covered with molecular species present in blood. They can absorb proteins and these effects should be taken into account to define the right distribution and evaluate their stability and efficiency in developing a therapy strategy [29] of tumor tissues. Magnetic nano particles such as Fe3O4 exhibit a magnetization that oscillates up and down under the influence of an external field and induce heating of the surrounding medium. Other elements like cobalt and nickel can also be used combined with polymers. These results should be useful in prescribing efficient treatments of tumors in close collaboration with the medical team. It is hoped that these considerations will trigger interest in collecting precise experimental data to assess the validity of the results and the concomitant pre- dictive models. REFERENCES [1] J. H. Breasted, “Edwin’s Smith Chirurgical Papyrus,” University of Chicago, 1930. [2] L. Goldman, et al., Adv. Biomed. Eng. Med. Phys., Vol. 1, 1968, pp. 317. [3] M. Ferrari, Nat. Rev. Cancer, Vol. 5, pp. 161, 2005. [4] I. R. Hirsh et al., Proc. Nat. Acad. Sci. USA, Vol. 100, pp. 13549, 2003. [5] A.G. Cuenca et al., Am. Cancer Soc., Vol. 107, pp. 459 2006. [6] O. V. Salata, J. Nanobiotech., Vol. 2, pp. 1, 2004. [7] A. Hadj Sahraoui, et al., J. Appl. Phys., Vol. 82, pp. 12, 1997. [8] A. Boudene, S. Khaldi, J. Appl. Polym. Sci. Vol. 89, pp. 481, 2003. [9] X. Coqueret et al., Macromol. Theory Simul., Vol. 9, pp. 725, 2000. [10] P. Keblinski al., J. Appl. Phys., Vol. 100, pp. 054305, 2006. [11] C. M. Pitsillides et al. Biophys. J., Vol. 84, pp. 4023, 2003. [12] L. E. Vlerken, M. M. Amiji, Expert Opin. Drug Deliv., Vol. 3, pp. 205, 2006. [13] X. Huang et al., J. Adv. Res., Vol. 1, pp. 13, 2010. [14] D. Lapotko, Nanomed., Vol. 4, pp. 253, 2009. [15] S. Shenogin et al., J. Appl. Phys., Vol. 95, pp. 8136, 2004. [16] G. Mie, Ann. Phys., Vol. 25, pp. 377, 1908. [17] M. Kerker, “The Scattering of Light and Other Electromag Netic Radiations,” Academic Press, New York, 1969. [18] B.T. Draine et al., Astrophys. J., Vol. 405, pp. 685, 1993. [19] B.T. Draine, “Light Scattering by Non-Spherical Particles: Theory, Measurements and Applications,” Academic Press, San Diego, 2000. [20] U. Kreibig, M. Vollmer, “Optical Properties of Metal Clusters,” Springer Verlag, Berlin, 1995. [21] C.F. Bohren, D.R. Huffman, “Absorption and Scattering of Light by Small Particles,” Wiley, New York, 1983. [22] A. R. Sennoudi et al. unpublished. [23] I.-S. Gradshteyn and I. W. Ryghik, “Tables of Integrals, Series and Products,” Academic Press, New York, 1965. [24] H. Goldenberg, C.J. Tranter, Brit. J. Appl. Phys. 296, 1952. [25] G. Yin et al. , J. Kor. Phys. Soc., Vol. 49, pp. 2108, 2006. [26] G. Brix et al , Magn. Res. Imag., Vol. 20, pp. 65, 2002. [27] F. Hakem et al., Ber. Bunsenges. Phys. Chem., Vol. 100, pp. 815, 1996. [28] M. Benmouna et al., Phys. Chem. Liq., Vol. 16, pp. 235, 1987. [29] J. M. Dobson, “Manual of Small Animal Oncology”, RAS White Edit., Bsava, 1991. |

