 World Journal of AIDS, 2013, 3, 280-286 http://dx.doi.org/10.4236/wja.2013.33035 Published Online September 2013 (http://www.scirp.org/journal/wja) Darunavir Resistance in HI V Infec ting Prote ase Inhibitor-Experienced Mexican Patients* Carlos A. Agudelo1, Luis E. Soto-Ramírez1, Abraham Katime-Zúñiga1, Lorena Cabrera-Ruíz2, Hugo Lara-Sánchez3, Juan J. Calva3# 1Department of Infectious Diseases, “Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán”, Mexico City, Mexico; 2Department of Infectious Diseases, Hospital Médica Sur and GUIAR Group, Mexico City, Mexico; 3The Clinical Epidemiology Unit, “Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán”, Mexico City, Mexico. Email: #juanjcalva@gmail.com Received July 9th, 2013; revised August 9th, 2013; accepted August 19th, 2013 Copyright © 2013 Carlos A. Agudelo et al. This is an open access article distributed under the Creative Commons Attribution Li- cense, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Darunavir (DRV) is a useful antiretroviral treatment in the salvage therapy of multiclass-resistant HIV- infected patients. This study’s aim was to determine the frequency and risk factors for DRV resistance-associated muta- tions (DRV-RAM) among DRV-naïve Mexican patients with virologic failure after extensive antiretroviral treatment and exposure to at least one protease inhibitor (PI). Methods: HIV-infected patients with a history of at least 2 failed re- gimes were included and their clinical histories and genotype resistance tests were analyzed. Major PI resistance-associ- ated mutations (PI-RAM), DRV-RAM and resistance to DRV were defined according to the IAS-USA criteria. Previous exposure to PI was compared between patients with DRV-resistant HIV and DRV-susceptible HIV-infected controls. Results: The median number of major PI-RAM was 2 (IQR = 0 - 3). In 54.7% (95% CI = 50.0% - 59.4%) of 631 sub- jects, no DRV-RAM were found on viral genotyping and 6.7% (95% CI = 4.8% - 8.6%) had 3 or more DRV-RAM. The two most frequently found DRV-RAM were in codons I84V (in 22.7% of cases) and L33F (in 20% of cases) in the viral protease gene. The number of major PI-RAM (as a surrogate marker of duration and number of PI used) and previous exposure to (fos) amprenavir or tipranavir were independently associated with DRV-resistant HIV infection. Conclu- sions: In this Mexican population, despite a high prior PI exposure, HIV-DRV resistance rate is relatively low and suc- cessful viral control with DRV-containing combined salvage therapy is expected in most patients. Keywords: Darunavir; Resistance; Risk Factors; Prevalence; Mexico 1. Introduction Randomized clinical trials have shown that ritonavir- boosted darunavir (DRV/r) as a component of salvage re- gimens (and at least one other fully active drug), leads to significantly higher rates of lasting virological control when compared with conventional protease inhibitors (PI), among patients infected with multidrug-resistant HIV and an extensive treatment history [1-6]. Eleven specific darunavir resistance-associated muta- tions (DRV-RAM) in the viral protease gene have been linked to decrease in vitro HIV susceptibility as well as sub-optimal clinical responses to darunavir [7]. A loss of response begins to occur with one mutation but 3 or more mutations and additional numerous protease inhibitor (PI) resistance-associated mutations (PI-RAM) lead to a great- ly diminished virologic suppression rate. Participants in the POWER 1 and 2 trials (in the DRV/r arm) as well as the DUET-1 and DUET-2 studies, had a baseline preva- lence of 3 or more DRV-RAM of 22%, 41% and 44%, respectively [2-5]. In routine clinical practice, the rate of occurrence of these 11 DRV-RAM in PI-treated patients not participat- ing in clinical trials, has been reported in some popula- tions [8-10]. A low percentage (ranging between 4.1% and 6.7%) of individuals with 3 or more DRV-RAM, has consistently been found in these surveys. Furthermore, these studies have shown an association between the number of prior PI used, the total number of PI resistance mutations, previous treatment with (fos) amprenavir, and *Author Disclosure: L. E. S-R. has received payment for lectures from MSD, Janssen-Cilag and ViiV. J. J. C. has served as a Board member o the DSMB of the CADIRIS Study. None of the other authors had con- flicts of interest or funding sources. #Corresponding author. Copyright © 2013 SciRes. WJA  Darunavir Resistance in HIV Infecting Protease Inhibitor-Experienced Mexican Patients 281 a greater risk of developing DRV-RAM. In Mexico, scaling up of free access to antiretroviral treatment started approximately 15 years ago; during the first 4 years, about one third of total patient-months were treated with a PI (predominantly unboosted indinavir and unboosted saquinavir) [11]. Over the past few years, close to 29% of combined therapy prescriptions have in- cluded a PI with a broad range of agents, mostly: rito- navir-boosted lopinavir, ritonavir-boosted atazanavir, ri- tonavir-boosted saquinavir and ritonavir-boosted indina- vir [12]. This study’s aim was to assess the impact of this long and extensive use of diverse PI in our country, on the number of DRV-RAM in HIV infected DRV-naïve pa- tients and virologic failure with a long-standing history of antiretroviral therapy with several regimens. Also, we determined whether the presence of 3 or more DRV- RAM was associated with surrogate markers of the level of prior PI selective pressure. 2. Methods 2.1. Study Population A retrospective, cross-sectional study was conducted to estimate the prevalence of HIV DRV-RAM and by case- control analysis, the association between certain charac- teristics in study patients and DRV-resistant HIV was as- sessed. To be eligible, patients required: 1) to be considered as virologic failures (ongoing viral replication as defined by an HIV viral load above 50 copies/ml in at least two con- secutive measurements) and on antiretroviral therapy at the time of this survey, 2) to have a history of at least two antiretroviral failed regimes, 3) prior treatment with at least one PI, 4) no previous use of DRV, 5) an HIV ge- notype resistant test performed while receiving the last drug scheme, 6) at least one PI resistance mutation (PI- RAM) in this genotyping and 7) complete clinical data. Between 2008 and 2010, patients were selected from two populations of cases whose physician had requested and received a recommendation to optimize the salvage re- gimen of heavily-treated experienced patients, by one of two national antiretroviral therapy peer-advisory commit- tees. The Board for the rational use of antiretrovirals (CORESAR) provides advice to physicians caring for in- dividuals in the Mexican Ministry of Health system; the Inter-institutional group for antiretroviral treatment (GUIAR) provides help to practitioners caring for pa- tients in this health system or in two of the largest na- tional social security systems (IMSS and ISSSTE). Demographic, virologic, prior specific drug exposure and drug regimen at the time of last failure data were re- corded in each case. 2.2. Drug Resistance Testing Genetic sequencing of protease and reverse transcriptase HIV genes was conducted in plasma, using the Viroseq®, HIV-1 kit (Abbott Laboratories, Abbott Park, Illinois) in samples from the cases in CORESAR, and the Trugene®, HIV-1 kit (Siemens, Erlangen, Germany) in samples from GUIAR. Drug resistance mutations within the pol gene were interpreted following the 2011 International Anti- viral Society-USA panel list. Accordingly, the following 11 resistance mutations were evaluated for darunavir: V11I, V32I, L33F, I47V, I50V, I54L/M, T74P, L76V, I84V and L89V [13]. Darunavir resistance was defined as the presence of 3 or more of these mutations. 2.3. Statistical Analysis All data are reported as absolute numbers and percent- ages, as well as medians (Md) and interquartile ranges (IQR). Comparisons between 2 independent groups were established using the U-Mann-Whitney test for continu- ous variables and the Pearson χ2 or the Fisher exact test for categorical variables. Comparison of dimensional da- ta distribution across more than 2 independent groups, was analyzed with the Kruskal-Wallis non-parametric 1-way ANOVA test. Univariate and multivariate logistic regres- sion analysis were performed to assess factors associated to the presence of each DRV-RAM and DRV resistance. The magnitude of the association was expressed as the odds ratio (OR) and its 95% confidence interval (95% CI). Statistical significance was reflected by a P value < 0.05. Association between continuous variables was mea- sured with the Pearson correlation coefficient (r). A re- ceiver operating-characteristic (ROC) curve was plotted to assess the diagnostic performance of the number of major PI-RAM in the prediction of darunavir-resistance. Statistical analysis was carried out using the SPSS®16.0 (IBM Corp. Armonk, NY). 2.4. Ethical Considerations This study was approved by the Research Ethics Com- mittee of the “Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán” (Ref #62), and was conduc- ted in accordance with The Declaration of Helsinki. 3. Results 3.1. Study Population From a total of 868 referred cases (476 patients in CORESAR and 392 patients in GUIAR), 631 (73%) met the inclusion criteria. Study patient features are depicted in Table 1. Most had received various combinations of antiretroviral therapies without optimal viral control, over several years. Copyright © 2013 SciRes. WJA  Darunavir Resistance in HIV Infecting Protease Inhibitor-Experienced Mexican Patients Copyright © 2013 SciRes. WJA 282 Table 1. Characteristics of the 631 study subjects. Age (years): Md (IQR) 40 (33 - 46) Duration since HIV diagnosis (years): Md (IQR) 9 (6 - 11.6) Nadir T-CD4+ cell count (cells/mm3): Md (IQR) 61 (23 - 155) Patients with no documented HIV plasma viral load under the limit of detection at any time: n (%) 328 (52) Duration with ARV therapy (years): Md (IQR) 8 (5 - 11) Number of prior drug regimens: Md (IQR) 4 (3 - 5) Number of PI previously used: Md (IQR) 2 (2 - 3) Prior use of (fos) amprenavir: n (%) of cases 40 (6.3) Prior use of tipranavir: n (%) of cases 98 (15.5) Duration of prior use of any PI (years): Md (IQR) 5 (2.9 - 7.7) Plasma HIV viral load prior to genotyping (log10 cop/ml): Md (IQR) 4.52 (3.95 - 5) T-CD4+ cell count prior to genotyping (cells/mm3): Md (IQR) 192 (86.7 - 328.3) ARV regimen at time of genotyping: n (%) of cases NRTI + PI 429 (68%) NRTI + NN 118 (19%) NRTI + PI + NN 31 (5%) Other 53 (8%) Number of major PI-resistance associated mutations: Md (IQR) 2 (0 - 3) NRTI = Nucleos (t) ide analogue reverse transcriptase inhibitor; PI = Protease inhibitor; NN = Non-nucleoside reverse transcriptase inhibitor. 3.2. Prevalence of Major PI-RAM and DRV-RAM Table 2 shows the distribution of the 631 study patients according to the number of PI-RAM and DRV-RAM found in their HIV genotype resistance test. Two or more major PI-RAM and 4 or more major PI-RAM were iden- tified in 60% and 18% of the patients, respectively. More than half (54.7%, 95% CI = 49.5% - 59.9%) of patients had no DRV-RAM; 20.9% (95% CI = 14% - 27.8%) had more than one DRV-RAM and 6.7% (95% CI = 4.7% - 8.7%) were infected with HIV resistant to darunavir (ge- notype with 3 or more DRV-RAM). The two most frequently identified DRV-RAM in the overall population were mutation I84V (22.7% of cases) and mutation L33F (20% of cases). Other DRV-RAM was present in less than 10% of the overall population. Among the 42 patients with HIV resistant to DRV, the relative frequency of all DRV-RAM was: I84V (76.2% of cases), L33F (54.8% of cases) V32I (54.8% of cases), I47V (47.6% of cases), L89V (31.0% of cases), I54L (28.6% of cases), T74P (21.4% of cases), V11I (19.0% of cases), I54M (16.7% of cases), L76V (7.1% of cases) and I50V (2.4% of cases). 3.3. Association between DRV-RAM and Prior Exposure to Other PI An association between the presence of a particular DRV-RAM and prior use of other specific PI was estab- lished. There was a significant association between (fos) amprenavir use (vs. no prior use of these PI) and the identification of the following five DRV-RAM: I54M (OR = 13.9; 95% CI = 4.1 - 47.8), I50V (OR = 6.2; 95% CI = 1.1 - 32.8), V32I (OR = 3.2; 95% CI = 1.2 - 8), I47V (OR = 3.1; 95% CI = 1.3 - 7.4) and L33F (OR = 2.3; 95% CI = 1.2 - 4.6). Moreover, there was also a signifi- cant association between tipranavir use (vs. no prior use of this PI) and the identification of the following five DRV-RAM: I84V (OR = 4.9; 95% CI = 3.1 - 7.7), T74P (OR = 4.0; 95% CI = 1.5 - 11), L33F (OR = 3.1; 95% CI = 1.9 - 4.9), L32I (OR = 2.5; 95% CI = 1.2 - 5.2) and I47V (OR = 2.4; 95% CI = 1.2 - 4.7). Patients infected with HIV and harboring 3 or more DRV-RAM compared with those with less than 3 DRV- RAM, had been treated with a significantly greater num- ber of PI and had a longer cumulative time period of pro- tease inhibitor exposure; moreover, these individuals were significantly more likely to have used a regimen with two  Darunavir Resistance in HIV Infecting Protease Inhibitor-Experienced Mexican Patients 283 PI (independently of ritonavir use as a booster), (fos) am- prenavir, lopinavir/ritonavir, and tipranavir (see Table 3). The median number of major PI-RAM was significantly greater in patients with 3 or more DRV-RAM. A significant correlation between the number of major PI-RAM and the previous amount and duration of PI ad- ministration was also detected (Pearson correlation coef- ficients = 0.30, p < 0.01 and 0.23, p < 0.01, respectively). Hence, the number of major PI-RAM was used as a sur- rogate marker of the level of prior PI pharmacologic se- lective pressure. On multivariate analysis, prior use of (fos) amprenavir, the use of tipranavir and the number of ma- jor PI-RAM were independently associated with 3 or more DRV-RAM (see Table 4). There is a 3.7 fold in- crease in the risk of having a darunavir resistant HIV in- fection per one extra major PI-RAM. As Figure 1 shows, the median number of DRV-RAM rose as the number of major PI-RAM increased (Pearson correlation coefficient = 0.73; P < 0.001). The distribu- tion of the number of DRV-RAM was significantly dif- ferent across categories of the number of major PI-RAM Table 2. Distribution of 631 cases according to the number of major protease inhibitor-resistance associated (PI-RAM) and darunavir-resistance associated mutations (DRV-RAM) in genotype testing. Number of RAM Number (%) of patient with Major PI-RAM DRV-RAM n (%) n (%) 0 185 (29.3) 345 (54.7) 1 72 (11.4) 154 (24.4) 2 115 (18.2) 90 (14.3) 3 148 (23.5) 28 (4.4) 4 70 (11.1) 5 (0.8) 5 29 (4.6) 7 (1.1) 6 9 (1.4) 2 (0.3) 7 3 (0.5) 0 (0) 631 (100) 631 (100) Table 3. Comparison of prior exposure to other protease inhibitors (PI) between subjects infected with HIV harboring 3 or more darunavir-resistance associated mutations (DRV-RAM) vs with less than 3 DRV-RAM. Variable HIV with 3 or more DRV-RAM (42 subjects) HIV with less than 3 DRV-RAM (589 subjects) P value Number of PI used: median (IQR) 3 (2, 4) 2 (1, 3) <0.01 Duration of PI use (years): median (IQR) 7.1 (4.6, 8.4) 4.9 (2.8, 7.7) 0.02 (fos) amprenavir use: % of patients 24 5 <0.001 Tipranavir use: % of patients 40 14 <0.001 Lopinavir/ritonavir use: % of patients 81 65 0.03 Double PI use: % of patients 26 15 0.05 Number of major PI-RAM*: median (IQR) 5 (3, 6) 2 (0, 3) <0.001 *Protease inhibitor resistance-associated mutation. Table 4. Association between infection by HIV harboring 3 or more darunavir-resistance associated mutations (DRV-RAM) and prior exposure to other protease inhibitors (PI) in 631 subjects. Variable Univariate Multivariate Odds ratio95% CI p value Odds ratio 95% CI p value Number of PI used, per one PI increase 1.9 1.4 - 2.5 <0.001 Duration of PI use, per one year increase 1.1 1.0 - 1.2 0.03 (fos) amprenavir use/no use 5.8 2.6 - 12.9 <0.001 7.3 2.4 - 22 <0.001 Tipranavir use/no use 4.3 2.2 -8.2 <0.001 2.4 1.0 - 5.7 0.04 Lopinavir/ritonavir use/no use 2.3 1.0 - 5.0 0.04 0.7 0.2 - 2.1 NS Double PI use/no use 2 1.0 - 4.2 0.05 1.5 0.6 - 4.3 NS Number of major PI-RAM*, per one mutation increase 4.3 3.0 - 6.2 <0.001 4.7 3.0 - 7.1 <0.001 *Protease inhibitor resistance-associated mutation. Copyright © 2013 SciRes. WJA 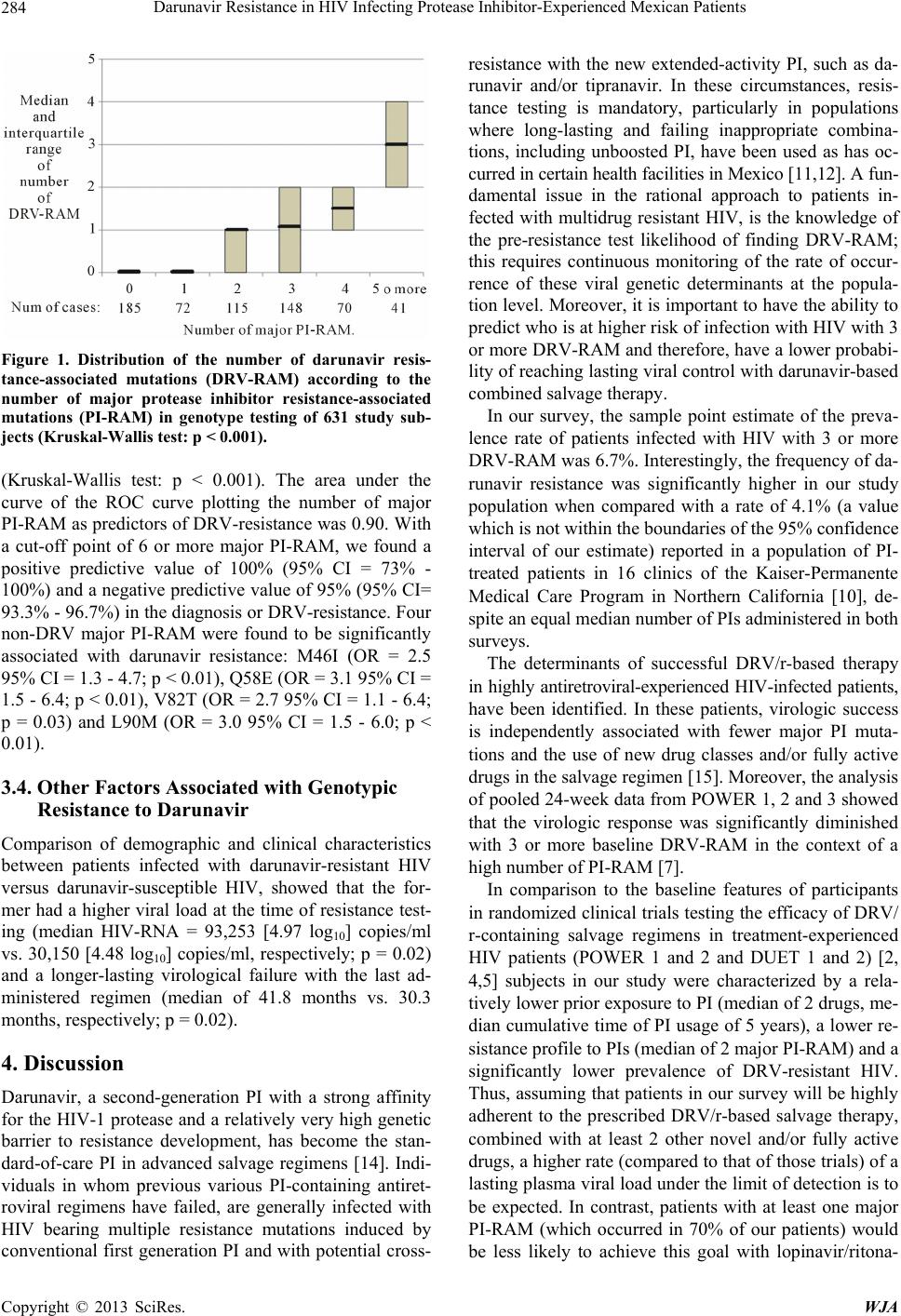 Darunavir Resistance in HIV Infecting Protease Inhibitor-Experienced Mexican Patients 284 Figure 1. Distribution of the number of darunavir resis- tance-associated mutations (DRV-RAM) according to the number of major protease inhibitor resistance-associated mutations (PI-RAM) in genotype testing of 631 study sub- jects (Kruskal-Wallis test: p < 0.001). (Kruskal-Wallis test: p < 0.001). The area under the curve of the ROC curve plotting the number of major PI-RAM as predictors of DRV-resistance was 0.90. With a cut-off point of 6 or more major PI-RAM, we found a positive predictive value of 100% (95% CI = 73% - 100%) and a negative predictive value of 95% (95% CI= 93.3% - 96.7%) in the diagnosis or DRV-resistance. Four non-DRV major PI-RAM were found to be significantly associated with darunavir resistance: M46I (OR = 2.5 95% CI = 1.3 - 4.7; p < 0.01), Q58E (OR = 3.1 95% CI = 1.5 - 6.4; p < 0.01), V82T (OR = 2.7 95% CI = 1.1 - 6.4; p = 0.03) and L90M (OR = 3.0 95% CI = 1.5 - 6.0; p < 0.01). 3.4. Other Factors Associated with Genotypic Resistance to Darunavir Comparison of demographic and clinical characteristics between patients infected with darunavir-resistant HIV versus darunavir-susceptible HIV, showed that the for- mer had a higher viral load at the time of resistance test- ing (median HIV-RNA = 93,253 [4.97 log10] copies/ml vs. 30,150 [4.48 log10] copies/ml, respectively; p = 0.02) and a longer-lasting virological failure with the last ad- ministered regimen (median of 41.8 months vs. 30.3 months, respectively; p = 0.02). 4. Discussion Darunavir, a second-generation PI with a strong affinity for the HIV-1 protease and a relatively very high genetic barrier to resistance development, has become the stan- dard-of-care PI in advanced salvage regimens [14]. Indi- viduals in whom previous various PI-containing antiret- roviral regimens have failed, are generally infected with HIV bearing multiple resistance mutations induced by conventional first generation PI and with potential cross- resistance with the new extended-activity PI, such as da- runavir and/or tipranavir. In these circumstances, resis- tance testing is mandatory, particularly in populations where long-lasting and failing inappropriate combina- tions, including unboosted PI, have been used as has oc- curred in certain health facilities in Mexico [11,12]. A fun- damental issue in the rational approach to patients in- fected with multidrug resistant HIV, is the knowledge of the pre-resistance test likelihood of finding DRV-RAM; this requires continuous monitoring of the rate of occur- rence of these viral genetic determinants at the popula- tion level. Moreover, it is important to have the ability to predict who is at higher risk of infection with HIV with 3 or more DRV-RAM and therefore, have a lower probabi- lity of reaching lasting viral control with darunavir-based combined salvage therapy. In our survey, the sample point estimate of the preva- lence rate of patients infected with HIV with 3 or more DRV-RAM was 6.7%. Interestingly, the frequency of da- runavir resistance was significantly higher in our study population when compared with a rate of 4.1% (a value which is not within the boundaries of the 95% confidence interval of our estimate) reported in a population of PI- treated patients in 16 clinics of the Kaiser-Permanente Medical Care Program in Northern California [10], de- spite an equal median number of PIs administered in both surveys. The determinants of successful DRV/r-based therapy in highly antiretroviral-experienced HIV-infected patients, have been identified. In these patients, virologic success is independently associated with fewer major PI muta- tions and the use of new drug classes and/or fully active drugs in the salvage regimen [15]. Moreover, the analysis of pooled 24-week data from POWER 1, 2 and 3 showed that the virologic response was significantly diminished with 3 or more baseline DRV-RAM in the context of a high number of PI-RAM [7]. In comparison to the baseline features of participants in randomized clinical trials testing the efficacy of DRV/ r-containing salvage regimens in treatment-experienced HIV patients (POWER 1 and 2 and DUET 1 and 2) [2, 4,5] subjects in our study were characterized by a rela- tively lower prior exposure to PI (median of 2 drugs, me- dian cumulative time of PI usage of 5 years), a lower re- sistance profile to PIs (median of 2 major PI-RAM) and a significantly lower prevalence of DRV-resistant HIV. Thus, assuming that patients in our survey will be highly adherent to the prescribed DRV/r-based salvage therapy, combined with at least 2 other novel and/or fully active drugs, a higher rate (compared to that of those trials) of a lasting plasma viral load under the limit of detection is to be expected. In contrast, patients with at least one major PI-RAM (which occurred in 70% of our patients) would be less likely to achieve this goal with lopinavir/ritona- Copyright © 2013 SciRes. WJA  Darunavir Resistance in HIV Infecting Protease Inhibitor-Experienced Mexican Patients 285 vir-containing salvage combined therapy [16]. Our data show that the number of major PI-RAM de- tected by genotype testing is an accurate proxy of the magnitude of prior PI exposure/failure and of pharmaco- logic PI selective pressure during long periods of treat- ment with PI-containing failing regimens. Our findings provide further support to the concept that the risk of in- fection with darunavir-resistant HIV is directly related to the level and duration of previous PI exposure; there is a 3.7 fold increase in this risk per each additional major PI- RAM identified by genotype testing. We also found that specific non-darunavir PI-RAM (such as M46I, Q58E, V82T and L90M) were associated with darunavir resis- tance, as was also shown by Mitsuya et al. [10]. By multivariate analysis, we were able to establish that previous use of (fos) amprenavir and/or tipranavir sig- nificantly increases the risk of darunavir resistance, inde- pendently of the number of major PI-RAM (as an indica- tor of the prior overall load of PI selective pressure). Both molecules, (fos) amprenavir and darunavir, are structur- ally related and hence, share some specific resistance mu- tations [17]. In our study sample, this effect of (fos) am- prenavir might be mediated through the selection of mu- tations I50V and I54M which were more common in in- dividuals who had received these agents versus other PI. Interestingly, to our knowledge this is the first report of the previous use of tipranavir as an independent risk factor for the loss of viral susceptibility to darunavir. A possible explanation of this finding is that a failing ti- pranavir-containing regimen increases the likelihood of selecting certain PI-RAM conferring cross-resistance with darunavir (such as mutations L33F, I47V, T74P and I84V). During the last six years, the prescription of this PI in Mexico has been unrestricted and commonly used inap- propriately since it has been added to other antiretroviral agents without full antiviral activity (functional monothe- rapy); we are now seeing the deleterious effect of this er- roneous clinical practice on darunavir-viral susceptibili- ty. A potential methodological drawback of this study is the possible lack of representativeness of our study sam- ple (sample selection bias) which may limit the extrapo- lation of our results to other multi-drug exposed patients but with a different degree of previous PI use. The preva- lence of DRV-RAM-bearing HIV needs to be determined in other populations with a diverse history of PI selective pressure. 5. Conclusion In summary, in this sample of heavily antiretroviral treated patients, despite the fact that most had a history of several years under selective pressure by PI-including failing regimens, the emergence of HIV variants with DRV-RAM was of relatively low magnitude. DRV/r- based salvage therapy with at least 2 other novel and/or fully active drugs is likely to be successful in a signifi- cant proportion of these patients, with expected higher rates of virologic control compared to those found in cli- nical trial participants. Future similar surveys are neces- sary in order to continuously monitor this dynamic phe- nomenon; a rise in the incidence of darunavir-resistant HIV infections is likely to occur with longer patient sur- vival and an increase in overall PI (including tipranavir and darunavir) exposure. 6. Source of Research Funds The Inter-institutional group for antiretroviral treatment (GUIAR) group was funded with a grant provided by Merck Sharp & Dohme and The Board for the rational use of antiretrovirals (CORESAR), with subsidies provi- ded by the Centro Nacional para la Prevención y el Con- trol del VIH/SIDA (CENSIDA-México). 7. Acknowledgements We thank Dr. Wendy K. Moncada, Dr. Yukie García- Kishi, Dr. Elsa Vidal-Laurencio and Luis Fuentes-Rome- ro, BSc, from the Department of Infectious Diseases, Ins- tituto Nacional de Ciencias Médicas y Nutrición Salva- dor Zubirán, Mexico City, Mexico, for their technical as- sistance. We are particularly grateful to the physicians who referred clinical cases for peer-advising on salvage antiretroviral therapy. REFERENCES [1] A. Imaz, V. Falcó and E. Ribera, “Antiretroviral Salvage Therapy for Multiclass Drug-Resistant HIV-1-Infected Patients: From Clinical Trials to Daily Clinical Practice,” AIDS, Vol. 13, 2011, pp. 180-193. [2] B. Clotet, N. Bellos, J. M. Molina, et al., “Efficacy and Safety of Darunavir-ritonavir at Week 48 in Treatment- Experienced Patients with HIV-1 Infection in POWER 1 and 2: A Pooled Subgroup Analysis of Data from Two Randomized Trials,” Lancet, Vol. 369, No. 9568, 2007, pp. 1169-1178. doi:10.1016/S0140-6736(07)60497-8 [3] K. Arastéh, P. Yeni, A. Pozniak, et al., “Efficacy and Sa- fety of Darunavir/Ritonavir in Treatment-Experienced HIV Type-1 Patients in the POWER 1, 2 and 3 Trials at Week 96,” Antiviral Therapy, Vol. 14, 2009, pp. 859-864. doi:10.3851/IMP1301 [4] J. V. Madruga, P. Cahn, B. Grinztejn, et al., “Efficacy and Safety of TMC125 (Etravirine) in Treatment-Experienced HIV-1-Infected Patients in DUET-1: 24-Week Results from a Randomized, Double Blind, Placebo-Controlled Trial,” Lancet, Vol. 370, No. 9581, 2007, pp. 29-38. doi:10.1016/S0140-6736(07)61047-2 [5] A. Lazzarin, T. Campell, B. Clotet, et al., “Efficacy and Safety of TMC125 (Etravirine) in Treatment-Experienced HIV-1-Infected Patients in DUET-2: 24-Week Results Copyright © 2013 SciRes. WJA  Darunavir Resistance in HIV Infecting Protease Inhibitor-Experienced Mexican Patients Copyright © 2013 SciRes. WJA 286 from a Randomized, Double Blind, Placebo-Controlled Trial,” Lancet, Vol. 370, No. 9581, 2007, pp. 39-48. doi:10.1016/S0140-6736(07)61048-4 [6] C. Katlama, B. Clotet, A. Mills, et al., “Efficacy and Sa- fety of Etravirine at Week 96 in Treatment-Experienced HIV Type-1-Infected Patients in the DUET-1 and DUET- 2 Trials,” Antiviral Therapy, Vol. 15, 2010, pp. 1045- 1052. doi:10.3851/IMP1662 [7] S. De Meyer, T. Vangeneugden, B. van Baelen, et al., “Resistance Profile of Darunavir: Combined 24-Week Results from the POWER Trials,” AIDS Research and Human Retroviruses, Vol. 24, No. 3, 2008, pp. 379-388. doi:10.1089/aid.2007.0173 [8] J. E. Vidal, A. C. Freitas, A. T. W. Song, S. V. Campos, M. Dalben and A. V. Hernandez, “Prevalence and Factors Associated with Darunavir Resistance Mutations in Mul- ti-Experienced HIV-1-Infected Patients Failing Other Pro- tease Inhibitors in a Referral Teaching Center in Brazil,” Brazilian Journal of Infectious Diseases, Vol. 15, No. 3, 2011, pp. 245-248. doi:10.1016/S1413-8670(11)70183-0 [9] E. Poveda, C. de Mendoza, L. Martin-Carbonero, et al., “Prevalence of Darunavir Resistance Mutations in HIV-1- Infected Patients Failing Other Protease Inhibitors,” Jour- nal of Antimicrobial Chemotherapy, Vol. 60, No. 4, 2007, pp. 885-888. doi:10.1093/jac/dkm276 [10] Y. Mitsuya, T. F. Liu, S. Y. Rhee, W. J. Fessel and R. W. Shafer, “Prevalence of Darunavir Resistance-Associated Mutations: Patterns of Occurrence and Association with Past Treatment,” The Journal of Infectious Diseases, Vol. 196, No. 8, 2007, pp. 1177-1179. doi:10.1086/521624 [11] S. Bautista-Arredondo, A. Mane and S. M. Bertozzi, “Eco- nomic Impact of Antiretroviral Therapy Prescription De- cisions in the Context of Rapid Scaling-Up of Access to Treatment: Lessons from Mexico,” AIDS, Vol. 20, No. 1, 2006, pp. 101-109. doi:10.1097/01.aids.0000198096.08444.53 [12] J. J. Calva and Y. Vargas-Infante, “Cobertura Universal con la Terapia Antirretroviral Combinada. Logros y Desa- fíos en la Secretaría de Salud de Mexico,” In: J. A. Cor- dova-Villalobos, S. Ponce de Leon-Rosales and J. L. Val- despino, Eds., 25 años de SIDA en Mexico. Logros, Desa- ciertos y Retos, 2nd Edition, Instituto Nacional de Salud Pública, Cuernavaca, Morelos, 2009, pp. 333-353. [13] V. A. Johnson, V. Calvez, H. F. Gunthard, et al., “2011 Up- date of the Drug Resistance Mutations in HIV-1,” Topics in Antiviral Medicine, Vol. 19, No. 4, 2011, pp. 156-164. [14] L. E. Wilson and J. E. Gallant, “The Management of Treat- ment-Experienced HIV-Infected Patients: New Drugs and Drug Combinations,” Clinical Infectious Diseases, Vol. 48, 2009, pp. 214-221. [15] C. Delaugerre, J. F. Buyck, G. Peytavin, et al., “Factors Pre- dictive of Successful Darunavir/Ritonavir-Based Therapy in Highly Antiretroviral-Experienced HIV-1-Infected Pa- tients (the DARWEST Study),” Journal of Clinical Vi- rology, Vol. 47, No. 3, 2010, pp. 248-252. doi:10.1016/j.jcv.2009.12.022 [16] S. De Meyer, A. Hill, G. Picchio, R. DeMasi, E. De Pa- epe and M. P. de Bethune, “Influence of Baseline Prote- ase Inhibitor Resistance on the Efficacy of Darunavir/ ri- tonavir or Lopinavir/Ritonavir in the TITAN Trial,” Jour- nal of Acquired Immune Deficiency Syndromes, Vol. 49, No. 5, 2008, pp. 563-564. doi:10.1097/QAI.0b013e318183ac9c [17] S. Y. Rhee, J. Taylor, W. J. Fessel, et al., “HIV-1 Prote- ase Mutations and Protease Inhibitor Cross-Resistance,” Antimicrobial Agents and Chemotherapy, Vol. 54, No. 10, 2010, pp. 4253-4261.
|