 Advances in Infectious Diseases, 2013, 3, 200-204 http://dx.doi.org/10.4236/aid.2013.33029 Published Online September 2013 (http://www.scirp.org/journal/aid) Identification of Bifidobacterium animalis, Bifidobacterium adolescentis and Bifidobacterium bifidum from Stool of Children and Detection of Their Antibacterial Properties Mansour Amin1,2*, Ahmad Farajzadeh Sheikh1, Ham e d Goodarzi1,2, Mehdi Sormeh2 1Department of Microbiology, School of Medicine, Ahvaz Jundishapur University of Medical Sciences (AJUMS), Ahvaz, Iran; 2Infectious and Tropical Diseases Research Center, Ahvaz Jundishapur University of Medical Sciences (AJUMS), Ahvaz, Iran. Email: *mnsamin@yahoo.com Received April 6th, 2013; revised May 6th, 2013; accepted June 6th, 2013 Copyright © 2013 Mansour Amin et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Introduction and Objective: The genus Bifidobacterium can generally be found in quantity in the habitats such as hu- man and animal gastrointestinal tract, dental caries, vagina and oral cavity. The aim of this study was to isolate Bifido- bacterium from stool and determine their inhibitory effect against some pathogens. Materials and Methods: 130 sam- ples were collected by wet swabs and kept in sterile tubes containing MRS broth media. And Bifidobacterium isolated from stool was enriched in Man-Rogosa-Sharpe medium (MRS) broth and isolated by growing on MRS agar medium and characterized by phenotypic characteristics and PCR technique at genus and species levels. The antimicrobial sub- stance was extracted from ethyl acetate solvent and the antimicrobial activity against some pathogenic bacteria, such as Salmonella typhi and Shigella sonnei, were investigated. Results: Eleven Bifidobacterium bifidum and four Bifidobac- terium adolescentis, which were isolated from fresh stool, were identified by PCR. Antimicrobial substance from MRS broth medium was extracted. This antimicrobial compound showed a potent inhibitory activity against four tested bac- teria. These bacteria produced acetic acid and lactic acid as inhibitory substances that were different from bacteriocins. Conclusion: Fresh stool may be used as a source of antimicrobial lactic acids bacteria, Bifidobacterium bifidum and adolescentis as two probiotics can establish themselves in gut and urogenital tract to prevent the human body from ad- verse effects of pathogens. Keywords: Bifidobacterium; PCR; Antimicrobial Substance; Stool 1. Introduction Bifidobacteria were first discovered in infant feces by Tissier and named it Bacillus bifidus [1]. Bifidobacteria are a natural resident of the human and animal gastroin- testinal tract, dental caries and vagina, oral cavity, urine and blood [2]. Several Bifidobacterium strains are now being used as probiotics by using established criteria which belong to the Bifidobacterium animalis, Bifido- bacterium bifidum, Bifidobacterium breve, Bifidobacte- rium longum fermentum and some other species of this genus [2,3]. For human nutrition, probiotics are defined as live microbial food supplements or components of bac- teria which have been shown to have beneficial effects on human health [4]. Some of the health benefits which have been claimed for probiotics include the following: Diarrhea Diseases, Radiation induced diarrhea, Inflam- matory Bowel Disease, Crohn’s disease, Ulcerative coli- tis, Prevention of colon cancer, Lactose Intolerance, Irri- table Bowel Syndrome (IBS), Pouchitis, Constipation, Helicobacter pylori, Pancreatitis, Hepatic encephalopa- thy, Nonalcoholic fatty liver disease (NASH), Allergy, Urogenital infections and HIV, Probiotics in Pregnancy, reduction of serum cholesterol and improvement of the normal microflora [5]. Certain strain of bacteria has been discovered over the years to have probiotic properties, mainly consisting of lactic acid producing bacteria (Lac- tobacilli, Streptococci, Enterococci, Lactococci, Bifido- bacteria), Bacillus and fungi such as Saccharomyces and Aspergillus [6]. The key enzyme of hexose catabolism in *Corresponding author. Copyright © 2013 SciRes. AID  Identification of Bifidobacterium animalis, Bifidobacterium adolescentis and Bifidobacterium bifidum from Stool of Children and Detection of Their Antibacterial Properties 201 Bifidobacterium is the fructose-6-phosphate phosphoke- tolase which splits the hexose phosphate to erythrose- 4-phosphosphate and acetyl phosphate. Several species, including Bifidobacterium adolescentis, are members of the normal intestinal tract and appendix, dental caries and vagina flora of healthy humans. Other species, such as Bifidobacterium animalis subsp. lactis and Bifidobacte- rium urinalis, are commonly isolated from dairy products as well as from human urine and they play an important role as probiotics in human and animal nutrition [2]. Bi- fidobacterium produces acids, hydrogen peroxide, bacte- riocins and biosurfactants, and thus confers protection of the host. Also Bifidobacterium as a natural vaginal flora plays an important role in the reproductive health of woman by maintaining acidic vaginal PH, providing colonization resistance and preventing the growth of pathogens [7-9]. The acidic PH itself act as a natural de- fense against sexually transmitted disease and AIDS [10,11]. The lactic acid Bacteria (LAB) is one of the most important groups of microorganisms to mankind, being involved in the products (cheese, yogurt and kefir) [12] and stool, blood, urine, dental caries [2]. The aim of this study was to isolate Bifidobacterium animalis, Bifi- dobacterium adolescentis and Bifidobacterium bifidum from fresh stool by PCR and to examine their antimicro- bial activity against some microorganisms such as Sal- monella typhi, Shigella dysenteriae. 2. Material and Method In total, 130 samples were collected by wet swabs and kept in sterile tubes containing MRS broth media. The sources of samples were fresh stool. The entire sample tubes where Man-Rogosa-Sharpe medium incubated at 37˚C and 5% CO2 conditions for 1days, then subcultured on (MRS) agar (Hi-media, India) for 24 h. The colonies were characterized by phenotypical properties including morphology, gram positive staining and absence of cata- lase, oxidase and motility [13]. The DNA of the bacteria was extracted from single colonies after growing the Bi- fidobacterium on MRS agar under anaerobes conditions overnight as described previously [14]. For preliminary detection of Bifidobacterium, the PCR assay was per- formed using the primers cited in Table 1. The composition of PCR mixture was 50 mM KCl, 10 mM HCL (PH: 8.5), 1.5 µl MgCl2, 12 µl dNTPs, 2 µl of each primer, 0.5 µl of Taq polymerase and 10 µl of DNA template in final volume of 25 µl. The PCR conditions were initial denaturation one cycle of 94˚C for 5 min, followed by 35 cycles of 94˚C for 20 s, annealing of 55˚C for 20 s, extension of 72˚C for 30 s and then a final extension at 72˚C for 5 min for Bifidobacterium adoles- centis and Bifidobacterium bifidum and for Bifidobacte- rium animalis 9 min at 94˚C for initial denaturation and 35 cycles of 30 s at 94˚C for denaturation, 30 s at 62˚C for annealing, 30 s at 72˚C for extension, followed by 10 min at 72˚C for a final extension using a thermocycler (Techgene, UK). The PCRproducts were analyzed on 1% agarose gel. The confirmed species by PCR were tested for antimicrobial properties. Antimicrobial compound was isolated using ethyl acetate solvent from Bifidobac- terium adolescentis and Bifidobacterium bifidum sepa- rately. After 4 days incubation, the MRS broth media containing bacteria was mixed with ethyl acetate and agitated with a magnatic stirrer for 1 day. Then the media was allowed to settle for 30 min. following settlement, the solution was separated into two phases, which the supernatant was comprised of the extracted antimicrobial compound. The color of ethyl acetate was turned yellow after agitation. The supernatant then was dried at 45˚C. The quantity of antimicrobial compound was determined as 70 mg. The Minimal Inhibitory Concentration (MIC) of this antimicrobial substance determined using modified E. test, by incorporating 20 µl of the each extract in paper discs. The final concentration of the extract in disc was estimated as 1.06 mg in total incorporated volume [17]. Table 1. The primers used in this study [15,16]. Species primer size Pbi F1:5’-CCGGAATAGCTCC-3’ Bifidobacterium spp. Pbi R2:5’-GACCATGCACCACCTGTGAAA-3’ (914 bp) Ban F2:5’-AACCTGCCCTGTG-3’ B. animalis Pbi R1:5’-GCACCACCTGTGAACCG-3’ (925 bp) BIA-1:5’- GGAAAGATTCTATCGGTATGG-3’ B. adolescentis BIA-2:5’-CTCCCAGTCAAAAGCGGTT-3’ (244 bp) BiBIF-1:5’-CCACATGATCGCATGTGATTG-3’ B. bifidum BiBIF-2:5’-CCGAAGGCTTGCTCCCAAA-3’ (278 bp) The standard strains of B. animalis, B. adolescentis and B. bifidum as positive controls were included in each set of PCR amplification; DNA template in a final volme of 25 µl. All the reagents were purchased from Cinnagen Company, Tehran, Iran. u Copyright © 2013 SciRes. AID 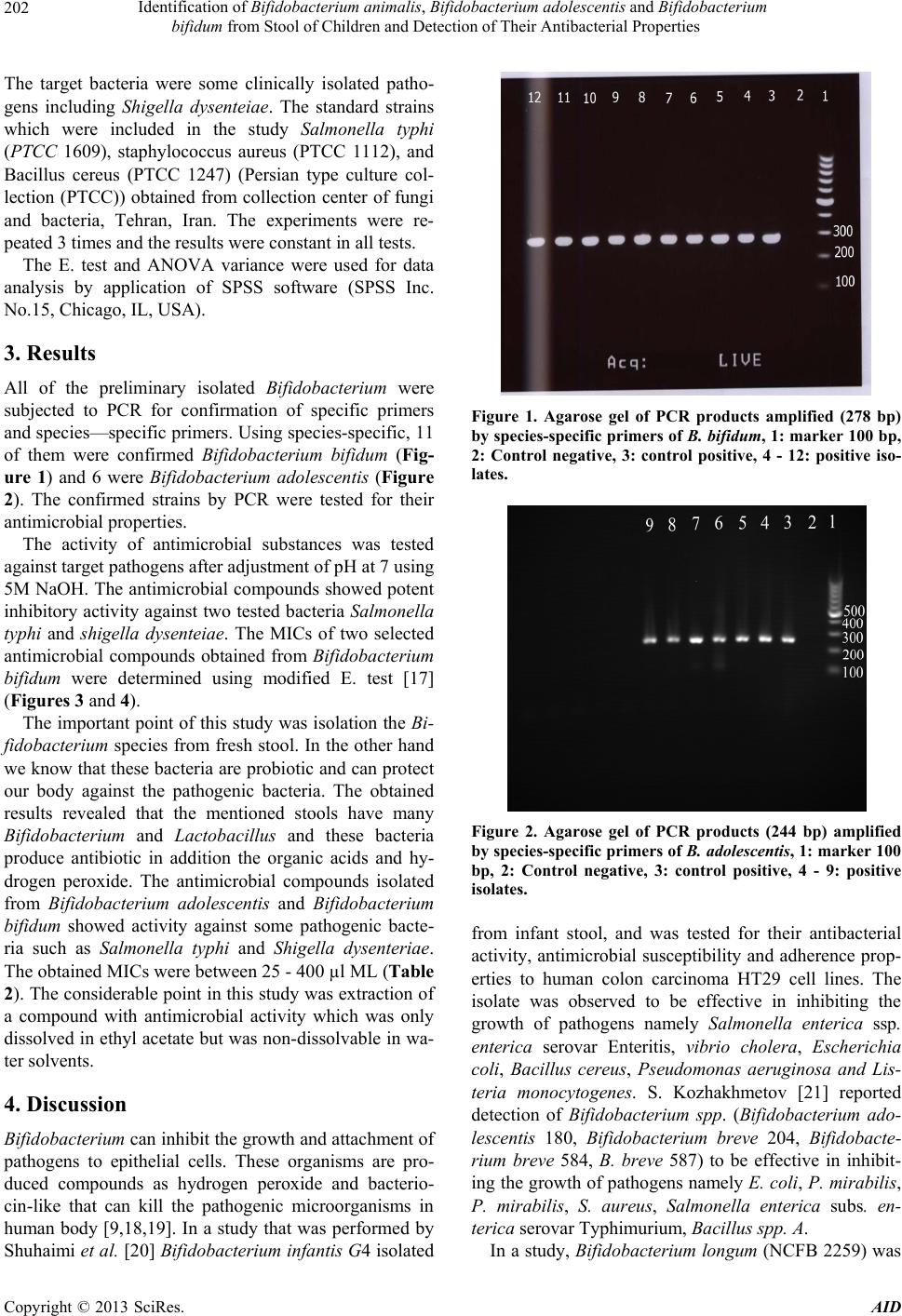 Identification of Bifidobacterium animalis, Bifidobacterium adolescentis and Bifidobacterium bifidum from Stool of Children and Detection of Their Antibacterial Properties 202 The target bacteria were some clinically isolated patho- gens including Shigella dysenteiae. The standard strains which were included in the study Salmonella typhi (PTCC 1609), staphylococcus aureus (PTCC 1112), and Bacillus cereus (PTCC 1247) (Persian type culture col- lection (PTCC)) obtained from collection center of fungi and bacteria, Tehran, Iran. The experiments were re- peated 3 times and the results were constant in all tests. The E. test and ANOVA variance were used for data analysis by application of SPSS software (SPSS Inc. No.15, Chicago, IL, USA). 3. Results All of the preliminary isolated Bifidobacterium were subjected to PCR for confirmation of specific primers and species—specific primers. Using species-specific, 11 of them were confirmed Bifidobacterium bifidum (Fig- ure 1) and 6 were Bifidobacterium adolescentis (Figure 2). The confirmed strains by PCR were tested for their antimicrobial properties. The activity of antimicrobial substances was tested against target pathogens after adjustment of pH at 7 using 5M NaOH. The antimicrobial compounds showed potent inhibitory activity against two tested bacteria Salmonella typhi and shigella dysenteiae. The MICs of two selected antimicrobial compounds obtained from Bifidobacterium bifidum were determined using modified E. test [17] (Figures 3 and 4). The important point of this study was isolation the Bi- fidobacterium species from fresh stool. In the other hand we know that these bacteria are probiotic and can protect our body against the pathogenic bacteria. The obtained results revealed that the mentioned stools have many Bifidobacterium and Lactobacillus and these bacteria produce antibiotic in addition the organic acids and hy- drogen peroxide. The antimicrobial compounds isolated from Bifidobacterium adolescentis and Bifidobacterium bifidum showed activity against some pathogenic bacte- ria such as Salmonella typhi and Shigella dysenteriae. The obtained MICs were between 25 - 400 µl ML (Table 2). The considerable point in this study was extraction of a compound with antimicrobial activity which was only dissolved in ethyl acetate but was non-dissolvable in wa- ter solvents. 4. Discussion Bifidobacterium can inhibit the growth and attachment of pathogens to epithelial cells. These organisms are pro- duced compounds as hydrogen peroxide and bacterio- cin-like that can kill the pathogenic microorganisms in human body [9,18,19]. In a study that was performed by Shuhaimi et al. [20] Bifidobacterium infantis G4 isolated Figure 1. Agarose gel of PCR products amplified (278 bp) by species-specific primers of B. bifidum, 1: marker 100 bp, 2: Control negative, 3: control positive, 4 - 12: positive iso- lates. Figure 2. Agarose gel of PCR products (244 bp) amplified by species-specific primers of B. adolescentis, 1: marker 100 bp, 2: Control negative, 3: control positive, 4 - 9: positive isolates. from infant stool, and was tested for their antibacterial activity, antimicrobial susceptibility and adherence prop- erties to human colon carcinoma HT29 cell lines. The isolate was observed to be effective in inhibiting the growth of pathogens namely Salmonella enterica ssp. enterica serovar Enteritis, vibrio cholera, Escherichia coli, Bacillus cereus, Pseudomonas aeruginosa and Lis- teria monocytogenes. S. Kozhakhmetov [21] reported detection of Bifidobacterium spp. (Bifidobacterium ado- lescentis 180, Bifidobacterium breve 204, Bifidobacte- rium breve 584, B. breve 587) to be effective in inhibit- ing the growth of pathogens namely E. coli, P. mirabilis, P. mirabilis, S. aureus, Salmonella enterica subs. en- terica serovar Typhimurium, Bacillus spp. Α. In a study, Bifidobacterium longum (NCFB 2259) was Copyright © 2013 SciRes. AID  Identification of Bifidobacterium animalis, Bifidobacterium adolescentis and Bifidobacterium bifidum from Stool of Children and Detection of Their Antibacterial Properties 203 Figure 3. E test representing MIC of antimicrobial com- pound obtained from B. bifidum against Shigella dysen- teriea. Figure 4. E test representing MIC of antimicrobial com- pound obtained from B. bifidum against Salmonella typhi. effective in inhibiting the growth of pathogens E. coli O157:H7, one of the leading causes of bacterial food borne diseases [22]. It is expected that the antimicrobial compound of B. adolescentis was not much different from active compounds Bifidobacterium bifidum. The MIC values for gram negative and gram positive indi- cated that gram positive and negative bacillus were more sensitive to bifidoba cterium antimicrobial compound than gram positive cocci. 5. Conclusion The objectives of this study showed that about 13% of Table 2. MIC of antimicrobial compound obtained from B. bifidum and B. adolescentis against pathogenic bacteria using E. test. Bacteria MIC (µg·mL−1) B. bifidum B. adolescentis Salmonella typhi 200 200 Shiglla dysentriae 200 200 Bacillus cereus 120 140 Staphylococcus aureus180 220 healthy peoples in Ahvaz city—Iran can be supported from rectal pathogens by Bifidobacterium probiotics but others are at risk of being attacked by harmful microbes. The food containing probiotics may be colonized by the Bifidobacterium and Lactobacillus species in the rectum through oral—fecal track. REFERENCES [1] B. Biavati, M. Vescovo, S. Torriani and V. Bottazzi, “Bi- fidobacteria History, Ecology, Physiology and Applica- tions,” Annals of Microbiology, Vol. 50, No. 2, 2000, pp. 117-131. [2] E. S. Klaassens, W. M. de Vos and E. E. Vaughan, “Mo- lecular Approaches to Assess Activity and Functionality of Commensal and Ingested Bifidobacteria in the Human Intestinal Tract,” In: M. Saarela, Ed., Functional Dairy Products (Volume 2), Woodhead Publishing Limited, Cambridge, 2007, 153-159. [3] S. Anuradha and K. Rajeshwan, “Probiotics in Health and Disease,” JIACM, Vol. 6, No. 1, 2005, pp. 67-72. [4] S. Salminen, “Human Studies on Probiotics: Aspects of Scientific Documentation,” Scandinavian Journal of Nu- trition, Vol. 45, 2001, pp. 8-14. [5] K. Harish and T. Varghese, “Probiotics in Humans— Evidence Based Review,” Calicut Medical Journal, Vol. 4, No. 4, 2006, p. e3. [6] M.-T. Liong, “Roles of Probiotics and Prebiotics in Colon Cancer Prevention: Postulated Mechanisms and In-Vivo Evidence,” International Journal of Molecular Sciences, Vol. 9, No. 5, 2008, pp. 854-863. doi:10.3390/ijms9050854 [7] G. Marelli, E. Papaleo and A. Ferrari, “Lactobacilli for Prevention of Urogenital Infection: A Review,” European Review for Medical and Pharmacological Sciences, Vol. 8, No. 2, 2004, pp. 87-95. [8] M. E. Falagas, G. I. Betsi and S. Athanasiou, “Prebiotics for Prevention of Recurrent Vulvovaginal Candidiasis: A Review,” Journal of Antimicrobial Chemotherapy, Vol. 58, No. 2, 2006, pp. 266-272. doi:10.1093/jac/dkl246 [9] B. Biavati, M. Vescovo, S. Torriani and V. Bottazzi, “Bi- fidobacteria: History, Ecology, Physiology and Applica- tions,” Annals of Microbiology, Vol. 50, No. 2, 2000, pp. 117-131. Copyright © 2013 SciRes. AID  Identification of Bifidobacterium animalis, Bifidobacterium adolescentis and Bifidobacterium bifidum from Stool of Children and Detection of Their Antibacterial Properties Copyright © 2013 SciRes. AID 204 [10] E. V. Mahmoud, L. O. Svensson, S. E. Olsson and A. Mardh, “Antichlamydial Activity of Vaginal Secretion,” American Journal of Obstetrics & Gynecology, Vol. 172, No. 4, 1995, pp. 1268-1272. doi:10.1016/0002-9378(95)91491-9 [11] S. Garg, R. A. Anderson, J. Chany, D. P. Waller, X. H. Diao, K. Vermani and L. J. Zaneveld, “Properties of a New Acid-Buffering Bioadhesive Vaginal Formulation (ACID- FORM),” Contraception, Vol. 64, No. 1, 2001, pp. 67-75. doi:10.1016/S0010-7824(01)00217-7 [12] W. M. De Vos and J. Hugenholtz, “Engineering Meta- bolic Highways in Lactococci and Other Lactic Acid Bacteria,” Trends in Biotechnology, Vol. 22, No. 2, 2004, pp. 72-79. doi:10.1016/j.tibtech.2003.11.011 [13] B. A. Forbes, D. F. Sahm and A. S. Welssfeld, “Baily and Scotts Diagnostic Microbiology,” 12th Edition, Mosby Inc., St. Louis, 2007, pp. 109-214. [14] D. Roy and S. Sirois, “Molecular Differentiation of Bifi- dobacterium Species with Amplified Ribosomal DNA Restriction Analysis and Alignment of Short Regions, of the ldh Gene,” FEMS Microbiology Letters, Vol. 181, No. 1, 2000, pp. 17-24. [15] T. Matsuki, K. Watanabe, R. Tanaka and H. Oyaizu, “Rapid Identification of Human Intestinal Bifidobacteria by 16S rRNA-Targeted Species- and Group-Specific Primers,” FEMS Microbiology Letters, Vol. 167, No. 2, 1998, pp. 113-121. doi:10.1111/j.1574-6968.1998.tb13216.x [16] R. F. Wang, W. W. Cao and C. E. Cerniglia, “PCR De- tection and Quantitation of Predominant Anaerobic Bac- teria in Human and Animal Fecal Samples,” Applied and Environmental Microbiology, Vol. 62, No. 4, 1996, pp. 1242-1247. [17] M. Amin and B. P. Kapadnis, “Heat Stable Antimicrobial Activity of Allium ascalonicum against Bacteria and Fungi,” Indian Journal of Experimental Biology, Vol. 43, No. 8, 2005, pp. 751-754. [18] I. V. Chervinec, V. M. Bondarenco, H. A. Shabanova, A. M. Samoukina and V. N. Chervinec, “Bacteriocinogenic High Antagonistic Strains of Lactobacillus,” Zhurnal Mikrobiologii Epidemiologii i Immunobiologii, Vol. 7, 2006, pp. 79-81. [19] E. Kheadr, N. Dabour, C. Le Lay, C. Lacroix and I. Fliss, “Antibiotic Susceptibility Profile of Bifidobacteria as Af- fected by Oxgall, Acid, and Hydrogen Peroxide Stress,” Antimicrobial Agents and Chemotherapy, Vol. 51, No. 1, 2007, pp. 169-174. doi:10.1128/AAC.00261-06 [20] M. Shuhaimi, A. M. Yazid, A. M. Ali, M. H. Ghazali, H. Zaitum and N. A. Nur Atiqah, “Antibacterial Activity, Antimicrobial Susceptibility and Adherence Properties of Bifidobacterium infantis G4,” Pakistan Journal of Bio- logical Sciences, Vol. 2, No. 4, 1999, pp. 1231-1235. doi:10.3923/pjbs.1999.1231.1235 [21] S. Kozhakhmetov, S. S. Oralbayeva, A. R. Kushugulova, K. Almagambetov, A. K. Abzhalelov and E. M. Raman- kulov, “Creation of the Probiotic Consortium on the Base of Strains of Bifidobacterium spp.,” Malaysian Journal of Microbiology, Vol. 5, No. 2, 2009, pp. 67-72. [22] S. A. Ibrahim, S. R. Dharmavaram, C. W. Seo and G. Shahbazi, “Antimicrobial Activity of Bifidobacterium Longum (NCFB 2259) as Influenced by Spices Internet,” Journal of Food Safety, Vol. 2, 2001, pp. 6-8.
|