 Journal of Cancer Therapy, 2013, 4, 1262-1271 http://dx.doi.org/10.4236/jct.2013.47149 Published Online September 2013 (http://www.scirp.org/journal/jct) Controlled Local Hyperthermia and Magnetic Hyperthermia of Surface (Skin) Cancer Diseases Zviad Kovziridze*, Paata Khorava, Nunu Mitskevich Department of Chemical and Biological Technology, Technical University of Georgia, Tbilisi, Georgia. Email: *kowsiri@gtu.ge Received May 23rd, 2013; revised June 25th, 2013; accepted July 1st, 2013 Copyright © 2013 Zviad Kovziridze et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Average size of hematite and magnetite micro and nanopowders and polydispersity index, zeta potential and distribu- tion of particles were studied. Analysis showed that averag e size of th e obtained particles for magnetite is 740 .9 nm, for hematite particles 30 - 35 nm. Alternate current feed source was created for hyperthermia. Proceeding from the re- quirements of the objectives, the U type MnZn material magneto conductors were selected, in which 10.0 and 8.0 mm width gaps were cut and glass test tubes with magnetite or hematite suspensions were placed in them. Series of experi- ments at various field intensity and frequencies showed that for efficient magnetic hyperthermia therapy more powerful device was needed with frequency of up to 10 Mega Hertz to achieve the temperature 43˚C - 45˚C necessary for full activation of Neel and Brown mechanisms in particles. At the next stage, on the basis of experimental material the anti- cancer mono-therapeutic effect of hyperthermia and its adjuvant action in poly chemotherapeutic treatment was pre- sented by the use of a device created by us “Lezi”. As a result of the experiment it was shown that in all animals (out- bred albino mice, 3 month s old) inhibition of can cer growth was fixed and intratumoral necro sis was developed, while after 7 and 10 sessions tumors were ulcerated, which refers to positive effect of the experiment (Conclusion of Patho- logicanatomical Laboratory “PATGEO”, Tbilisi, Georgia ). Keywords: Magnetic Hyperthermia; Nanopowder; Malignant Cancer; Necrosis; Ulceration; Controlled Local Hyper Thermia 1. Introduction It is known that malignant cancers consist of cancer cells, which differ from normal ones by uncontrolled and un- limited propagation and growth of cells. Therefore inten- sity of metabolic processes in malignancies and, corre- spondingly, energetic requirements are higher than in common healthy tissues. Taking into consideration this factor, it is perspective to use chemical and biophysical effects on cancer tissues and its neighboring tissues, which in definite time period will exhaust energetic po- tential of degenerated cells, result in denaturation (death) of their proteins, preserve viability of healthy cells. Such biophysical impact might be the local hyper- thermia (43˚C - 45˚C). Cancer cells die at about 43˚C, since delivery of oxy- gen via blood vessels is insufficient, while normal cells are not injured even at higher temperature. Alongside with it, cancer is heated easier th an nor mal tissues aro und it, since blood vessels and nervous systems are less de- veloped in cancer [1-3]. Ceramic Microspheres for Cancer Radiotherapy Y2O3-Al2O3-SiO2 Glass Microspheres In 1987 Hyatt and Day [4] and Erbe and Day [5] proved for the first time that it was possible to use 17Y2O3-19Al2O3-64 SiO2 (mol%) 20 - 30 mcm diameter glass microspheres for in situ irradiation of cancer. In this glass Yttrium-89 (89Y) is nonradioactive isotope, which is found in nature at 100%, but neutron irradiation results in activation of 89Y, which results in creation of β-irradiating 90Y, half-life of which equals to 64.1 hr. When these 20 - 30 mcm diameter radioactive glass mi- crospheres are injected into organism (e.g. liver cancer) they fall into narrow blood vessels of cancer and block delivery of nutrients. Alongside with it, it gives high- ionized β-rays acting at short distances. β-ray does not affect other chemical elements and it has short, 2.5 mm penetration range into live tissue and thus it is not dan- gerous for healthy tissues. These microspheres are char- *Corresponding author. Copyright © 2013 SciRes. JCT  Controlled Local Hyperthermia and Magnetic Hyperthermia of Surface (Skin) Cancer Diseases 1263 acterized by high chemical durability and therefore the radioactive 90Y microsphere stays in diseased body and doesn’t affect surrounding healthy tissues. Radioactivity of 90Y at neutron irradiation [6] decreases to insignificant level in 21 days; therefore microspheres lose activity after treatment of cancer. They are used already clinically for liver cancer therapy in Canada, USA and China. They are used in clinical experiments for treatment of affected kidney and spleen and in cinoectomy irradiation of ar- thritis joints [7-20]. Ceramic Microspheres for Cancer Hyperthermia, Ferromagnetic Glass Ceramics Currently lithium ferrite (LiFe5O8) containing glass ce- ramic in hematite (α-F2O3) bio-compatible matrix and SiO2-P2O5 glass phase [21-27], magnetite (F3O4) in β- volastonite (β-CaSiO3) matrix and CaO-SiO2-B2O3-P2O5 glass phase [28-35], α-Fe2O3 [36], in Fe3O4 B 2O3-free CaO-SiO2-P2O5 glass phase [37] and zinc-iron ferrite in CaO-SiO2 glass phase [38] are developed as thermo grain to be used in hyperthermia. Thus, e.g. glass ceramic that contains F3O4 in β-CaSiO3 matrix and CaO-SiO2-B2O3-P2O5 glass phase was efficient [29-31] in destruction of cancer cells implanted in rabbit femoral bone, when it was in- troduced in the pin-form into brain channel and [36] was placed in alternate magnetic field [33]. But such glass- ceramic pins can’t be used clinically, since cancer cells can be scattered around. Normal cells and injection of glass-ceramic pins can result in cancer metastasis. 20 - 30 mcm diameter ferromagnetic microspheres might be used for local heating of cancer through loss of hysteresis by ferromagnetic materials, without initiation of cancer metastasis. Microspheres can be introduced into cancer via blood vessels [39] and then placed in alternate mag- netic field. But up to now, 20 - 30 mcm size microspheres are not obtained and they have not revealed high heat formation capacity. Currently precise mechanism of hy- perthermia for cancer therapy is not known. Unknown is the size of magnetite or hematite particles too. There are no data in special references about it. Index of morbidity and lethality conditioned by melig- nancies in the whole world is increasing permanently. Early diagnostics is rather difficult and majority of pa- tients address hospitals because of generalized cancers, when they need combined surgery, radiation and drug therapy and complex treatment. Number of patients who address physician-oncologists with manifestation of cli- nical signs and various metabolic derangements inherent to complicated cancer processes has increased. Development of new methods of treatment of melig- nancies is the most urgent task of oncology Inculcation of drugs and methods of treatment possessing positive ef- fects which are proved by experimental and clinical stud- ies into clinical practice are forward steps in the sphere of treatment of oncologic patients. 2. Main Part 2.1. Goal and Objectives of Hyperthermal Studies The present research pursues improvement of modern and recent results in the sphere of treatment of patients suffering from cancer, by the application of hyperthermia on cancer formation. To achieve this goal we plan to resolve the following objectives: 1) Study of anticancer therapeutic effect on experi- mental cancers; 2) Determination of adjuvant anticancer effect of hy- perthermia in experiment, in combination with polyche- motherapy; 3) Study of various regimes of hyperthermia consider- ing immediate and recent results. 2.2. Experimental (Stage I) To implement the first stage works, first of all, we’ll take X-ray of the used magnetite powder and hematite nano- particles obtained on the rotation kathode device created by us (Patent, receiving method, registration number 11731, 15.03.2010. Georgian National Patent Center “SAQPATENTI”); Figures 1 and 2. As is seen from the X-ray analysis magnetite consists mainly of Fe3O4, dhkl = 2954; 1520; 2095; 1710; 1612; 1483 Å, hematite dhkl = 2690; 2520; 2422; 1710; 1694; 1600 Å, traces of CaCO3 d hkl = 3030; 1910; 1873 and traces of SiO2, but the main mass is magnetite, therefore powder is of dark black color. The obtained hematite powder is red, and the mass completely consists of α-Fe2O3. With “Nanophox” device accumulation curve of hematite particle distribution and particle density-normal Gauss distribution was deter- mined (Figures 3 and 4). From Figures is shown, that in powder the particles (agglomerate ) with sizes till 300 nm are 62% - 63%. The “Nanophox” device fixd also the ag- glomerated nanopowder. Figure 5 shows graphical rep- resentation of analysis of hematite powder stability (Pa- tent, receiving method-registration number 11731, 15.03. 2010. Georgian National Patent Center “SAQPATENTI”) Figure 6 shows scanning electron-microscopy represen- tation of hematite agglomeration. Average size of the particle 30 - 35 nm. Through sedimentation we have re- ceived the pa r ticle si ze wi th 30 - 35 nm. After phase analysis the physical properties of mag- netite powder were studied on the apparatus “Nano sizer” of Great Britain origin. Powder was screened through # 0063-8270 mesh sieve in advance. Intensity on the offered Figures is that of the transmit- ted laser ray, Width is a peak width and shows the parti- cle distribution according to dimensions. The narrower a peak, the more homogeneous is spreading. Particle characterization is based on the average size Copyright © 2013 SciRes. JCT 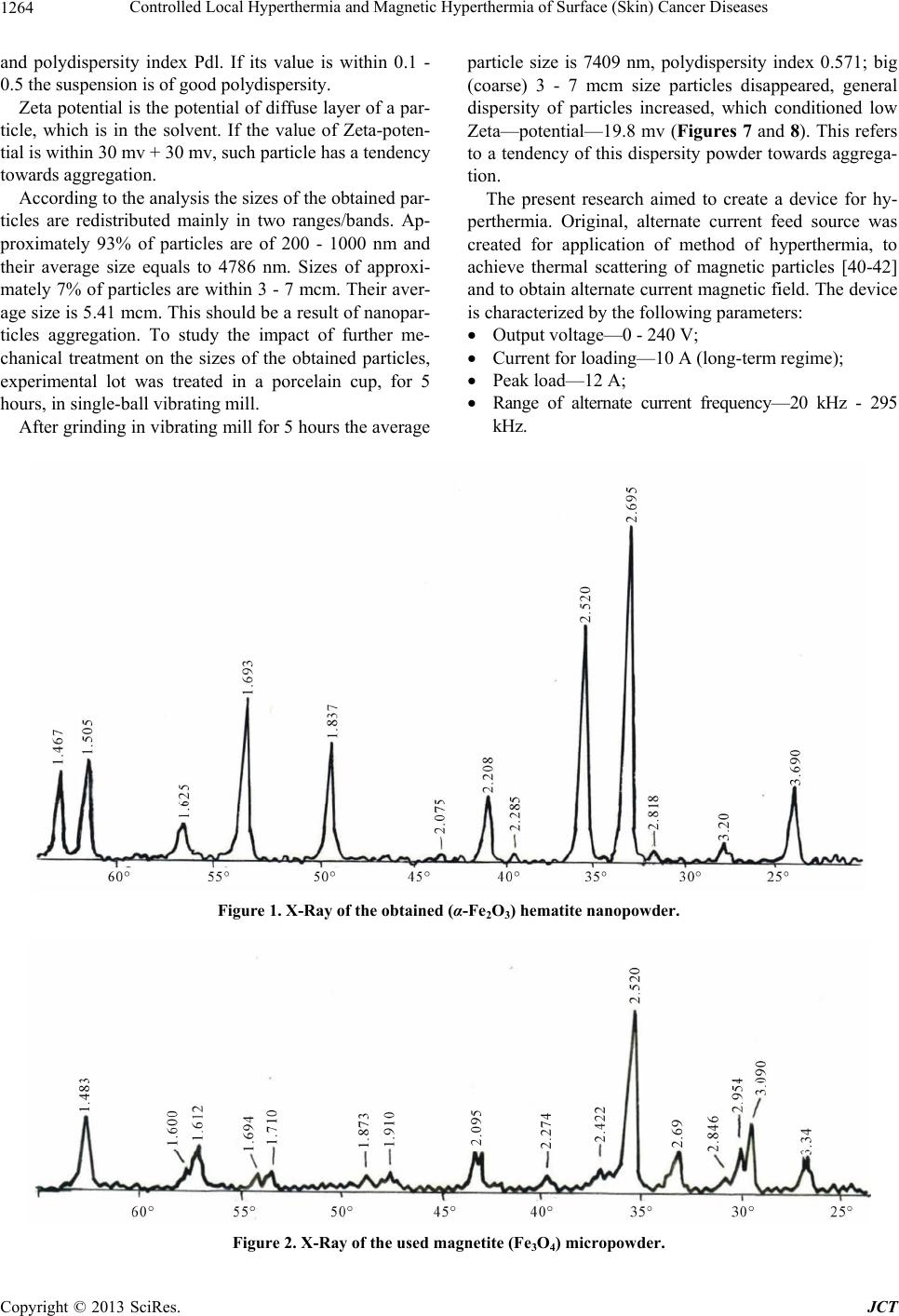 Controlled Local Hyperthermia and Magnetic Hyperthermia of Surface (Skin) Cancer Diseases Copyright © 2013 SciRes. JCT 1264 and polydispersity index Pdl. If its value is within 0.1 - 0.5 the suspension is of good polydispersity. Zeta potential is the poten tial of diffuse layer of a par- ticle, which is in the solvent. If the value of Zeta-poten- tial is within 30 mv + 30 mv, such particle has a tendency towards aggregation. According to the analysis the sizes of the obtained par- ticles are redistributed mainly in two ranges/bands. Ap- proximately 93% of particles are of 200 - 1000 nm and their average size equals to 4786 nm. Sizes of approxi- mately 7% of particles are within 3 - 7 mcm. Their aver- age size is 5.41 mcm. This should be a result of nanopar- ticles aggregation. To study the impact of further me- chanical treatment on the sizes of the obtained particles, experimental lot was treated in a porcelain cup, for 5 hours, in single-ball vibrating mill. After grinding in vibrating mill for 5 hours the average particle size is 7409 nm, polydispersity index 0.571; big (coarse) 3 - 7 mcm size particles disappeared, general dispersity of particles increased, which conditioned low Zeta—potential—19.8 mv (Figures 7 and 8). This refers to a tendency of this dispersity powder towards aggrega- tion. The present research aimed to create a device for hy- perthermia. Original, alternate current feed source was created for application of method of hyperthermia, to achieve thermal scattering of magnetic particles [40-42] and to obtain alternate current magnetic field. The device is characterized by the following parameters: Output voltage—0 - 240 V; Current for loading—10 A (long-term regime); Peak load—12 A; Range of alternate current frequency—20 kHz - 295 kHz. Figure 1. X-Ray of the obtained (α-Fe2O3) hematite nanopowder. Figure 2. X-Ray of the used magnetite (Fe3O4) micropowder. 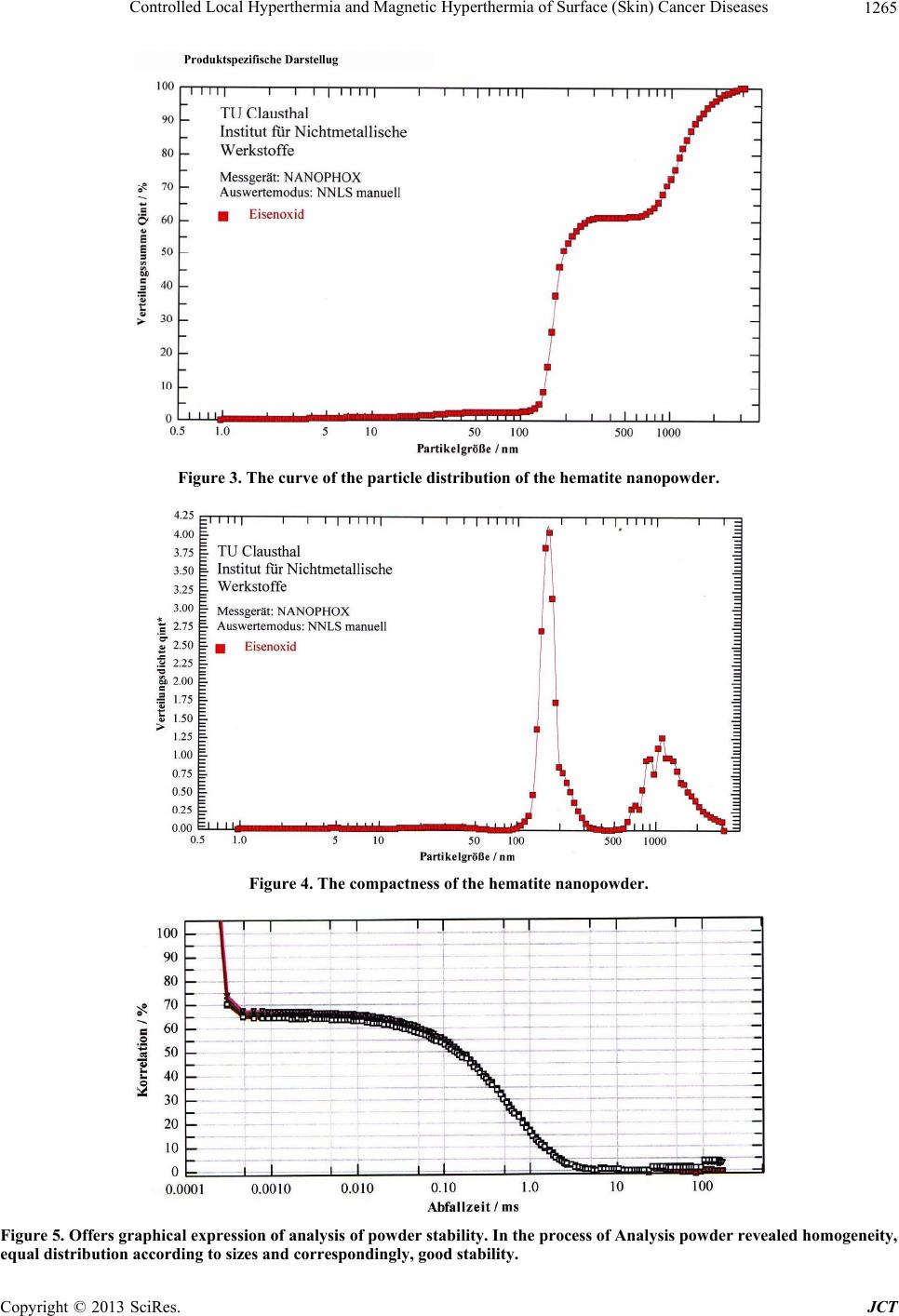 Controlled Local Hyperthermia and Magnetic Hyperthermia of Surface (Skin) Cancer Diseases 1265 Figure 3. The curve of the particle distribution of the hematite nanopowder. Figure 4. The compactness of the hematite nanopowder. Figure 5. Offers graphical expression of analysis of powder stability. In the process of Analysis powder revealed homogeneity, equal distribution according to sizes and correspondingly, good stability. Copyright © 2013 SciRes. JCT 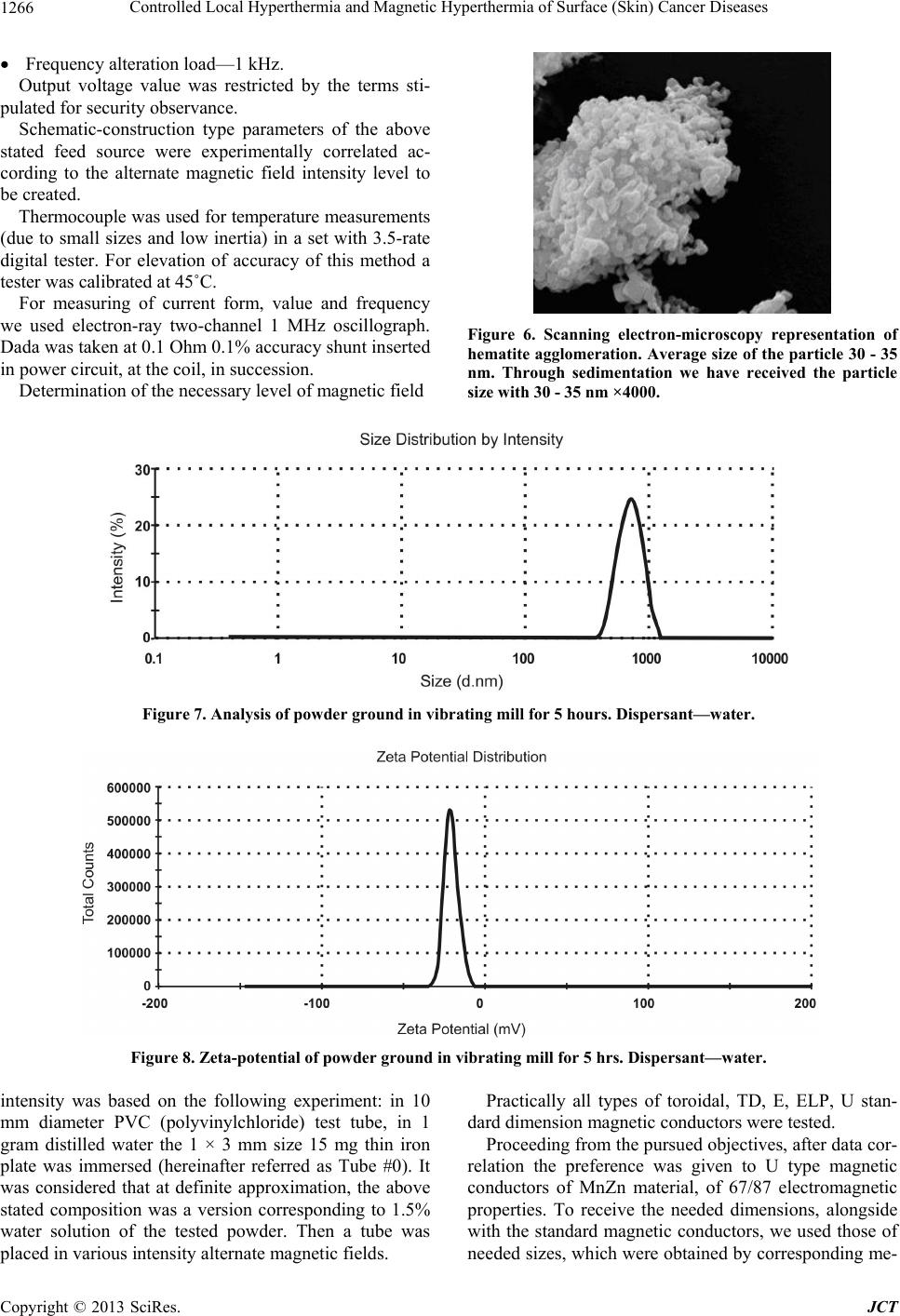 Controlled Local Hyperthermia and Magnetic Hyperthermia of Surface (Skin) Cancer Diseases Copyright © 2013 SciRes. JCT 1266 Frequency alteration load—1 kHz. Output voltage value was restricted by the terms sti- pulated for security observance. Schematic-construction type parameters of the above stated feed source were experimentally correlated ac- cording to the alternate magnetic field intensity level to be created. Thermocouple was used for temperature measurements (due to small sizes and low inertia) in a set with 3.5-rate digital tester. For elevation of accuracy of this method a tester was calibrated at 45˚C. For measuring of current form, value and frequency we used electron-ray two-channel 1 MHz oscillograph. Dada was taken at 0.1 Ohm 0.1% accuracy shunt inserted in power circuit, at the coil, in succession. Figure 6. Scanning electron-microscopy representation of hematite agglomeration. Average size of the particle 30 - 35 nm. Through sedimentation we have received the particle ize with 30 - 35 nm ×4000. Determination of the necessary level of magnetic field s Figure 7. Analysis of powder ground in vibrating mill for 5 hours. Dispersant—w a ter . Figure 8. Zeta-potential of powder ground in vibrating mill for 5 hrs. Dispersant—w ater . intensity was based on the following experiment: in 10 mm diameter PVC (polyvinylchloride) test tube, in 1 gram distilled water the 1 × 3 mm size 15 mg thin iron plate was immersed (hereinafter referred as Tube #0). It was considered that at definite approximation, the above stated composition was a version corresponding to 1.5% water solution of the tested powder. Then a tube was placed in various intensity alternate magnetic fields. Practically all types of toroidal, TD, E, ELP, U stan- dard dimension magnetic conductors were tested. Proceeding from the pursued objectives, after data cor- relation the preference was given to U type magnetic conductors of MnZn material, of 67/87 electromagnetic properties. To receive the needed dimensions, alongside with the standard magnetic conductors, we used those of needed sizes, which were obtained by corresponding me-  Controlled Local Hyperthermia and Magnetic Hyperthermia of Surface (Skin) Cancer Diseases 1267 chanical treatment of ETD34, E42 and E52 standard magnetic conductors. In the bundle of magnetic conductors various size gaps were cut: 20 mm, 16 mm, 12 mm, 10 mm and 8 mm. On the basis of experience acquired in the process of experi- ments 10.0 mm and 8.0 mm width gaps were given pref- erence as working ones. Similarly, optimal dimensions of magnetic conductors in millimeters were selected: 1) 50 × 50 × 11; 2) 60 ×50 × 12; 3) 40 × 40—area of magnetic conductor section 32 mm2; 4) 40 × 30—area of magnetic conductor section 32 mm2. To cover the whole range of 20 kHz - 295 kHz fre- quency electric coils were prepared for every magnetic conductor: 1) 42 windings—2.0 mm transform er copper conductor; 2) 36 windings—2.0 mm transform er copper conductor; 3) 34 windings—2.0 mm transform er copper conductor; 4) 30 windings—2.0 mm transform er copper conductor; 5) 30 windings—3 × 0.6 mm transformer copper con- ductor; 6) 26 windings—2.0 mm transform er copper conductor; 7) 22 windings—2.0 mm transform er copper conductor; 8) 18 windings—1.5 mm2 section mounting multithr e a d copper conductor; 9) 16 windings—1.5 mm2 section mounting multithr e a d copper conductor; 10) 12 windi ngs—1.5 mm 2 section m ounting multithread copper conductor. Magnetic conductors prepared by us together with the above stated alternate current feed source enabled us to carry out sets of experiments at various field intensity and frequency. #1 and #2, that is 5% and 10% suspensions were pre- pared from hematite and magnetite, correspondingly. From 8 mm diameter glass pipe the tubes were made in which the above r eferred suspensions were pour ed. Initially experiments were conducted at the frequen- cies: 27.7 kHz, 33.3 kHz, 40.0 kHz, 50.0 kHz, 66.0 kHz, 100.0 kHz, 166.0 kHz, 200.0 kHz, 250.0 kHz and 290.0 kHz. By variation of voltage coming from power source we fixed 4.0. 5.0, 8.0, 9.0 and 10.0 A current in electric coils. Temperature monitoring was performed in 1 min, 5 min, 10 min and 15 min intervals. Expediency of continuation of experiments in alternate magnetic field of lower than 8.0 A was practically excluded. Similarly was excluded application of gaps width of which exceeded 10 mm. Magnetic field intensity in 8 mm and 10 mm gaps for magnetic conductors of the above frequencies were ob- tained: 4200 A/winding, 4000 A/winding 3800 A/wind- ing, 3400 A/winding, 3200 A/winding, 3000 A/winding, 2800 A/winding, 2600 A/winding. The first series of experiments showed that tempera- ture of solutions in tubes placed in magnetic field com- pared to the environment temperature used to increase within 15 minutes only by 8.0˚ - 10.0˚, inclusive 6˚ - 7˚, within the first 5 minutes. Tube #0, when placed in ana- logous intensity field within the first 5 minutes yielded 25˚ - 30˚ increase, inclusive 15˚ - 20˚ in the first minute. Intensity of increase of temperature is directly propor- tional to magnetic field intensity. In the process of ex- periments, in gaps, at high magnetic field intensity, sig- nificant overheating of magnetic conductors and power winding was observed. Acco rding to measurements, tem- perature on the surface of magnetic conductors at gaps used to increase up to 50˚C - 70˚C during experiments. Therefore, the impact on tubes would have been signifi- cant too. A series of experiments was carried out that showed that increase of temperature by 6˚C - 7˚C in 5 min period in tubes was conditioned by thermal effect of magnetic conductor in g ap s . As it was shown by the experiments, more powerful device of 5 - 10 mega Herz is needed for efficient treat- ment by magnetic hyperthermia to enable thorough acti- vation of Neel and Brawn mechanisms at the impact of alternate magnetic field on micro- and nanopowders ob- tained by us. The works in this direction will be contin- ued. We have developed also other method, a new device for hyperthermia therapy and further works were contin- ued at the second stage. Conditionally we called the de- vice “Lezi” (Georgian Inteligent Privecy National Center “SAQPATENTI”. Deponing Certificate 5054. Work: “Con- trol Local Hyperthermia and Magnetic Hyperthermia for Therapy of Maligna nc i es”). Antitumoral effect of hyperthermia in experimental cancers at the treatment by a device “Lezi”. 2.3. Experimental (Stage II) Scientific novelty. On the basis of experimental material the anticancer mono-therapeutic effect of hyperthermia and its adjuvant action in polychemotherapeutic treatment was presented for the first time in Georgia. With this in view rational schemes of hyperthermia were developed. 3. Materials and Methods 3 months old 18 - 20 g albino mice (outbred, non-linear) were used in experiments. After selection for experiments, the mice were kept in vivarium for 10 - 14 days at quarantine regime, accord- ing to sex. Individual protocols were executed for each animal. Animals were kept at similar feeding and care conditions. Copyright © 2013 SciRes. JCT  Controlled Local Hyperthermia and Magnetic Hyperthermia of Surface (Skin) Cancer Diseases Copyright © 2013 SciRes. JCT 1268 Experiments were carried out by the use of cancer strain of Erlich adenocarcinoma. Inoculation of Erlich adenocarcinoma was performed in mices, subcutaneously, in infrascapular region. was observed, the so called “intratumoral necrosis” was developed in cancers—necrosis of cancer cells. This, according to our opinion is conditioned by the effect of hyperthermia. Experiments were carried out by the methods widely applied in experimental oncology. The anticancer effect of hyperthermia was evaluated according to cancer gro wth inhibition, frequency of intratumoral necrosis and chang es in the data of animal life prolongation. In the second experimental group we again studied an- ticancer therapeutic effect of hyperthermia. On the first day of the experiment, on 03.01.2012, subcutaneous in- oculation of EAT cancer strain was performed. Cancer developed in all three experimental animals. The results will be processed by variation statistics methods. 13.01.2012, we measured animal cancers (see Table #2 and Figure 9). Measurements were performed after every 3 sessions. On 13.01.2012 the first session of hy- perthermia was carried out. These sessions were carried out every second day. A device was placed on cancer for- mation; at the ends of a device 43˚C - 45˚C was fixed. Length of hyperthermia manipulation equaled to 30, 40 and 50 min, correspondingly, on I, II and III animals. The first mouse was two-humped. Treatment was per- formed on the right hump. After 3 sessions of the ex- periment it was found that in all three animals inhibition (stopping) of cancer growth was fixed, while the II and III animals revealed development of intratumoral necro- sis in cancers (Figure 10). In this case, again, inhibition of cancer growth and intratumoral necrosis were condi- tioned by the effect of hyperthermia. 4. Obtained Results and Discussion Anticancer hyperthermic therapeutic effect of hyperther- mia In the I group of mice we studied anticancer therapeu- tic effect of hyperthermia. On the first day of the experi- ment, on 18.11.2011, subcutaneous inoculation of EAT cancer strain was performed. Cancer was developed in all experimental animals. On 28.11.2011 we measured cancers in mice (see Ta- ble 1). Measurements were made once in three days, while on 01.12.2011 the first session of hyperthermia was performed. Such sessions lasted till 11.12.2011, includ- ing this day. On a cancer formation we placed our device, at the ends of which 43˚C - 45˚C was fixed. Length of hyperthermia manipulation equaled to 5 - 5 minutes. In these animals measurements were made after seven sessions. According to Table 2 and visually necrosis and ulceration are observed, which refers to positive effect of the experiment. After ten sessions, again vivid necrosis and ulceration of the tumor was observed, see Figure 11. Experiments fixed inhibition of cancer growth in the I, III and V animals, while in the II, IV and VI animals, where progressive growth of a size of cancer formation Table 1. Number of sessions and date of measuring size. 28.11.11 01.12.11 04.12.11 07.12.11 11.12.11 14.12.11 17.12.11 1 3 × 3 × 3 mm 5 × 3 × 3 mm 5 × 3 × 3 mm 5 × 3 × 3 mm 5 × 3 × 3 mm 5 × 3 × 3 mm 5 × 3 × 3 mm 2 13 × 10 × 5 mm 16 × 12 × 8 mm 18 × 12 × 10 (ulceration) 1 9 × 13 × 8 mm20 × 14 × 10 mm23 × 16 × 10 mm 25 × 16 × 10 mm 3 8 × 5 × 5 mm 10 × 8 × 5 mm 11 × 8 × 5 mm 11 × 8 × 5 mm11 × 8 × 5 mm12 × 10 × 5 mm 12 × 10 × 5 mm 4 12 × 10 × 5 mm 14 × 10 × 8 mm 16 × 12 × 10 mm (ulceration)18 × 13 × 10 mm20 × 15 × 10 mm22 × 17 × 10 mm 24 × 18 × 10 mm 5 8 × 5 × 5 mm 10 × 8 × 5 mm 12 × 8 × 5 mm 12 × 8 × 5 mm12 × 8 × 5 mm12 × 10 × 5 mm 12 × 10 × 8 mm 6 12 × 8 × 8 mm 13 × 10 × 8 mm 15 × 10 × 8 mm (ulceration)16 × 10 × 8 mm18 × 10 × 8 mm19 × 10 × 8 mm 21 × 10 × 8 mm (#1) (#2) (#3) Figure 9. # 1 (two humped), #2 and #3 anima l s after one ses sion of treatment . 1 3.01.20 12. 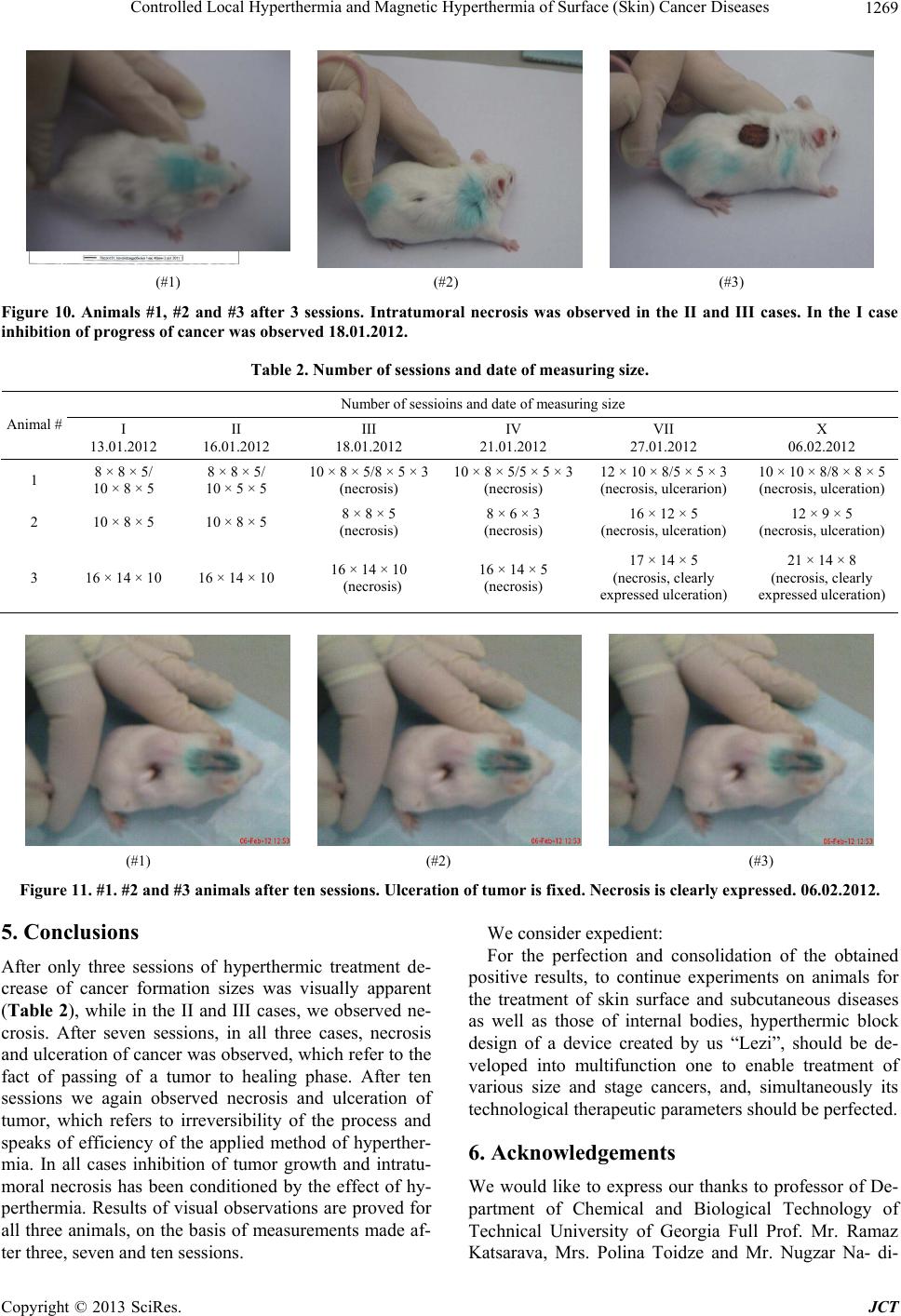 Controlled Local Hyperthermia and Magnetic Hyperthermia of Surface (Skin) Cancer Diseases 1269 (#1) (#2) (#3) Figure 10. Animals #1, #2 and #3 after 3 sessions. Intratumoral necrosis was observed in the II and III cases. In the I case inhibition of progress of cancer was observed 18.01.2012. Table 2. Number of sessions and date of measuring size. Number of sessioins and date of measuring size Animal # I 13.01.2012 II 16.01.2012 III 18.01.2012 IV 21.01.2012 VII 27.01.2012 X 06.02.2012 1 8 × 8 × 5/ 10 × 8 × 5 8 × 8 × 5/ 10 × 5 × 5 10 × 8 × 5/8 × 5 × 3 (necrosis) 10 × 8 × 5/5 × 5 × 3 (necrosis) 12 × 10 × 8/5 × 5 × 3 (necrosis, ulcerarion) 10 × 10 × 8/8 × 8 × 5 (necrosis, ulceration) 2 10 × 8 × 5 10 × 8 × 5 8 × 8 × 5 (necrosis) 8 × 6 × 3 (necrosis) 16 × 12 × 5 (necrosis, ulceration) 12 × 9 × 5 (necrosis, ulceration) 3 16 × 14 × 10 16 × 14 × 10 16 × 14 × 10 (necrosis) 16 × 14 × 5 (necrosis) 17 × 14 × 5 (necrosis, clearly expressed ulceration) 21 × 14 × 8 (necrosis, clearly expressed ulceration) (#1) (#2) (#3) Figure 11. #1. #2 and #3 animals after ten sessions. Ulceration of tumor is fixed. Necrosis is clearly expressed. 06.02.2012. 5. Conclusions After only three sessions of hyperthermic treatment de- crease of cancer formation sizes was visually apparent (Table 2), while in the II and III cases, we observed ne- crosis. After seven sessions, in all three cases, necrosis and ulceration of cancer was observed, which refer to the fact of passing of a tumor to healing phase. After ten sessions we again observed necrosis and ulceration of tumor, which refers to irreversibility of the process and speaks of efficiency of the applied method of hyperther- mia. In all cases inhibition of tumor growth and intratu- moral necrosis has been conditioned by the effect of hy- perthermia. Results of visual observations are proved for all three animals, on the basis of measurements made af- ter three, seven and ten sessions. We consider expedient: For the perfection and consolidation of the obtained positive results, to continue experiments on animals for the treatment of skin surface and subcutaneous diseases as well as those of internal bodies, hyperthermic block design of a device created by us “Lezi”, should be de- veloped into multifunction one to enable treatment of various size and stage cancers, and, simultaneously its technological therapeutic parameters should be perfected. 6. Acknowledgements We would like to express our thanks to professor of De- partment of Chemical and Biological Technology of Technical University of Georgia Full Prof. Mr. Ramaz Katsarava, Mrs. Polina Toidze and Mr. Nugzar Na- di- Copyright © 2013 SciRes. JCT  Controlled Local Hyperthermia and Magnetic Hyperthermia of Surface (Skin) Cancer Diseases 1270 rashvili for delivery of necessary material, for interest- ing research and support. The authors thank Prof. Dr. Jurgen Heinrich at the Institute of Non metallic Materials, Clausthal Technical University, Germany, for His sup- port during fulfilling the experimental works. We thank Powder Hause of Clausthal-Zellerfeld, Germany, for per- formed interesting research and assistance. REFERENCES [1] R. Cavaliere, E. C. Ciocatto, B. C. Giovanella, C. Hei- delberger, R. O. Johnson, M. Margottini, B. Mondovi, G. Moricca and A. Rossi-Fanelli, “Selective Heat Sensitivity of Cancer Cells. Biochemical and Clinical Studies,” Cancer, Vol. 20, No. 9, 1967, pp. 1351-1381. doi:10.1002/1097-0142(196709)20:9<1351::AID-CNCR2 820200902>3.0.CO;2-# [2] K. Overgaard and J. Overgaard, “Investigation on the Possibility of a Thermic Tumour Therapy. II. Action of Combined Heat-Roentgen Treatment on a Transplanted Mouse Mammary Carcinoma,” European Journal of Cancer, Vol. 8, 1972, pp. 573-575. doi:10.1016/0014-2964(72)90111-9 [3] J. Overgaard, “Effect of Hyperthermia on Malignant Cells in Vivo. A Review and a Hypothesis,” Cancer, Vol. 39, 1977, pp. 2637-2646. doi:10.1002/1097-0142(197706)39:6<2637::AID-CNCR2 820390650>3.0.CO;2-S [4] M. J. Hyatt and D. E. Day, “Glass Properties of Yttria- Alumina-Silica System,” Journal of the American Ce- ramic Society, Vol. 70, No. 10, 1987, pp. 283-287. doi:10.1111/j.1151-2916.1987.tb04901.x [5] E. M. Erbe and D. E. Day, “Chemical Durability of Y2O3- Al2O3-SiO2 Glasses for the in Vivo Delivery of Beta Ra- diation,” Journal of Biomedical Materials Research, Vol. 27, No. 10, 1993, pp. 1301-1308. doi:10.1002/jbm.820271010 [6] D. E. Day and T. E. Day, “Radiotherapy Glasses,” In: L. L. Hench and J. Wilson, Eds., An Introduction to Bioce- ramics, World Science, Singapore, 1993, pp. 305-317. doi:10.1142/9789814317351_0017 [7] G. J. Ehrhardt and D. E. Day, “Therapeutic Use of 90Y Microspheres,” Journal of Nuclear Medicine, Vol. 14, 1987, pp. 233-242. [8] R. V. Mantravadi, D. G. Spigos, W. S. Tan and E. L. Felix, “Intraarterial Yttrium 90 in the Treatment of He- patic Malignancy,” Radiology, Vol. 142, No. 3, 1982, pp. 783-786. [9] M. J. Herba, F. F. Illescas, M. P. Thirlwell, G. J. Boos, L. Rosenthall, M. Atri and P. M. Bret, “Hepatic Malignan- cies: Improved Treatment with Intraarterial Y-90,” Radi- ology, Vol. 169, No. 2, 1988, pp. 311-314. [10] I. Wollner, C. Knutsen, P. Smith, D. Prieskorn, C. Chrisp, J. Andrews, J. Juni, S. Warber, J. Klevering, J. Crudup and W. Ensminger, “Effects of Hepatic Arterial Yttrium 90 Glass Microspheres in Dogs,” Cancer, Vol. 61, No. 7, 1988, pp. 1336-1344. doi:10.1002/1097-0142(19880401)61:7<1336::AID-CNC R2820610711>3.0.CO;2-K [11] S. Houle, T. K. Yip, F. A. Shepherd, L. E. Rotstein, K. W. Sniderman, E. Theis, R. H. Cawthorn and K. Richmond- Cox, “Hepatocellular Carcinoma: Pilot Trial of Trea tment with Y-90 Microspheres,” Radiology, Vol. 172, No. 3, 1989, pp. 857-860. [12] J. H. Anderson, J. A. Goldberg, R. G. Bessent, D. J. Kerr, J. H. McKillop, I. Stewart, T. G. Cooke and C. S. Mc- Ardle, “Glass Yttrium-90 Microspheres for Patients with Colorectal Liver Metastases,” Radiology and Oncology, Vol. 25, No. 2, 1992, pp. 137-139. doi:10.1016/0167-8140(92)90020-U [13] M. A. Burton, B. N. Gray, C. Jones and A. Coletti, “In- traoperative Dosimetry of 90Y in Liver Tissue,” Journal of Nuclear Medicine, Vol. 16, 1989, pp. 495-498. [14] F. A. Shepherd, L. E. Rotstein, S. Houle, T. C. Yip, K. Paul and K. W. Sniderman, “A Phase I Dose Escalation Trial of Yttrium-90 Microspheres in the Treatment of Primary Hepatocellular Carcinoma,” Cancer, Vol. 70, No. 9, 1992, pp. 2250-2254. doi:10.1002/1097-0142(19921101)70:9<2250::AID-CNC R2820700906>3.0.CO;2-4 [15] Z. P. Yan, G. Lin, H. Y. Zhao and Y. H. Dong, “An Ex- perimental Study and Clinical Pilot Trials on Yttrium-90 Glass Microspheres through the Hepatic Artery for Treatment of Primary Liver Cancer,” Cancer, Vol. 72, No. 11, 1993, pp. 3210-3215. doi:10.1002/1097-0142(19931201)72:11<3210::AID-CN CR2820721113>3.0.CO;2-6 [16] Z. P. Yan, G. Lin, H. Y. Zhao and Y. H. Dong, “Yttrium- 90 Glass Microspheres Injected via the Portal Vein,” Acta Radiologica, Vol. 34, No. 4, 1993, pp. 395-398. [17] J. C. Andrews, S. C. Walker, R. J. Ackermann, L. A. Cotton, W. D. Ensminger and B. Shapiro, “Hepatic Ra- dioembolization with Yttrium-90 Containing Glass Mi- crospheres: Preliminary Results and Clinical Follow-Up,” Journal of Nuclear Medicine, Vol. 35, No. 10, 1994, pp. 1637-1644. [18] J. H. Tian, B. X. Xu, J. M. Zhang, B. W. Dong, P. Liang and X. D. Wang, “Ultrasound-Guided Internal Radio- therapy Using Yttrium-90-Glass Microspheres for Liver Malignancies,” Journal of Nuclear Medicine, Vol. 37, No. 6, 1996, pp. 958-963. [19] X. Cao, N. He, J. Sun, J. Tan, C. Zhang, J. Yang, T. Lu and J. Li, “Hepatic Radioembolization with Yttrium-90 Glass Microspheres for Treatment of Primary Liver Can- cer,” Chinese Medical Journal, Vol. 112, 1999, pp. 430- 432. [20] S. D. Chen, J. F. Hsieh, S. C. Tsai, W. Y. Lin, K. Y. Cheng and S. J. Wang, “Intra-Tumoural Injection of 90Y Microspheres into an Animal Model of Hepatoma,” Nu- clear Medicine Communications, Vol. 22, No. 2, 2001, pp. 121-125. doi:10.1097/00006231-200102000-00002 [21] M. Kawashita, F. Miyaji, T. Kokubo, G. H. Takaoka, I. Yamada, Y. Suzuki and K. Kajiyama, “Phosphorus-Im- planted Glass for Radiotherapy: Effect of Implantation Energy,” Journal of the American Ceramic Society, Vol. 82, No. 3, 1999, pp. 683-688. doi:10.1111/j.1151-2916.1999.tb01817.x Copyright © 2013 SciRes. JCT  Controlled Local Hyperthermia and Magnetic Hyperthermia of Surface (Skin) Cancer Diseases Copyright © 2013 SciRes. JCT 1271 [22] M. Kawashita, R. Shineha, H.-M. Kim, T. Kokubo, Y. Inoue, N. Araki, Y. Nagata, M. Hiraoka and Y. Sawada, “Preparation of Ceramic Microspheres for in Situ Radio- therapy of Deep-Seated Cancer,” Biomaterials, Vol. 24, 2003, pp. 2955-2963. doi:10.1016/S0142-9612(03)00094-2 [23] M. Hiraoka and G. M. Hahn, “Comparison between Tu- mor pH and Cell Sensitivity to Heat in RIF-1 Tumors,” Cancer Research, Vol. 49, No. 14, 1989, pp. 3734-3736. [24] N. Araki, Y. Nagata, M. Hiraoka, M. Kawashita, T. Ko- kubo, Y. Inoue and Y. Sawada, “Treatment of VX2 Tu- mors in Rabbit Liver by Radioactive Y2O3 Microspheres,” In: Transactions of the 7th World Biomaterials Congress, Australian Society for Biomaterials Inc., Sydney, 2004. [25] N. F. Borrelli, A. A. Luderer, J. N. Panzarino and H. L. Rittler, “Magnetic Glass-Ceramics for Tumor-Therapy by Hyperthermia,” American Ceramic Society Bulletin, Vol. 61, 1982, pp. 819-819. [26] A. A. Luderer, N. F. Borrelli, J. N. Panzarino, G. R. Mansfield, D. M. Hess, J. L. Brown, E. H. Barnett and E. W. Hahn, “Glass-Ceramic-Mediated, Magnetic-Field-In- duced Localized Hyperthermia—Response of a Murine Mammary-Carcinoma,” Radiation Research, Vol. 94, 1983, pp. 190-198. doi:10.2307/3575874 [27] N. F. Borrelli, A. A. Luderer and J. N. Panzarino, “Hys- teresis Heating for the Treatment of Tumors,” Physics in Medicine and Biology, Vol. 29, No. 5, 1984, pp. 487-494. doi:10.1088/0031-9155/29/5/001 [28] Y. Ebisawa, T. Kokubo, K. Ohura and T. Yamamuro, “Bioactivity of CaO-SiO2-Based Glasses—In Vitro Eva- luation,” Journal of Materials Science: Materials in Me- dicine, Vol. 1, No. 4, 1990, pp. 239-244. doi:10.1007/BF00701083 [29] Y. Ebisawa, Y. Sugimoto, T. Hayashi, T. Kokubo, K. Ohura and T. Yamamuro, “Cry stallization of, ( Fe O,F e2O3)- CaO-SiO2 Glasses and Magnetic 182 Properties of Their Crystallized Products,” Journal of the Ceramic Society of Japan, Vol. 99, 1991, pp. 7-13. doi:10.2109/jcersj.99.7 [30] K. Ohura, M. Ikenaga, T. Nakamura, T. Yamamuro, Y. Ebisawa, T. Kokubo, Y. Kotoura and M. Oka, “A Heat- Generating Bioactive Glass-Ceramic for Hyperthermia,” Journal of Applied Biomaterials, Vol. 2, No. 3, 1991, pp. 153-159. doi:10.1002/jab.770020303 [31] T. Kokubo, Y. Ebisawa, Y. Sugimoto, M. Kiyama, K. Ohura, T. Yamamuro, M. Hiraoka and M. Abe, “Prepara- tion of Bioactive and Ferrimagnetic Glass-Ceramic for Hyperthermia,” Bioceramics, Vol. 3, 1992, pp. 213-223. [32] Y. Ebisawa, T. Kokubo, K. Ohura and T. Yamamuro, “Bioactivity of Fe2O3-Containing CaO-SiO2 Glasses— In-Vitro Evaluation,” Journal of Materials Science: Ma- terials in Medicine, Vol. 4, No. 3, 1993, pp. 225-232. doi:10.1007/BF00122273 [33] M. Ikenaga, K. Ohura, T. Yamamuro, Y. Kotoura, M. Oka and T. J. Kokubo, Journal of Orthopaedic Research, Vol. 11, 1993, p. 849. [34] Y. Ebisawa, F. Miyaji, T. Kokubo, K. Ohura and T. Na- kamura, “Surface Reaction of Bioactive and Ferrimag- netic Glass-Ceramics in the System FeO-Fe2O3-CaO- SiO2,” Journal of the Ceramic Society of Japan, Vol. 105, 1997, pp. 947-951. doi:10.2109/jcersj.105.947 [35] Y. Ebisaw, F. Miyaji, T. Kokubo, K. Ohura and T. Naka- mura, “Bioactivity of Ferrimagnetic Glass-Ceramics in the System FeO-Fe2O3-CaO-SiO2,” Biomaterials, Vol. 18, 1997, pp. 1277-1284. doi:10.1016/S0142-9612(97)00067-7 [36] H. Konaka, F. Miyaji and T. Kokubo, “Preparation and Magnetic Properties of Glass-Ceramics Containing a-Fe for Hyperthermia,” Journal of the Ceramic Society of Ja- pan, Vol. 105, 1997, pp. 833-836. doi:10.2109/jcersj.105.833 [37] M. Kawashita, H. Takaoka, T. Kokubo, T. Yao, S. Ha- mada and T. Shinjo, “Preparation of Magnetite-Contain- ing Glass-Ceramics in Controlled Atmosphere for Hy- perthermia of Cancer,” Journal of the Ceramic Society of Japan, Vol. 109, No. 1, 2001, pp. 39-44. doi:10.2109/jcersj.109.39 [38] M. Kawashita, Y. Iwahashi, T. Kokubo, T. Yao, S. Ha- mada and T. Shinjo, “Preparation of Glass-Ceramics Containing Ferrimagnetic Zinc-Iron Ferrite for the Hy- perthermal Treatment of Cancer,” Journal of the Ceramic Society of Japan, Vol. 112, No. 1307, 2004, pp. 373-379. doi:10.2109/jcersj.112.373 [39] M. Kawashita, “Ceramic Microspheres for Biomedical Applications,” International Journal of Applied Ceramic Technology, Vol. 2, No. 3, 2005, pp. 173-183. doi:10.1111/j.1744-7402.2005.02023.x [40] Z. Kovziridze, G. Donadze, G. Mamniashvili, A. Akhal- katsi, D. Daraselia, D. Japharidze, O. Romelashvili, A. Shengelaia, C. Gavasheli and J. G. Heinrich, “The Re- ceiving and Study of Hematite Nanoparticles for Hyper- thermia,” 1st International Conference for Students and Young Scientists on Materials Processing Science, Tbilisi, 10-13 October 2010, pp. 37-46. [41] Z. Kovziridze, J. Heinrich, R. Goerke, G. Mamniashvili, Z. Chachkhiani, N. Mitskevich and G. Donadze, “Produc- tion of Superparamagnetic Nanospheres for Hyperthermic Therapy of Surface (Skin) Cancer Diseases,” 3rd Inter- national Congress on Ceramics, Osaka, 14-18 November 2010. [42] Z. Kovziridze, J. Heinrich, R. Goerke, G. Mamniashvili, A. Akhalkatsi, Z. Chachkhiani, N. Mitskevich and G. Do- nadze, “production of Bionanoceramic Superparamagnet- ics for Creation of Controlled. Local Hyperthermia and Their Use, as Therapeutic Agents, for Purposeful Trans- portation in Living Organisms in Surface (Skin) Cancer Treatment,” Journal of Georgian Ceramists Association “Ceramics”, Vol. 1, No. 22, 2010, pp. 43-51.
|