 Journal of Materials Science and Chemical Engineering, 2013, 1, 8-13 http://dx.doi.org/10.4236/msce.2013.14002 Published Online September 2013 (http://www.scirp.org/journal/msce) Microwave Absorbing Properties of W-Type Hexaferrite Ba(MnZn)xCo2(1−x)Fe16O27 Xianming Qin1, Ying Cheng2, Kesheng Zhou2, Shengxiang Huang2, Xia Hui2* 1Xiamen Hongye Project Build Technique Co. Ltd., Xiamen, China 2School of Physics and Electronics, Central South University, Changsha, China Email: *xhui73@csu.edu.cn, 5430@csu.edu.cn Received June 18, 2013; revised July 18, 2013; accepted August 25, 2013 Copyright © 2013 Xianming Qin et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT MnZn-doped W-type barium cobalt ferrite powder composites of Ba(MnZn)xCo2(1−x)Fe16O27 (x = 0.1, 0.2, 0.3, 0.4, and 0.5) were prepared in a sol-gel process. The microwave absorbing properties of the composites in the range of 2 - 18 GHz and their electromagnetic loss mechanisms were studied. The results demonstrated that the synthesized Ba(MnZn)xCo2(1−x)Fe16O27 samples possess a W-type phase of the crystal structure with a hexagonal flaky shape in mi- cro-morphology, and the samples exhibited a soft magnetic trait that enables improving their microwave absorption properties through suitable MnZn doping. For Ba(MnZn)0.4Co1.2Fe16O27 with a thickness of 2.8 mm, the reflection loss peak was −40.7 dB at a frequency of 7.3 GHz, with a bandwidth of 6.6 GHz at a loss of less than −10 dB. The micro- wave absorption primarily resulted from magnetic losses caused by magnetization relaxation, domain wall resonance, and natural resonance. Keywords: Sol-Gel Process; W-Type Ferrite; Microwave Absorbing Materials; Electromagnetic Loss 1. Introduction Ferrite is one type of the important electromagnetic-wave absorbing materials, of which spinel and magnetoplum- bite hexagonal ferrites are the most widely used in appli- cations. However, because of Snoek’s limitation, the working frequency of those spinel ferrites is generally confined to the range below 3 GHz [1-5]. Hexagonal ferrites, which exhibit a planar anisotropy and ferromag- netic resonance in the GHz range caused by a high mag- netocrystalline anisotropy field, are applicable for use as electromagnetic-wave absorption materials working in the GHz wavelength range [5-9]. Hexagonal ferrites can be classified into many types, including M-, Y-, W-, X-, Z-, and U-type phase, according to their chemical for- mulas and crystal structures. The M-type is the most studied among those hexagonal ferrites. It has been con- firmed that different ions can be substituted for Ba2+ and Fe3+ in M-type phase ferrite, such as La for Ba2+ and Co-Ti, Mn-Ti, Mg-Ti, Co-Mn-Ti, Mn-Cu-Ti, Mn-Cu-Zr and Zn-Zr-Sn for Fe3+. Consequently, the electromag- netic parameters of the ferrites can be adjusted or con- trolled, and their microwave absorption performance can be improved [2-7,10-17]. W-type phase ferrite can be viewed as a complex fer- rite consisting of M-type phase and spinel phase block, which is M + 2S (M: BaFe12O19, S: Fe2+Fe2O4). In BaFe12O19, chemical doping is commonly utilized by replacing Fe2+ with Co2+ to improve its thermal stability and other properties, which results in a W-type barium- cobalt ferrite of BaCo2Fe16O27. Because of the existing Fe2+ and Fe3+ cation sites, Fe cations are easily substi- tuted by other divalent and trivalent cations; the mag- netic parameters, including saturation magnetization, Curie temperature, coercivity, magnetic anisotropy and ferromagnetic resonance frequency, are thus more ad- justable. However, there are fewer studies on microwave electromagnetic characteristics of W-type phase ferrites than on the characteristics of M-type ferrites. The substi- tution of Co2+ or Ba2+ by other metal ions was deter- mined to help improve the microwave absorption proper- ties of BaCo2Fe16O27. Materials with improved micro- wave absorption include Ba(ZnxCo1−x)2Fe16O27 [10], BaZn1.1Co0.9Fe16O27 [13], Ba1−xErx(Zn0.3Co0.7)2Fe16O27 [18] and Ba(Zn0.5Co0.5)2Fe16O27 [19]. The microwave electromagnetic effects are expected to be richer when Co2+ in BaCo2Fe16O27 is substituted by two or more metal ions. In this paper, MnZn-doped W-type barium cobalt fer- *Corresponding author. C opyright © 2013 SciRes. MSCE  X. M. QIN ET AL. 9 rite composites of Ba(MnZn)xCo2(1−x)Fe16O27 were pre- pared using a sol-gel process. The microwave absorption properties of Ba(MnZn)xCo2(1−x)Fe16O27 in the frequency range of 2 - 18 GHz are investigated, and the electro- magnetic loss mechanism is discussed. 2. Experiments 2.1. Preparation of Ba(MnZn)xCo2(1−x)Fe16O27 Powders Ba(MnZn)xCo2(1−x)Fe16O27 (x = 0.1, 0.2, 0.3, 0.4 and 0.5) were synthesized using a sol-gel process. According to the stoichiometric ratio of Ba(MnZn)xCo2(1−x)Fe16O27, the weighed Fe3+, Co2+, Zn2+, and Ba2+ nitrate and manganese acetate were dissolved in deionized water and stirred to form a uniform brown transparent solution. Citric acid was added to the above solution according to the molar ratio of 1:1.5 between the metal ions and the citric acid, and the ammonia water was dripped to ensure the pH value that was in the range of 6 - 7. Next, the solution was strung for 4 h in a water bath at 80˚C to form a sol. The solution was then dried in an oven (100˚C) to obtain the dry gels. The dry gels were slightly grinded after ashing at 450˚C. Finally, the black Ba(MnZn)xCo2(1−x)Fe16O27 ferrites were obtained by cooling the powder, which was sintered in an electric furnace at 1235˚C for 4 h under the atmosphere circumstances. 2.2. Measurements The crystal structure of Ba(MnZn)xCo2(1−x)Fe16O27 (x = 0.1, 0.2, 0.3, 0.4 and 0.5) was investigated through X-ray diffraction (XRD) using a system from X’pert PRO of Holland PANalytical that utilized a Cu target at a volt- age/current of 35-kV/25-mA. Scanning electron micros- copy (SEM) examination was performed using QUANTA 200 to obtain the morphology and size of the powder particles of Ba(MnZn)xCo2(1−x)Fe16O27. The hysteresis loops of the Ba(MnZn)0.4Co1.2Fe16O27 particles were measured using a vibrating sample magnetometer (VSM). The prepared crystalline powders were mixed with paraffin wax at a ratio of 8:3 in weight by heating and pressed into annular samples. The complex permittivity and permeability of the samples were measured using an AV3629 microwave vector network analyzer in the fre- quency range of 2 - 18 GHz. The relationship between microwave reflectivity (R) and frequency (f) can be ob- tained according to a previous formula [20,21] that used the measured data. 3. Results and Discussion 3.1. Crystal Structure, Morphology and Hysteresis Loop of the Powders Figure 1 shows the XRD pattern of the ˚ Figure 1. XRD pattern of Ba(MnZn)xCo2(1−x)Fe16O27. Ba(MnZn)xCo2(1−x)Fe16O27 (x = 0.1, 0.2, 0.3, 0.4 and 0.5) ferrites calcined at 1235˚C for 4 h. Compared with the characteristic diffraction peaks of the standard XRD card (PDF#19-0098) for W-type phase ferrite, the crystal structures of Ba(MnZn)xCo2(1−x)Fe16O27 samples were measured to be a single W-type phase structure. Nearly no variations of the crystal structure were observed for all MnZn-doped Ba(MnZn)xCo2(1−x)Fe16O27 samples when x was equal to 0.1, 0.2, 0.3 0.4 and 0.5. Figure 2 show SEM images of Ba(MnZn)0.4Co1.2Fe16O27 and Ba(MnZn)0.3Co1.4Fe16O27 powders after high-temperature annealing at 1235˚C for 4 h. The SEM images indicate that both the morphology of the Ba(MnZn)0.4Co1.2Fe16O27 and Ba(MnZn)0.3Co1.4Fe16O27 are hexagonal flaky with a clear outline. The average size was 5 μm with a narrow distribution. As a result, doping with a small amount of MnZn does not significantly affect the grain size and morphology. Figure 3 shows the hysteresis loop of Ba(MnZn)0.4Co1.2Fe16O27 powders calcined at 1235˚C for 4 h. The coercitity (Hc), magnetization (Ms) and reten- tivity (Mr) are 28.702 Oe, 67.320 emu/g, 3.7964 emu/g, respectively. The hysteresis loop indicates that Ba(MnZn)0.4Co1.2Fe16O27 is a soft magnetic material. 3.2. The Microwave Absorption Properties Figure 4 shows the variation of reflection loss versus frequency for Ba(MnZn)xCo2(1−x)Fe16O27 (x = 0.1, 0.2, 0.3, 0.4 and 0.5) at a thickness of 2.8 mm. For x = 0.1, the reflection loss exhibits a peak at approximately −12 dB at a frequency of 8.1 GHz, with a bandwidth of 5 GHz for losses less than −10 dB. For x = 0.2, the reflec- tion loss exhibits a peak at approximately −16 dB at 5.0 GHz, with a bandwidth of 6 GHz for losses less than −10 dB. For x = 0.3, the reflection loss exhibits a peak at ap- Copyright © 2013 SciRes. MSCE 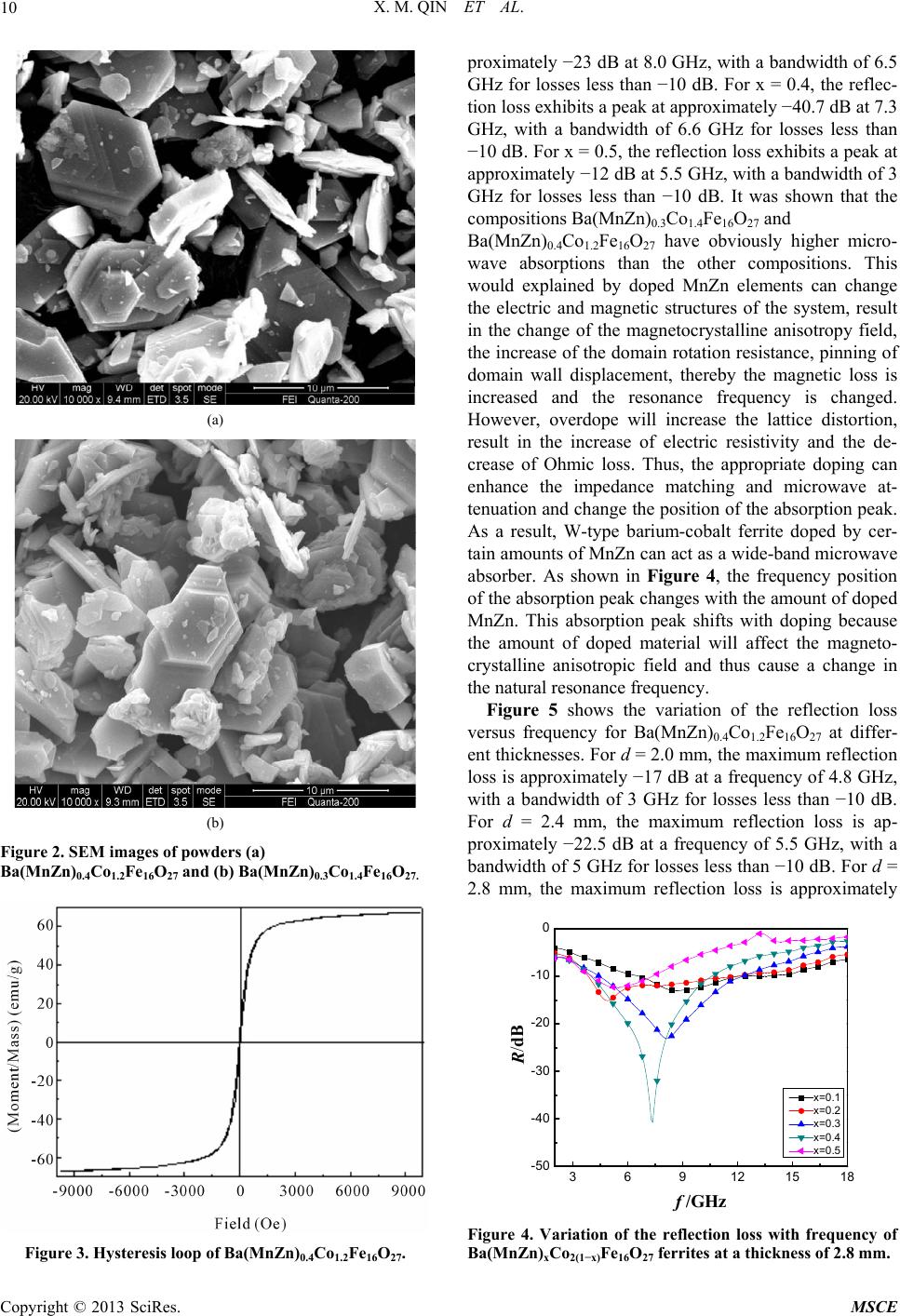 X. M. QIN ET AL. 10 (a) (b) Figure 2. SEM images of powders (a) Ba(MnZn)0.4Co1.2Fe16O27 and (b) Ba(MnZn)0.3Co1.4Fe16O27. Figure 3. Hysteresis loop of Ba(MnZn)0.4Co1.2Fe16O27. proximately −23 dB at 8.0 GHz, with a bandwidth of 6.5 GHz for losses less than −10 dB. For x = 0.4, the reflec- tion loss exhibits a peak at approximately −40.7 dB at 7.3 GHz, with a bandwidth of 6.6 GHz for losses less than −10 dB. For x = 0.5, the reflection loss exhibits a peak at approximately −12 dB at 5.5 GHz, with a bandwidth of 3 GHz for losses less than −10 dB. It was shown that the compositions Ba(MnZn)0.3Co1.4Fe16O27 and Ba(MnZn)0.4Co1.2Fe16O27 have obviously higher micro- wave absorptions than the other compositions. This would explained by doped MnZn elements can change the electric and magnetic structures of the system, result in the change of the magnetocrystalline anisotropy field, the increase of the domain rotation resistance, pinning of domain wall displacement, thereby the magnetic loss is increased and the resonance frequency is changed. However, overdope will increase the lattice distortion, result in the increase of electric resistivity and the de- crease of Ohmic loss. Thus, the appropriate doping can enhance the impedance matching and microwave at- tenuation and change the position of the absorption peak. As a result, W-type barium-cobalt ferrite doped by cer- tain amounts of MnZn can act as a wide-band microwave absorber. As shown in Figure 4, the frequency position of the absorption peak changes with the amount of doped MnZn. This absorption peak shifts with doping because the amount of doped material will affect the magneto- crystalline anisotropic field and thus cause a change in the natural resonance frequency. Figure 5 shows the variation of the reflection loss versus frequency for Ba(MnZn)0.4Co1.2Fe16O27 at differ- ent thicknesses. For d = 2.0 mm, the maximum reflection loss is approximately −17 dB at a frequency of 4.8 GHz, with a bandwidth of 3 GHz for losses less than −10 dB. For d = 2.4 mm, the maximum reflection loss is ap- proximately −22.5 dB at a frequency of 5.5 GHz, with a bandwidth of 5 GHz for losses less than −10 dB. For d = 2.8 mm, the maximum reflection loss is approximately 369 12151 -50 -40 -30 -20 -10 0 8 R/dB f /GHz x=0.1 x=0.2 x=0.3 x=0.4 x=0.5 Figure 4. Variation of the reflection loss with frequency of Ba(MnZn)xCo2(1−x)Fe16O27 ferrites at a thickness of 2.8 mm. Copyright © 2013 SciRes. MSCE 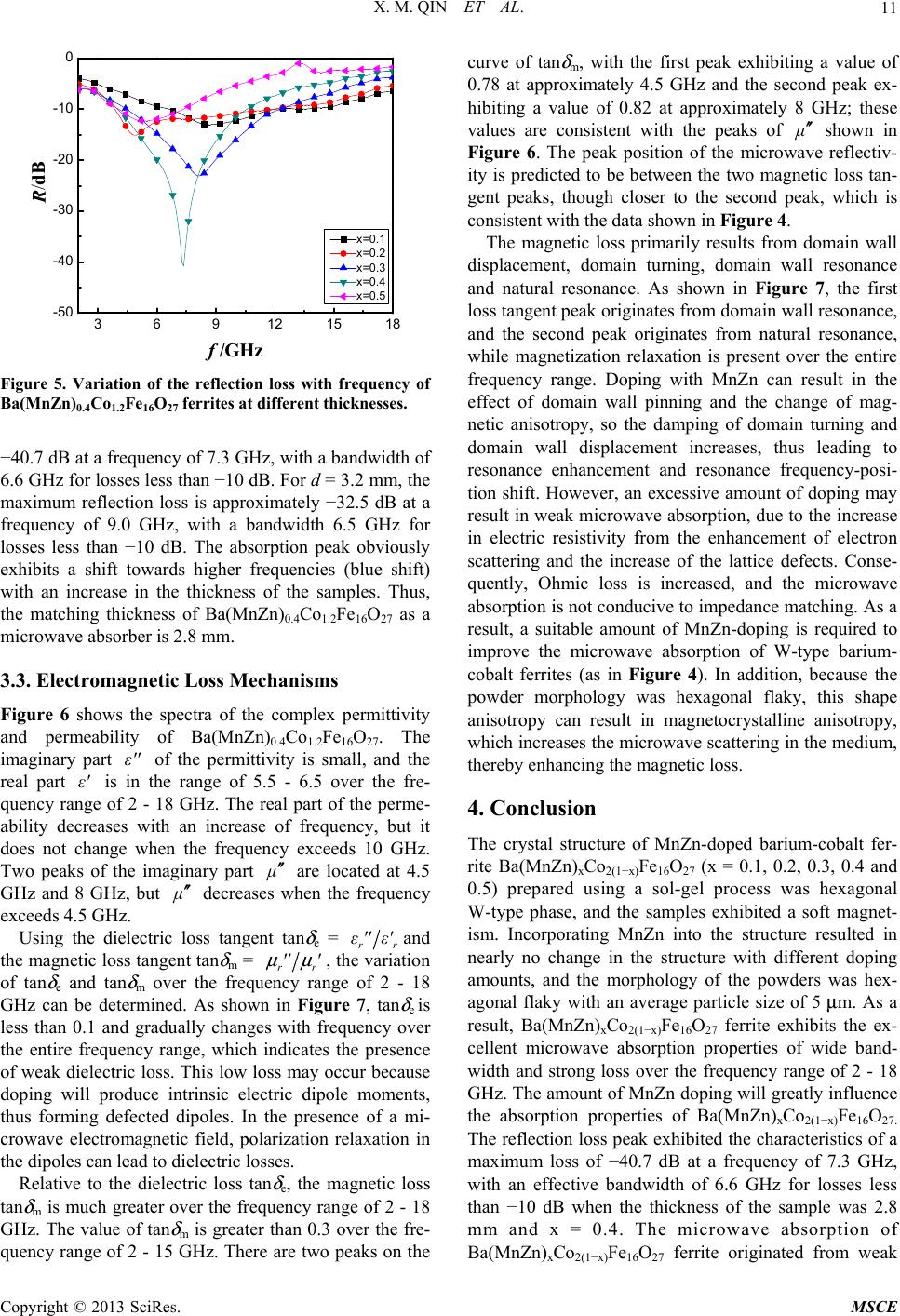 X. M. QIN ET AL. 11 36912 15 18 -50 -40 -30 -20 -10 0 R/dB f /GHz x=0.1 x=0.2 x=0.3 x=0.4 x=0.5 Figure 5. Variation of the reflection loss with frequency of Ba(MnZn)0.4Co1.2Fe16O27 ferrites at different thicknesses. −40.7 dB at a frequency of 7.3 GHz, with a bandwidth of 6.6 GHz for losses less than −10 dB. For d = 3.2 mm, the maximum reflection loss is approximately −32.5 dB at a frequency of 9.0 GHz, with a bandwidth 6.5 GHz for losses less than −10 dB. The absorption peak obviously exhibits a shift towards higher frequencies (blue shift) with an increase in the thickness of the samples. Thus, the matching thickness of Ba(MnZn)0.4Co1.2Fe16O27 as a microwave absorber is 2.8 mm. 3.3. Electromagnetic Loss Mechanisms Figure 6 shows the spectra of the complex permittivity and permeability of Ba(MnZn)0.4Co1.2Fe16O27. The imaginary part of the permittivity is small, and the real part is in the range of 5.5 - 6.5 over the fre- quency range of 2 - 18 GHz. The real part of the perme- ability decreases with an increase of frequency, but it does not change when the frequency exceeds 10 GHz. Two peaks of the imaginary part ε'' ε' ′′ are located at 4.5 GHz and 8 GHz, but ′′ decreases when the frequency exceeds 4.5 GHz. Using the dielectric loss tangent tan δ e = rr and the magnetic loss tangent tan δ m = ε'' ε' rr '' ' , the variation of tan δ e and tan δ m over the frequency range of 2 - 18 GHz can be determined. As shown in Figure 7, tan δ e is less than 0.1 and gradually changes with frequency over the entire frequency range, which indicates the presence of weak dielectric loss. This low loss may occur because doping will produce intrinsic electric dipole moments, thus forming defected dipoles. In the presence of a mi- crowave electromagnetic field, polarization relaxation in the dipoles can lead to dielectric losses. Relative to the dielectric loss tan δ e, the magnetic loss tan δ m is much greater over the frequency range of 2 - 18 GHz. The value of tan δ m is greater than 0.3 over the fre- quency range of 2 - 15 GHz. There are two peaks on the curve of tan δ m, with the first peak exhibiting a value of 0.78 at approximately 4.5 GHz and the second peak ex- hibiting a value of 0.82 at approximately 8 GHz; these values are consistent with the peaks of ′′ shown in Figure 6. The peak position of the microwave reflectiv- ity is predicted to be between the two magnetic loss tan- gent peaks, though closer to the second peak, which is consistent with the data shown in Figure 4. The magnetic loss primarily results from domain wall displacement, domain turning, domain wall resonance and natural resonance. As shown in Figure 7, the first loss tangent peak originates from domain wall resonance, and the second peak originates from natural resonance, while magnetization relaxation is present over the entire frequency range. Doping with MnZn can result in the effect of domain wall pinning and the change of mag- netic anisotropy, so the damping of domain turning and domain wall displacement increases, thus leading to resonance enhancement and resonance frequency-posi- tion shift. However, an excessive amount of doping may result in weak microwave absorption, due to the increase in electric resistivity from the enhancement of electron scattering and the increase of the lattice defects. Conse- quently, Ohmic loss is increased, and the microwave absorption is not conducive to impedance matching. As a result, a suitable amount of MnZn-doping is required to improve the microwave absorption of W-type barium- cobalt ferrites (as in Figure 4). In addition, because the powder morphology was hexagonal flaky, this shape anisotropy can result in magnetocrystalline anisotropy, which increases the microwave scattering in the medium, thereby enhancing the magnetic loss. 4. Conclusion The crystal structure of MnZn-doped barium-cobalt fer- rite Ba(MnZn)xCo2(1−x)Fe16O27 (x = 0.1, 0.2, 0.3, 0.4 and 0.5) prepared using a sol-gel process was hexagonal W-type phase, and the samples exhibited a soft magnet- ism. Incorporating MnZn into the structure resulted in nearly no change in the structure with different doping amounts, and the morphology of the powders was hex- agonal flaky with an average particle size of 5 μm. As a result, Ba(MnZn)xCo2(1−x)Fe16O27 ferrite exhibits the ex- cellent microwave absorption properties of wide band- width and strong loss over the frequency range of 2 - 18 GHz. The amount of MnZn doping will greatly influence the absorption properties of Ba(MnZn)xCo2(1−x)Fe16O27. The reflection loss peak exhibited the characteristics of a maximum loss of −40.7 dB at a frequency of 7.3 GHz, with an effective bandwidth of 6.6 GHz for losses less than −10 dB when the thickness of the sample was 2.8 mm and x = 0.4. The microwave absorption of Ba(MnZn)xCo2(1−x)Fe16O27 ferrite originated from weak Copyright © 2013 SciRes. MSCE 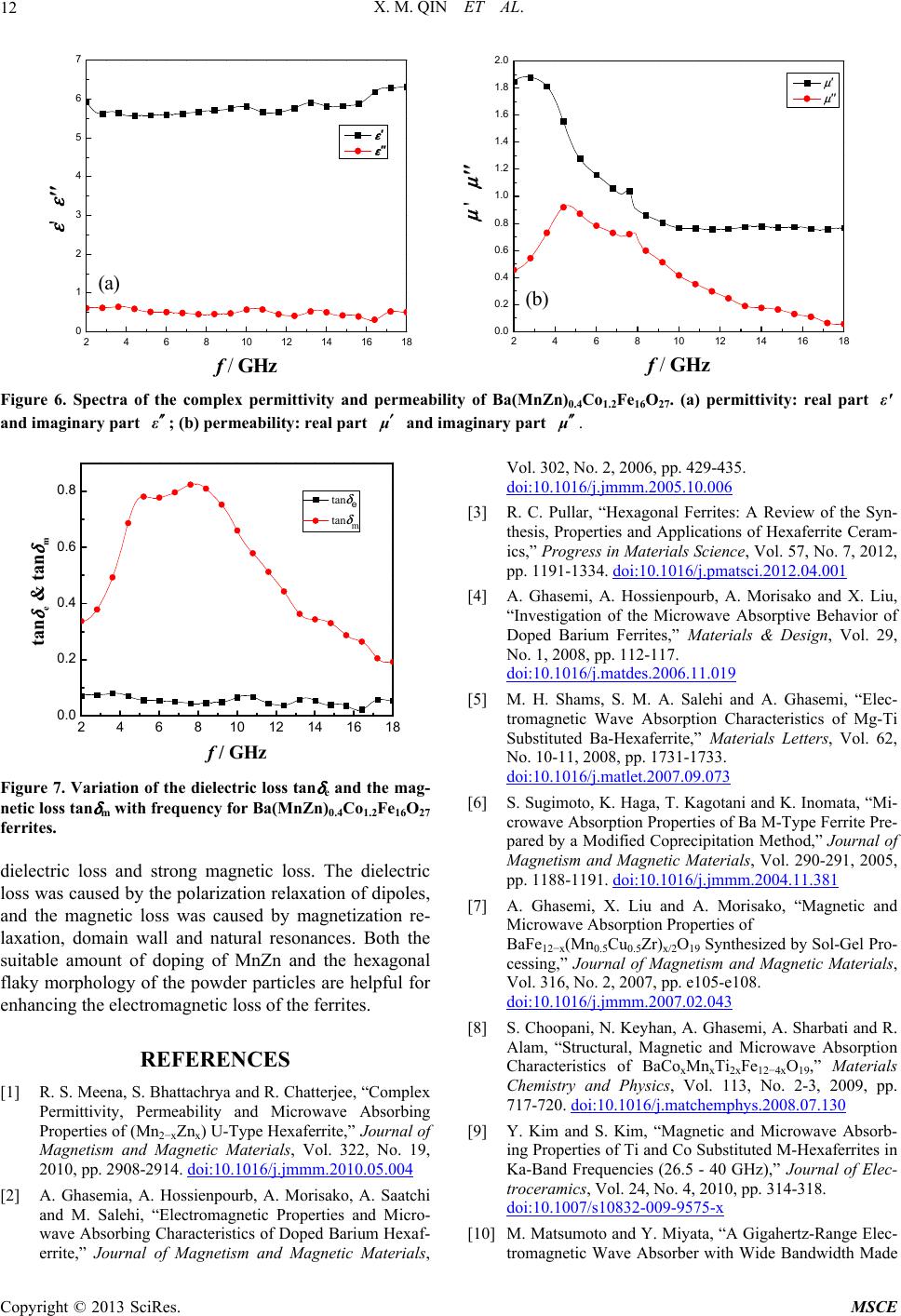 X. M. QIN ET AL. Copyright © 2013 SciRes. MSCE 12 246810 12 14 16 18 0 1 2 3 4 5 6 7 ' ' f / GHz ε ' ε '' (a) 246810 12 14 1618 0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 2.0 ' ' f / GHz μ ' μ '' (b) Figure 6. Spectra of the complex permittivity and permeability of Ba(MnZn)0.4Co1.2Fe16O27. (a) permittivity: real part and imaginary part ; (b) permeability: real part and imaginary part . ε' ′′ ε′ μ′′ μ 246810 12 14 16 18 0.0 0.2 0.4 0.6 0.8 tan δ e tan δ m tan e & tan m f / GHz Figure 7. Variation of the dielectric loss tan δ e and the mag- netic loss tan δ m with frequency for Ba(MnZn)0.4Co1.2Fe16O27 ferrites. dielectric loss and strong magnetic loss. The dielectric loss was caused by the polarization relaxation of dipoles, and the magnetic loss was caused by magnetization re- laxation, domain wall and natural resonances. Both the suitable amount of doping of MnZn and the hexagonal flaky morphology of the powder particles are helpful for enhancing the electromagnetic loss of the ferrites. REFERENCES [1] R. S. Meena, S. Bhattachrya and R. Chatterjee, “Complex Permittivity, Permeability and Microwave Absorbing Properties of (Mn2−xZnx) U-Type Hexaferrite,” Journal of Magnetism and Magnetic Materials, Vol. 322, No. 19, 2010, pp. 2908-2914. doi:10.1016/j.jmmm.2010.05.004 [2] A. Ghasemia, A. Hossienpourb, A. Morisako, A. Saatchi and M. Salehi, “Electromagnetic Properties and Micro- wave Absorbing Characteristics of Doped Barium Hexaf- errite,” Journal of Magnetism and Magnetic Materials, Vol. 302, No. 2, 2006, pp. 429-435. doi:10.1016/j.jmmm.2005.10.006 [3] R. C. Pullar, “Hexagonal Ferrites: A Review of the Syn- thesis, Properties and Applications of Hexaferrite Ceram- ics,” Progress in Materials Science, Vol. 57, No. 7, 2012, pp. 1191-1334. doi:10.1016/j.pmatsci.2012.04.001 [4] A. Ghasemi, A. Hossienpourb, A. Morisako and X. Liu, “Investigation of the Microwave Absorptive Behavior of Doped Barium Ferrites,” Materials & Design, Vol. 29, No. 1, 2008, pp. 112-117. doi:10.1016/j.matdes.2006.11.019 [5] M. H. Shams, S. M. A. Salehi and A. Ghasemi, “Elec- tromagnetic Wave Absorption Characteristics of Mg-Ti Substituted Ba-Hexaferrite,” Materials Letters, Vol. 62, No. 10-11, 2008, pp. 1731-1733. doi:10.1016/j.matlet.2007.09.073 [6] S. Sugimoto, K. Haga, T. Kagotani and K. Inomata, “Mi- crowave Absorption Properties of Ba M-Type Ferrite Pre- pared by a Modified Coprecipitation Method,” Journal of Magnetism and Magnetic Materials, Vol. 290-291, 2005, pp. 1188-1191. doi:10.1016/j.jmmm.2004.11.381 [7] A. Ghasemi, X. Liu and A. Morisako, “Magnetic and Microwave Absorption Properties of BaFe12−x(Mn0.5Cu0.5Zr)x/2O19 Synthesized by Sol-Gel Pro- cessing,” Journal of Magnetism and Magnetic Materials, Vol. 316, No. 2, 2007, pp. e105-e108. doi:10.1016/j.jmmm.2007.02.043 [8] S. Choopani, N. Keyhan, A. Ghasemi, A. Sharbati and R. Alam, “Structural, Magnetic and Microwave Absorption Characteristics of BaCoxMnxTi2xFe12−4xO19,” Materials Chemistry and Physics, Vol. 113, No. 2-3, 2009, pp. 717-720. doi:10.1016/j.matchemphys.2008.07.130 [9] Y. Kim and S. Kim, “Magnetic and Microwave Absorb- ing Properties of Ti and Co Substituted M-Hexaferrites in Ka-Band Frequencies (26.5 - 40 GHz),” Journal of Elec- troceramics, Vol. 24, No. 4, 2010, pp. 314-318. doi:10.1007/s10832-009-9575-x [10] M. Matsumoto and Y. Miyata, “A Gigahertz-Range Elec- tromagnetic Wave Absorber with Wide Bandwidth Made  X. M. QIN ET AL. 13 of Hexagonal Ferrite,” Journal of Applied Physics, Vol. 79, No. 8, 1996, pp. 5486-5488. doi:10.1063/1.362284 [11] Z. F. Zi, J. M. Dai,Q. C. Liu, H. Y. Liu, X. B. Zhu and Y. P. Sun, “Magnetic and Microwave Absorption Properties of W-Type Ba(ZnxCo1−x)2Fe16O27 Hexaferrite Platelets,” Journal of Applied Physics, Vol. 109, No. 7, 2011, Article ID: 07E536-1-3. [12] M. R. Meshram, N. K. Agrawal, B. Sinhaa and P. S. Misra, “Characterization of M-Type Barium Hexagonal Ferrite-Based Wide Band Microwave Absorber,” Journal of Magnetism and Magnetic Materials, Vol. 271, No. 2-3, 2004, pp. 207-214. doi:10.1016/j.jmmm.2003.09.045 [13] R. S. Meena1, S. Bhattachrya and R. Chatterjee, “Com- plex Permittivity, Permeability and Microwave Absorb- ing Studies of (Co2−xMnx) U-Type Hexaferrite for X- Band (8.2 - 12.4 GHz) Frequencies,” Materials Science and Engineering: B, Vol. 171, No. 1-3, 2010, pp. 133- 138. doi:10.1016/j.mseb.2010.03.086 [14] Y. Nie, H. H. He, Z. K. Feng, X. C. Zhang and X. M. Cheng, “Microwave Characterization of (Co, Zn)2 W Bar- ium Hexagonal Ferrite Particles,” Journal of Magnetism and Magnetic Materials, Vol. 303, No. 2, 2006, pp. e423- e427. [15] F. Tabatabaie, M. H. Fathi, A. Saatchi and A. Ghasemi, “Microwave Absorption Properties of Mn- and Ti-Doped Strontium Hexaferrite,” Journal of Alloys and Com- pounds, Vol. 470, No. 1-2, 2009, pp. 332-335. doi:10.1016/j.jallcom.2008.02.094 [16] S. Choopani, N. Keyhan, A.Ghasemi, A. Sharbathi, I. Maghsoudi and M. Eghbali, “Static and Dynamic Mag- netic Characteristics of BaCo0.5Mn0.5Ti1.0Fe10O19,” Jour- nal of Magnetism and Magnetic Materials, Vol. 321, No. 13, 2009, pp. 1996-2000. doi:10.1016/j.jmmm.2008.12.030 [17] S. P. Gairola, V. Verma, A. Singh , L. P. Purohit and R. K. Kotnala, “Modified Composition of Barium Ferrite to Act as a Microwave Absorber in X-Band Frequencies,” Solid State Communications, Vol. 150, No. 3-4, 2010, pp. 147- 151. doi:10.1016/j.ssc.2009.10.011 [18] T. Kagotani, D. Fujiwara, S. Sugimoto, K. Inomata and M. Homma, “Enhancement of GHz Electromagnetic Wave Absorption Characteristics in Aligned M-Type Bar- ium Ferrite Ba1−xLaxZnxFe12−x−y(Me0.5Mn0.5)yO19 (x = 0.0 - 0.5; y = 1.0 - 3.0; Me: Zr, Sn) by Metal Substitution,” Journal of Magnetism and Magnetic Materials, Vol. 272, 2004, pp. e1813-e1815. [19] X. Huang, J. Zhang, H. Wang, S. Yan, L. Wang and Q. Zhang, “Er3+-Substituted W-Type Barium Ferrite: Prepa- ration and Electromagnetic Properties,” Journal of Rare Earths, Vol. 28, No. 6, 2010, pp. 940-943. doi:10.1016/S1002-0721(09)60211-8 [20] A. Oikonomou, T. Giannakopoulou and G. Litsardakis, “Design, Fabrication and Characterization of Hexagonal Ferrite Multi-Layer Microwave Absorber,” Journal of Magnetism and Magnetic Materials, Vol. 316, No. 2, 2007, pp. e827-e830. doi:10.1016/j.jmmm.2007.03.114 [21] P. Singh, V. K. Babbar, A. Razdan, R. K. Puri and T. C. Goel, “Complex Permittivity, Permeability, and x-Band Microwave Absorption of CaCoTi Ferrite Composites,” Journal of Applied Physics, Vol. 87, No. 9, 2000, pp. 4362-4365. doi:10.1063/1.373079 Copyright © 2013 SciRes. MSCE
|