 American Journal of Plant Sciences, 2013, 4, 1879-1892 http://dx.doi.org/10.4236/ajps.2013.49231 Published Online September 2013 (http://www.scirp.org/journal/ajps) Low-Density Co-Inoculation of Myanmar Bradyrhizobium yuanmingense MAS34 and Streptomyces griseoflavus P4 to Enhance Symbiosis and Seed Yield in Soybean Varieties Khin Myat Soe1*, Takeo Yamakawa2 1Plant Nutrition Laboratory, Graduate School of Bioresource and Bioenvironmental Sciences, Faculty of Agriculture, Kyushu Uni- versity, Fukuoka, Japan; 2Plant Nutrition Laboratroy, Division of Molecular Biosciences, Department of Biosciences & Biotechnol- ogy, Faculty of Agriculture, Kyushu University, Fukuoka, Japan. Email: *khinmyatsoe@gmail.com Received June 23rd, 2013; revised July 23rd, 2013; accepted August 15th, 2013 Copyright © 2013 Khin Myat Soe, Takeo Yamakawa. This is an open access article distributed under the Creative Commons Attri- bution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT This study examined whether low-density co-inoculation of Myanmar Bradyrhizobium yuanmingense strain MAS34 and Streptomyces griseoflavus P4 would enhance nodulation, N2 fixation, and seed yield in two soybean varieties. A field experiment was conducted during the July to November 2012 growing season at Kyushu University Farm, Japan, using a split-plot design with three replications and the following four treatments: T1, an uninoculated treatment with peat moss (uninoculated); T2, a single inoculation with S. griseoflavus P4 (P4); T3, a single inoculation of B. yuanmin- gense MAS34 (MAS34); and T4, a dual inoculation of P4 with MAS34 (P4 + MAS34). Two varieties of soybean, Yezin-3 (Rj4) and Yezin-6 (non-Rj), were used. The N2 fixation activity of soybean was evaluated by the relative ureide method using xylem solute from root bled sap at the early pod-fill stage (R3.5). Dry matter production, N2 fixation, and seed yield were significantly (P < 0.01) different between the inoculated treatments. The effect of variety was also sig- nificant (P < 0.05) for nodule dry weight at the V6 stage, percentage of N derived from the atmosphere at the R3.5 stage, and seed yield at the maturity stage. The number of nodules on the tap roots was significantly higher in Yezin-3 than in Yezin-6. The single inoculation of P4 did not have a significant effect on dry matter production, N2 fixation, and seed yield in either soybean variety. The dry matter production, relative ureide index, percentage of N derived from the at- mosphere, and seed yield were significantly (P < 0.01) enhanced by a single inoculation of MAS34 in Yezin-3 and by dual inoculation of P4 + MAS34 in Yezin-6. These results indicate that low inoculum concentrations (105 cells seed−1) increase N2 fixation and seed yield in these soybean varieties under open field conditions. Myanmar B. yuanmingense MAS34 and S. griseoflavus P4 are expected to be useful biofertilizers for soybean production. Keywords: Bradyrhizobium yuan mingense; N2 Fixation; S. Griseoflavus P4; Seed Yield; Soybean 1. Introduction Soybean (Glycine max L. Merr.) is the most important grain legume crop in the world and an important protein source for both humans and livestock [1]. In Myanmar, soybean has been cultivated for centuries, providing not only a nutritious food, but also helping to increase the soil fertility [2]. With demands for this legume increasing domestically and abroad, soybean has become the second largest crop cultivated in Myanmar [3]. Soybean helps to maintain soil fertility by assimilating nitrogen from the atmosphere through symbiotic bio- logical N2 fixation (BNF) with bradyhizobia [4]. BNF is of agronomic importance because it reduces the need for chemical nitrogen fertilizer [5], and nitrogen fixation in the root nodule is thus an important area of study. The level of nitrogen fixation varies by cultivar in many le- gume crop species. Some crops, particularly combina- tions of strains and cultivars, have been shown to be es- pecially efficient at N2 fixation [6]. Several studies have also reported differences between Bradyrhizobium strains regarding their effectiveness with different soybean ge- notypes [7-9]. Weaver and Frederick [10] reported that to success- fully compete with indigenous rhizobium, the introduced *Corresponding author. Copyright © 2013 SciRes. AJPS  Low-Density Co-Inoculation of Myanmar Bradyrhizobium yuanmingense MAS34 and Streptomyces griseoflavus P4 to Enhance Symbiosis and Seed Yield in Soybean Varieties 1880 inoculum must be 1000 times greater. However, in Can- ada, the standard inoculum for soybean, kidney bean, and pea is applied at 105 cells·seed−1 [11]. Furthermore, Ya- makawa and Saeki [12] conducted a field experiment at Kyushu University Farm in Japan to examine the effect of inoculum density and the use of different inoculation methods on soybean production. They found that a higher inoculum density decreased acetylene reduction activity. Significantly increased yield was found at inoculation concentrations of 105 cell·seed−1 and 107 cell·seed−1, but there was no effect of increasing the inoculum above the latter density. Streptomyces griseoflavus P4 was identified by a full 16S rRNA sequencing, and was mostly related to S. griseoflavus (99.7% identical score) with the GenBank database accession number of JN102356 [13]. Significant stimulation effects were observed following the dual in- oculation of P4 and the Bradyrhizobium strain on N up- take in adzuki bean, Thai sweet pea [14], and soybean [15] compared to a bradyrhizobial single inoculation. Many studies have determined the dry weight and ni- trogen fixation efficiency of indigenous rhizobial strains in leguminous plants [16-18]. Our previous laboratory study revealed that the dual inoculation of S. griseoflavu s P4 with Myanmar B. yuanmingense MAS34 significantly influenced the nodule dry weight in Yezin-6 (non-Rj) soybean and the nodule dry weight and N2 fixation in Yezin-3 (Rj4) soybean [15]. The experimental data sug- gested the usefulness of further field investigations of selected S. griseoflavus P4 and indigenous B. yuanmin- gense MAS34 with Yezin-3 (Rj4) and Yezin-6 (non-Rj) soybean varieties. This study was designed to verify the previous finding and determine whether plant growth and nitrogen fixation ability were improved in these two soy- bean varieties under field conditions. Specifically, the main objective of this study was to evaluate the co-in- oculation of Myanmar B. yuanmingense MAS34 and S. griseoflavus P4 to enhance symbiosis and seed yield in two soybean varieties, using a low density of inoculum (105 cells·seed−1). 2. Materials and Methods 2.1. Experimental Site The experiment was conducted at Kyushu University Farm, Fukuoka Prefecture, Japan (33˚37'N, 130˚25'E), during the July to November 2012 growing season. The cultivated field had a clay loam soil containing kaolinite with a pH ranging from 6.1 to 6.4 (soil:water, 1:2.5) or 5.0 to 5.3 (soil:KCl, 1:2.5). The cation exchange capacity (CEC) of the soil was 20.20 cmolc·kg−1 [19]. The soil had a total nitrogen (N) content of 117 mg per 100 g of soil [20], total phosphorus (P) content of 76.5 mg per 100 g of soil [21], available phosphorus (P) content of 9.00 mg per 100 g of soil [22], and exchangeable potassium (K), calcium (Ca), and magnesium (Mg) contents of 0.94, 6.31, and 1.69 cmolc·kg−1, respectively [19]. To estimate the density of indigenous rhizobia, soil samples at 15 cm depth were collected from six locations in the experi- mental field before fertilization and the rhizobia density was measured by the most probable number (MPN) method [23] using two soybean cultivars: Yezin-3 (Rj4- genotype) and Yezin-6 (non-Rj-genotype). 2.2. Plant Materials The soybean cultivars Yezin-3 (Rj4) and Yezin-6 (non-Rj) [24] were collected from the Food Legumes Section, De- partment of Agricultural Research, Yezin, Myanmar. Rj4 and non-Rj in parentheses indicate the nodulation regu- latory genes [25]. The Rj genes play a role in controlling a plant’s compatibility with specific rhizobial strains and also the preference for indigenous soybean-nodulating rhizobia [26,27]. In addition, non-Rj is compatible with all bradyrhizobial strains, but Rj4 has unique features that restrict nodulation with specific strains of Bradyrhiz o- bium [28]. Yezin-3 (Rj4) and Yezin-6 (non-Rj) are well- known soybean cultivars in Myanmar. 2.3. Bacteria Maintenance The B. yuanmingense MAS34 was stored at −85°C on HM salts glycerol stock. It was cultured in A1E liquid media [29] on a rotary shaker (100 rpm) at 30°C for seven days. The stock culture of S. griseoflavus P4 was stored at 4°C in IMA-2 medium [30] and cultured in a broth medium on a rotary shaker (100 rpm) at 30°C for five days. 2.4. Land Preparation, Inoculation, and Planting Prior to planting the field was prepared according to con- ventional practices. Chemical fertilizers containing urea, super phosphate, and potassium chloride (30.87, 308.75, and 61.75 kg·ha−1) were applied at the rate recommended by the Department of Agricultural Research, Ministry of Agriculture and Irrigation, Myanmar. The land was then leveled and divided into individual plots. The site was 15 m long and 24 m wide. The row width and intra-row spacing were 60 and 20 cm, respectively. Peat moss inoculum for 100 soybean seeds was pre- pared from 1.5 mL deionized water and 10 mL of 12% aqueous solution of gum Arabic, 10 g of BM2 (raw ma- terials: peat moss, Group Berger Peat Moss Ltd., Canada), 0.01 mL of 1 × 109 cell·mL−1 of B. yuanmingense MAS 34 (1 × 105 cells seed−1), and 1 mL of 1 × 107 cell·mL−1 of S. griseoflavus P4 (1 × 105 cells·seed−1). All inocula- tion occurred just before planting. Seeds were allowed to Copyright © 2013 SciRes. AJPS  Low-Density Co-Inoculation of Myanmar Bradyrhizobium yuanmingense MAS34 and Streptomyces griseoflavus P4 to Enhance Symbiosis and Seed Yield in Soybean Varieties 1881 mix well with the peat moss inoculum prior to seed sow- ing. Three inoculated soybean seeds per hill of peat were sown in the field and immediately covered with rice ash. A split plot design was used with three replications and the following four treatments: T1, an uninoculated treat- ment with peat moss (uninoculated); T2, a single inocu- lation with S. griseoflavus P4 (P4), T3, a single inocula- tion of B. yuanmingense MAS34 (MAS34); and T4, a dual inoculation of P4 with MAS34 (P4 + MAS34). At the two trifoliate stage (V2 stage), the seedlings were thinned to two seedlings per hill, and inter-cultivation and manual weeding were undertaken. Some pesticides and insecticides were periodically applied when pests and insects were observed. 2.5. Plant Sampling Plant samples were collected from three growing stages: the six unfolded trifoliate leaves stage (V6 stage), early pod-fill stage (R3.5 stage), and the maturity stage. For the V6 and R3.5 stages, two plants in one hill per plot were sampled and separated into nodules, roots, and shoots. The soil was carefully removed from nodules and roots by sieving through a 1-mm sieve. Nodules, roots, and shoots of the plants from each plot were oven-dried at 70˚C for 72 h for the determination of dry weight. The shoot dry matter was powdered in a Cyclotec 1093 sam- ple mill (100 - 120 mesh, Tecator AB, Hoedanaes, Swe- den). The N content of the dry matter was analyzed using indophenol [20] after digestion with H2SO4-H2O [31]. For the collection of xylem sap at the R3.5 stage, root bled sap samples from the plants in each plot were taken. To collect the root bled sap, the shoot just under the coty- ledon node of each plant was cut with a very sharp cutter according to a previously reported method [32]. Sap sam- ples were kept on ice and frozen at −20˚C for long-term storage. The root bled sap samples were analyzed for amino N [33], NO3-N [34], and ureide-N [35]. The rela- tive ureide index (RUI) of root bled sap at the R3.5 stage was calculated according to the following formula [32]: RUI % 4ureide4ureideamino acidnitrate100 The percentage of N derived from N fixation was cal- culated from y = 21.3 + 0.67x in which y is relative ureide-N (%) and x is the percentage of N derived from N fixation (%) [36]. At maturity stage, ten continuous hills were harvested by cutting at the cotyledon node of the stem. The plants were harvested from each plot at physiological maturity, leaving the border rows. The yield components parame- ters of number of pods per plant, number of seeds per pod, and hundred-seed weight were determined. After recording the seed yield, the above-ground dry matters were oven-dried at 70˚C for 72 h for dry weight deter- mination. The dry matters were powdered and N content was analyzed in the same way as for the V6 and R3.5 growth stages. 2.6. Statistical Analysis Data for the dry weight of nodules, roots and shoots, total N accumulation, RUI, percentage of N derived from the atmosphere, and seed yield were statistically analyzed using STATISTIX 8 (Analytical Software, Tallahassee, FL, USA) and the means were compared by Tukey’s HDS test at P < 0.01. 3. Results 3.1. Indigenous Rhizobia in the Cultivated Soil The density of indigenous rhizobia from cultivated soil was estimated by the MPN method. Indigenous rhizobia in this soil nodulated to Yezin-3 (Rj4) and to Yezin-6 (non-Rj) were present at concentrations of 1.16 × 104 and 1.16 × 105 cells (g dry soil)−1, respectively. 3.2. Nodulation, Dry Matter Production, and N Accumulation at the V6 and R3.5 Stages The number of nodules on tap roots per hill was signifi- cantly (P < 0.01) different between varieties and treat- ments (Figure 1). The Yezin-3 variety had a greater number of nodules on the tap roots than the Yezin-6 soybean variety. A single inoculation of MAS34 resulted in a significantly (P < 0.01) higher number of nodules on the tap roots of the Yeizn-3 soybean variety compared to the uninoculated control at the V6 and R3.5 stages. There were no significant differences between P4, MAS34, and the dual inoculation of P4 with MAS34. However, after a single inoculation of MAS34, 32% and 19% of R3.5- stage tap roots of the Yezin-3 soybean variety had higher numbers of nodules than after a single inoculation of P4 and a dual inoculation of P4 with MAS34, respectively. In the Yezin-6 soybean, the dual inoculation of P4 with MAS34 was significantly (P < 0.01) higher than the un- inoculated control in terms of the number of nodules on the tap roots, but no significant difference was observed between a single inoculation of P4 and MAS34 at the R 3.5 stage. Nevertheless, following the dual inoculation of P4 with MAS34, 35% and 17% of tap roots had a higher number of nodules than after single inoculations of P4 and MAS34 at the R3.5 stage for the Yezin-6 soybean variety. There was no significant difference in the num- ber of nodules on lateral roots per hill (Figure 1). How- ever, a single inoculation of AS34 and the dual inocu- M Copyright © 2013 SciRes. AJPS 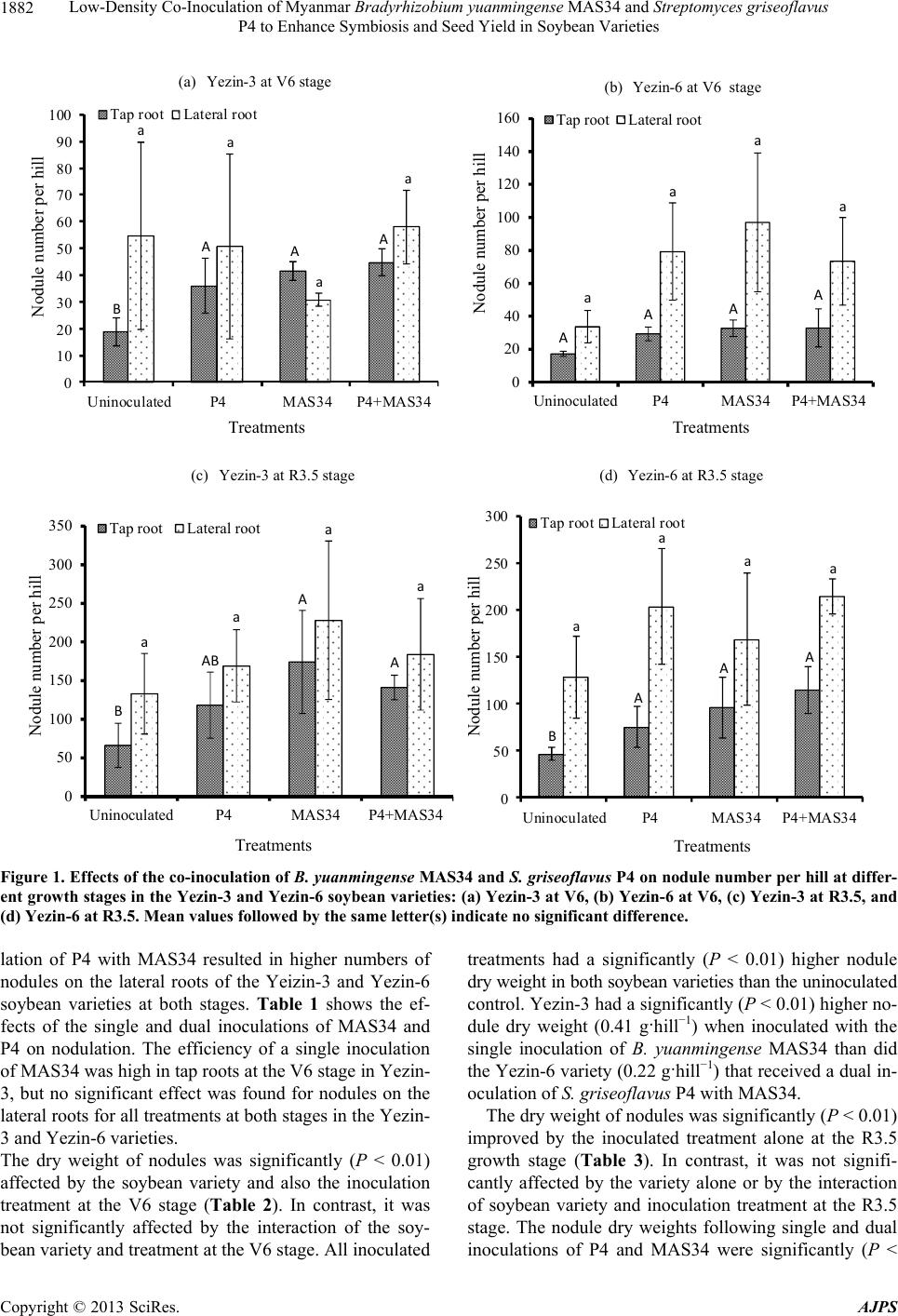 Low-Density Co-Inoculation of Myanmar Bradyrhizobium yuanmingense MAS34 and Streptomyces griseoflavus P4 to Enhance Symbiosis and Seed Yield in Soybean Varieties Copyright © 2013 SciRes. AJPS 1882 B AA A a a a a 0 10 20 30 40 50 60 70 80 90 100 UninoculatedP4MAS34 P4+MAS34 Tap rootLateral root A AA A a a a a 0 20 40 60 80 100 120 140 160 UninoculatedP4MAS34 P4+MAS34 Tap rootLateral root (a) Yezin-3 at V6 stage Nodule number per hill Treatments (b) Yezin-6 at V6 stage Nodule number per hill Treatments B AB A A a a a a 0 50 100 150 200 250 300 350 UninoculatedP4MAS34 P4+MAS34 Tap rootLateral root B A A A a a aa 0 50 100 150 200 250 300 UninoculatedP4MAS34 P4+MAS34 Tap rootLateral root (c) Yezin-3 at R3.5 stage Nodule number per hill Treatments (d) Yezin-6 at R3.5 stage Nodule number per hill Treatments Figure 1. Effects of the co-inoculation of B. yuanmingense MAS34 and S. griseoflavus P4 on nodule number pe r hill at differ- ent growth stages in the Yezin-3 and Yezin-6 soybean varieties: (a) Yezin-3 at V6, (b) Yezin-6 at V6, (c) Yezin-3 at R3.5, and (d) Yezin-6 at R3.5. Mean values followed by the same letter(s) indicate no significant difference. lation of P4 with MAS34 resulted in higher numbers of nodules on the lateral roots of the Yeizin-3 and Yezin-6 soybean varieties at both stages. Table 1 shows the ef- fects of the single and dual inoculations of MAS34 and P4 on nodulation. The efficiency of a single inoculation of MAS34 was high in tap roots at the V6 stage in Yezin- 3, but no significant effect was found for nodules on the lateral roots for all treatments at both stages in the Yezin- 3 and Yezin-6 varieties. The dry weight of nodules was significantly (P < 0.01) affected by the soybean variety and also the inoculation treatment at the V6 stage (Table 2). In contrast, it was not significantly affected by the interaction of the soy- bean variety and treatment at the V6 stage. All inoculated treatments had a significantly (P < 0.01) higher nodule dry weight in both soybean varieties than the uninoculated control. Yezin-3 had a significantly (P < 0.01) higher no- dule dry weight (0.41 g·hill−1) when inoculated with the single inoculation of B. yuanmingense MAS34 than did the Yezin-6 variety (0.22 g·hill−1) that received a dual in- oculation of S. griseoflavus P4 with MAS34. The dry weight of nodules was significantly (P < 0.01) improved by the inoculated treatment alone at the R3.5 growth stage (Table 3). In contrast, it was not signifi- cantly affected by the variety alone or by the interaction of soybean variety and inoculation treatment at the R3.5 stage. The nodule dry weights following single and dual inoculations of P4 and MAS34 were significantly (P <  Low-Density Co-Inoculation of Myanmar Bradyrhizobium yuanmingense MAS34 and Streptomyces griseoflavus P4 to Enhance Symbiosis and Seed Yield in Soybean Varieties 1883 Table 1. Effects of co-inoculation of B. yuanmingense MAS34 and S. griseoflavus P4 on weight per nodule in the Yezin-3 and Yezin-6 soybean varieties at the V6 and R3.5 stages. Weight per nodule (mg) V6 stage R3.5 stage Treatment Tap root Lateral root Tap root Lateral root Yezin-3 Uninoculated 6.27 abc 1.22 a 7.90 a 2.61 a P4 7.10 ab 1.91 a 7.70 ab 4.14 a MAS34 8.37 a 1.81 a 5.81 ab 3.60 a P4 + MAS34 6.74 ab 1.78 a 6.98 ab 3.44 a Yezin-6 Uninoculated 2.65 c 0.79 a 6.16 ab 2.16 a P4 3.70 bc 0.97 a 6.43 ab 2.43 a MAS34 4.12 bc 0.88 a 4.94 ab 2.37 a P4+MAS34 4.28 bc 0.93 a 4.58 b 2.82 a Means within a column followed by the same letter(s) indicate no significant difference. Table 2. Effects of co-inoculation of B. yuanmingense MAS34 and S. griseoflavus P4 on plant dry weight and total N accumu- lation in the Yezin-3 and Yezin-6 soybean varieties at the V6 stage. Plant dry weight (g·hill−1) Treatment Nodule Root Shoot Total N accumulation (mg N·hill−1) Varieties Yezin-3 0.32 a 1.24 a 5.62 a 172 a Yezin-6 0.18 b 1.10 a 4.00 a 119 a Treatment of Yezin-3 Uninoculated 0.17 b 0.87 b 3.51 b 108 b P4 0.33 a 1.31 ab 6.04 ab 186 ab MAS34 0.41 a 1.38 a 6.01 ab 183 ab P4 + MAS34 0.40 a 1.41 a 6.96 a 211 a Treatment of Yezin-6 Uninoculated 0.07 b 0.63 b 2.00 b 56.9 b P4 0.19 a 1.08 a 3.83 a 116 a MAS34 0.22 a 1.25 a 4.66 a 141 a P4 + MAS34 0.20 a 1.43 a 5.23 a 160 a Tukey HSD test Variety ** NS NS NS Treatment ** ** ** ** Variety × Treatment NS NS NS NS CV% 10.9 12.6 14.7 16.3 N S, **: nonsignificant or significant at P < 0.01 respectively. Mean values followed by the same letter(s) indicate no significant difference. Copyright © 2013 SciRes. AJPS  Low-Density Co-Inoculation of Myanmar Bradyrhizobium yuanmingense MAS34 and Streptomyces griseoflavus P4 to Enhance Symbiosis and Seed Yield in Soybean Varieties 1884 0.01) different in Yezin-3. However, in Yezin-6, the sin- gle P4 inoculation did not significantly affect nodulation at the R3.5 stage. The single inoculation of MAS34 pro- duced a significantly (P < 0.01) higher nodule dry weight in Yezin-3 but the dual inoculation of P4 + MAS34 did not produce the same result in Yezin-6. The dry matter production at the V6 stage was signifi- cantly (P < 0.01) different between the different inocula- tion treatments (Table 2). It was not significantly af- fected by the soybean variety. The Yezin-3 soybean vari- ety produced a significantly higher amount of dry matter (1.41 g·hill−1 in roots; 6.96 g·hill−1 in shoots) following the dual inoculation of P4 and MAS34. The same re- sponse was found in Yezin-6 (1.43 g·hill−1 in roots; 5.23 g·hill−1 in shoots) with the same dual inoculation of P4 and MAS34. The single inoculation of P4 produced a significant response compared to the uninoculated con- trol in Yezin-6, but there was no significant effect on dry matter production in Yezin-3 at the V6 stage. The dual inoculation of P4 and MAS34 resulted in the highest dry matter production regardless of soybean variety. How- ever, the interaction between variety and treatment was found to be not significant in relation to dry matter pro- duction. The dry matter production at the R3.5 growth stage was significantly (P < 0.01) increased following the in- oculation treatments (Table 3). However, there was no significant difference between the soybean variety and the interaction of variety and treatment. The Yezin-3 soybean variety produced a significantly higher amount of dry matter following the single inoculation of MAS34. However, the dual inoculation of P4 with MAS34 sig- nificantly increased dry matter production in Yezin-6. The single inoculation of P4 did not significantly affect dry matter production in Yezin-3 and Yezin-6 compared with the uninoculated control at the R3.5 stage. Table 3. Effects of co-inoculation of B. yuanmingense MAS34 and S. griseoflavus P4 on plant dr y weight and total N accumu- lation in the Yezin-3 and Yezin-6 soybean varieties at the R3.5 stage. Plant dry weight (g·hill−1) Treatment Nodule Root Shoot Total N accumulation (mg N·hill−1) Varieties Yezin-3 1.42 a 14.3 a 60.5 a 220 a Yezin-6 1.36 a 13.5 a 56.0 a 206 a Treatment of Yezin-3 Uninoculated 0.84 b 13.0 b 50.1 b 196 a P4 1.51 a 14.5 ab 61.5 ab 211 a MAS34 1.77 a 15.0 a 65.8 a 238 a P4 + MAS34 1.52 a 14.9 a 64.3 a 236 a Treatment of Yezin-6 Uninoculated 0.56 b 11.8 b 48.3 b 189 a P4 0.94 ab 13.5 ab 53.8 ab 198 a MAS34 0.83 ab 14.2 a 58.7 ab 211 a P4+MAS34 1.14 a 14.5 a 63.0 a 224 a Tukey HSD test Variety NS NS NS NS Treatment ** ** ** * Variety × Treatment NS NS NS NS CV% 10.9 4.29 7.76 9.07 N S, *, **: nonsignificant or significant at P < 0.05 or P < 0.01 respectively. Mean values followed by the same letter(s) indicate no significant difference. Copyright © 2013 SciRes. AJPS  Low-Density Co-Inoculation of Myanmar Bradyrhizobium yuanmingense MAS34 and Streptomyces griseoflavus P4 to Enhance Symbiosis and Seed Yield in Soybean Varieties 1885 The effects of the co-inoculation of Myanmar B. yuan- mingense MAS34 and S. griseoflavus P4 in enhancing the total N accumulation at the V6 and R3.5 stages of the two soybean varieties are presented in Tables 2 and 3. The total N accumulation by the soybeans at the V6 stage was significantly (P < 0.01) different between the inocu- lated treatments. The effect of variety and the interaction of variety with treatment had no significant influence on the total N accumulation by the soybeans at the V6 stage (Table 2). The dual inoculation of P4 with MAS34 in Yezin-3 significantly (P < 0.01) influenced the total N accumulation compared to the other treatments and the uninoculated control. All inoculated treatments were sig- nificantly (P < 0.01) better than the uninoculated control at the V6 stage in Yezin-6. The total N accumulation at the R3.5 stage was also observed to be significantly (P < 0.01) affected by the inoculation treatments. There was no effect of the variety or of the interaction of variety with treatment on the total N accumulation by soybeans at the R3.5 stage (Table 3). There was an insignificant effect on the total N accumu- lation in Yezin-3 and Yezin-6 compared with the union- culated control at the R3.5 stage (Table 3). A higher total N accumulation was observed following the dual inocu- lation of P4 with MAS34 in Yezin-6 and the single in- oculation of MAS34 in Yezin-3. 3.3. N2 Fixation at R3.5 Stage The RUI of root bled sap of soybean at the R3.5 growth stage [37] was used to indicate the extent of N2 fixation resulting from the inoculation treatment and the percent- age of plant N derived from the atmosphere within the soybean growing season. The RUI and the percentage of plant N derived from the atmosphere resulting from the co-inoculation of B. yuanmingense MAS34 and S. gri- seoflavus P4 in Yezin-3 and Yezin-6 at the R3.5 stage are presented in Table 4. Table 4. Effects of co-inoculation of B. yuanmingense MAS34 and S. griseoflavus P4 on N2 fixation in the Yezin-3 and Yezin-6 soybean varieties at the R3.5 stage. Treatment Relative ureide index (%) Percentage of plant N derived from atmosphere N (%) Varieties Yezin-3 67.9 a 69.5 b Yezin-6 79.2 a 86.5 a Treatment of Yezin-3 Uninoculated 53.4 b 47.8 b P4 68.9 ab 71.1 ab MAS34 77.8 a 84.4 a P4 + MAS34 71.4 ab 74.8 ab Treatment of Yezin-6 Uninoculated 74.3 b 79.1 b P4 74.5 ab 79.4 ab MAS34 80.3 ab 88.1 ab P4+MAS34 87.8 a 99.3 a Tukey HSD test Variety NS * Treatment ** ** Variety × Treatment NS NS CV% 7.98 11.7 N S, *, **: nonsignificant or significant at P < 0.05 or P < 0.01 respectively. Mean values followed by the same letter(s) indicate no significant difference. Copyright © 2013 SciRes. AJPS  Low-Density Co-Inoculation of Myanmar Bradyrhizobium yuanmingense MAS34 and Streptomyces griseoflavus P4 to Enhance Symbiosis and Seed Yield in Soybean Varieties 1886 The RUI and percentage of plant N derived from the atmosphere were significantly (P < 0.01) different be- tween the inoculation treatments. They were not signifi- cantly (P < 0.01) affected by the variety and the interac- tion of variety and inoculation treatment. In Yezin-3, a single inoculation of MAS34 (77.8%) significantly (P < 0.01) improved the RUI at the R.5 stage compared to the uninoculated control (53.2%). The percentage of plant N derived from the atmosphere from the single inoculation of MAS34 was about 84.4%. There was no significant difference between single and dual inoculations of P4 on the RUI of root bled sap from Yezin-3. The RUI values of the treatments were within the range of 68.9% - 71.4%, and plant N derived from N2 fixation ranged between 71.1% and 74.8%. The single inoculation of P4 did not improve the N2 fixation of soybean, but this treatment did increase N2 fixation by about 15% over that of the uninoculated control. Yezin-6 from the uninoculated control had an RUI value of about 74.3% for root bled sap at the R3.5 stage. The single inoculations of P4 and MAS34 had no sig- nificant effect on the RUI, and they were statistically equivalent to the uninoculated control. The dual inocula- tion of P4 with MAS34 (87.8%) resulted in a significant (P < 0.01) increase in the RUI compared to the other inoculated treatments and the uninoculated control (74.1%) in Yezin-6. The percentage of plant N derived from the atmosphere in the Yezin-6 soybean variety following the dual inoculation of P4 with MAS34 was about 99.3% and significantly different (P < 0.01) from the other treat- ments. The percentages of plant N derived from the at- mosphere from P4 (79.4%) and MAS34 (88.1%) were higher than that of the uninoculated control (79.1%). 3.4. Above-Ground Dry Biomass at the Maturity Stage The treatment and variety-treatment interaction had a significant (P < 0.05) effect on the above-ground dry bio- mass yield. The single inoculation of MAS34 produced a significantly larger dry biomass yield (80.4 g·hill−1) in Yezin-3 compared to P4 (62.3 g·hill−1) and the uninocu- lated control (41.4 g·hill−1). In Yezin-6, the dual inocula- tion of P4 + MAS 34 significantly increased the dry biomass (84.7 g·hill−1) compared to P4 (66.3 g·hill−1) and the uninoculated control (44.7 g·hill−1). In this study, the variety alone did not produce a significant response in the above-ground dry biomass yield (Figure 2). 3.5. Total N Accumulation at Maturity The total nitrogen uptake by soybean plants at maturity was significantly (P < 0.01) different between the inocu- lated treatments and the variety-treatment interaction. The inoculation of B. yuanmingense MAS34 resulted in the maximum N accumulation (139 kg·N·ha−1) at maturity in Yezin-3, which was significantly higher than with the inoculation of P4 and in the uninoculated control (Table 4). As in Yezin-6, the dual inoculation of P4 with MAS 34 resulted in a higher N accumulation (148 kg·N·ha−1) at maturity than in the uninoculated control (Figure 3). c b aab c bab a 0 10 20 30 40 50 60 70 80 90 Uninoculated P4 MAS34 P4+MAS34 Uninoculated P4 MAS34 P4+MAS34 Incomplete Seed Complete seed Unfilled Pod Shell St em Dry matter at Maturity stage (g·hill −1 ) Yezin-3(Rj 4 ) Yezin-6 (non-Rj) Figure 2. Effects of the co-inoculation of B. yuanmingense MAS34 and S. griseoflavus P4 on dry matter at the maturity stage in the Yezin-3 and Yezin-6 soybean varieties; the four treatments of uninoculated, P4, MAS34, and P4 + MAS34 are shown on the horizontal axis. Mean values followed by the same letter(s) indicate no significant difference. Copyright © 2013 SciRes. AJPS  Low-Density Co-Inoculation of Myanmar Bradyrhizobium yuanmingense MAS34 and Streptomyces griseoflavus P4 to Enhance Symbiosis and Seed Yield in Soybean Varieties 1887 c bc a ab b ab ab a 0 20 40 60 80 100 120 140 160 U ninoculated P4 MAS3 4 P4+MAS34 U ninoculated P4 MAS3 4 P4+MAS34 Incomplete Seed Complete seed Unfilled Pod Shell Stem Yezin-6 (non-Rj) accumulation at the maturity stage (kg·N·ha −1 ) Yezin-3( j 4 ) Figure 3. Effects of the co-inoculation of B. yuanmingense MAS34 and S. griseoflavus P4 on N accumulation at the maturity stage in the Yezin-3 and Yezin-6 soybean varieties; the four treatments of uninoculated, P4, MAS34, and P4 + MAS34 are shown on the The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/rqZzin. 3.6. Yield and Yield-Related Parameters The number of pods per plant was significantly (P < 0.01) different among the inoculation treatments in both Yezin- 3 and Yezin-6. The single inoculation of MAS34 resulted in a significantly higher number of pods per plant than the single inoculation of P4 and the uninoculated control in Yezin-3, but it was not statistically different from the P4 + MAS 34 treatment. In Yezin-6, the dual inoculation of P4 + MAS34 was significantly different to the union- culated control. The effect of the soybean variety alone was not significant in terms of the number of pods per plant, and the interaction of variety and inoculation treat- ment also had a non-significant influence on the number of pods per plant (Table 5). Although the interaction of variety and treatment did not significantly influence the number of seeds per pod, the effect of inoculation treatment and variety alone was significant (P < 0.01). The single inoculation of MAS34 and the dual inoculation of P4 + MAS34 resulted in a significantly higher number of seeds per pod than in the uninoculated control in Yezin-3. In Yezin-6, there was no significant difference between each treatment (Table 5). Hundred-seed weight was found to be significantly (P < 0.01) affected by the effect of inoculation treatment as well as the interaction between soybean variety and treat- ment. The single inoculation of MAS34 in Yezin-3 and the dual inoculation of P4 + MAS34 in Yezin-6 responded significantly. However, the main effect of soybean varie- ties alone was non-significant in relation to the hundred- seed weight (Table 5). Seed yield varied significantly (P < 0.01) due to the effects of both soybean variety and inoculation treatment (Table 5). The higher seed yield in Yezin-3 may be at- tributed to the larger number of pods per plant recorded for this variety. The analysis of variance indicated that variety and treatment had an interaction effect on the seed yield. The increase in seed yield was significant (P < 0.01) following the single inoculation of MAS34 (3.45 ton·ha−1) in Yezin-3 compared to the uninoculated con- trol. Significant (P < 0.01) increases in seed yield were recorded following the dual inoculation of P4 + MAS34 (2.90 ton·ha−1) in Yezin-6. 4. Discussion A successful Rhizobium-legume symbiosis largely de- pends on the presence of a specific and compatible strain in the soil for a particular legume. Several studies have reported a significant increase in soybean growth para- meters and yield due to the inoculation of bradyrhizobial isolates [38-40]. Many studies noted that soybean plant nodulation was inhibited when plants were inoculated with high-density cell inoculum [12,41-43]. Furthermore, significantly igher nodulation, fixed nitrogen, and seed yields were h Copyright © 2013 SciRes. AJPS  Low-Density Co-Inoculation of Myanmar Bradyrhizobium yuanmingense MAS34 and Streptomyces griseoflavus P4 to Enhance Symbiosis and Seed Yield in Soybean Varieties 1888 Table 5. Effects of co-inoculation of B. yuanmingense MAS34 and S. griseoflavus P4 on seed yield and yield components at the maturity stage in theYezin-3 and Yezin-6 soybean varieties. Treatment Pods per hill Seeds per pod Hundred-seed weight (g) Yield (ton·ha −1)) Varieties Yezin-3 151 a 1.54 a 14.0 a 2.76 a Yezin-6 165 a 1.11 b 13.0 a 2.02 b Treatment of Yezin-3 Uninoculated 131 c 1.39 b 12.5 b 1.93 c P4 MAS34 139 bc 177 a 1.54 ab 1.65 a 14.9 a 14.9 a 2.52 bc 3.45 a P4 + MAS34 158 ab 1.57 a 14.5 a 3.14 ab Treatment of Yezin-6 Uninoculated 145 b 1.01 a 11.7 b 1.42 b P4 151 b 1.13 a 13.2 ab 1.89 ab MAS34 170 ab 1.07 a 12.4 ab 1.86 ab P4 + MAS34 191 a 1.24 a 14.5 a 2.90 a Tukey HSD test Variety NS ** NS ** Treatment ** ** ** ** Variety × Treatment NS NS * * CV% 6.74 6.94 4.81 12.9 NS, *, **: nonsignificant or significant at P < 0.05 or P < 0.01 respectively. Mean values followed by the same letter(s) indicate no significant difference; 8.33 hills per square meter. observed in plants inoculated by low concentrations of USDA110 under field conditions [12]. In our study, plant biomass, nitrogen uptake, and seed yield in the Yezin-3 (Rj4) and Yezin-6 (non-Rj) soybean varieties were clearly enhanced at Kyushu University Farm when they were inoculated with a low density of (105 cell·seed−1) inocu- lums of B. yuanmingense MAS34 and S. griseoflavus P4. In this field experiment, soybean plants were grown in cultivated soil in an open field using ordinary water for irrigation throughout the experimental period. The plants from the uninoculated control and the S. griseoflavus P4 treatment were able to form root nodules, indicating that the indigenous rhizobia in the soil is nodulated to soy- bean varieties in the open field. This is in line with pre- vious findings [44] that the response of legumes to in- oculation depends largely on the number of rhizobia al- ready established in the soil, the availability of soil N, and the demand for N by the crop. The first root nodules formed on the basal part of the primary roots becoming visible about 10 days after plant- ing. They started to fix nitrogen (N2) at about 15 - 20 days after planting when the diameter reached approxi- mately 2 mm [45]. In the later stages, the nodules formed at the basal part of primary roots degrade, and a large number of new nodules formed on the lateral roots near the soil surface. These nodules play an important role in supplying N during the pod filling stage. In our study, a number of nodules formed on the tap roots and lateral roots of soybeans in accordance with the above descrip- tion and resulted in an improved seed yield. Souleimanov et al. [46] found that the Nod factor of B. japonicum had an effect on root growth that resulted in a 34% - 44% longer root in soybean. Significant effects on root biomass improvement in pea and lentil following seed treatment with strains of Rhizobium legumanosarum bv. viceae in field experiments have also been reported [47]. Studies have also shown results similar to our find- ing that a single inoculation of B. yuanmingense MAS34 increases root dry weight in Yezin-3 and Yezin-6 soy- beans at the V6 and R3.5 stages. In our study, a single inoculation of B. yuanmingense MAS34 resulted in a significant improvement in N2 fixa- Copyright © 2013 SciRes. AJPS  Low-Density Co-Inoculation of Myanmar Bradyrhizobium yuanmingense MAS34 and Streptomyces griseoflavus P4 to Enhance Symbiosis and Seed Yield in Soybean Varieties 1889 tion in Yezin-3 but only a positive trend in Yezin-6. This supports the conclusions of Wani et al. [48] that within grain legume species, genotypic variability affects the nodule number or nitrogenase activity. Comparative as- sessment of the field trial results suggested that B. yuan- mingense MAS34 consistently resulted in significant im- provements in nodulation, dry matter production, N2 fix- ation, and seed yield in Yezin-3. Increased nodulation and subsequent nitrogen fixation resulted in increased plant growth and grain yield. Similarly, Egamberdiyeva et al. [49] noticed a positive effect of inoculation with B. japonicum S2492 on growth, nodule number, and yield of soybean under field conditions. Milic et al. [50] also reported variability in the dry matter mass and nitrogen content in the nodules of soy- bean varieties following the use of different bradyrhizo- bial strains. Such variations may be attributed to differ- ences in the genomic constitution of the host or bacteria or both, which control symbiosis, or there might be more than one affinity group within the legume rhizobia. Ac- cording to Okereke et al. [7] the inoculation response of Bradyrhizobium in different soybean cultivars is cultivar- and site-specific. We also observed variations between the two tested soybean varieties in terms of their response to bradyrhizobial strain. In both soybean varieties, S. griseoflavu s P4 had a sig- nificant effect only on the improvement of plant dry weight at the V6 stage and dry matter production at ma- turity was significantly better than in the uninoculated control. Soe et al. [15] stated that S. griseoflavus P4 alone could improve the shoot dry weight of Myanmar soybean varieties. In the present study, the co-inoculation of MAS34 with P4 significantly improved nodule dry weight, plant biomass, N2 fixation, and seed yield in Yezin-6 but not in Yezin-3. This supports the results of our previous study [51], in which the dual inoculation of endophytic actinomycetes (Streptomeces sp. strain, P4) with bradyrhizobial strains increased the nodulation and nitrogen fixation in some soybean varieties but not in other varieties. Rj genes play a role in controlling the plant’s compati- bility with specific rhizobial strains and also the prefer- ence for indigenous soybean-nodulating rhizobia [26,27]. In addition, non-Rj is compatible with all bradyrhizobial strains but Rj4 has unique features that restrict nodulation with specific strains of Bradyrhizobium [28]. In this study, Yezin-3 (Rj4) responded significantly to the single inocu- lation of B. yua nmingen se MAS34. The MAS34 was iso- lated from a Rj4-genotype host [15]. These findings sup- port the results of previous studies [26-28]. The Rj-geno- type of soybeans appears to be compatible with the pref- erence of bradyrhizobial strains for soybean cultivation. Inoculation with effective strains of bradyrhizobia may promote soybean growth and seed yield. We found that the symbiotic interaction of the P4 with B. yuanmingense MAS34 significantly improved nodule dry weight, ni- trogen fixation, and seed yield in Yezin-6. This synergis- tic efficacy of S. griseoflavus P4 with B. yuanmingense MAS34 was found in Yezin-6 (non-Rj). This also sup- ports previous findings showing that the dual inoculation of bradyrhizobial strains and S. griseoflavu s P4 increased nodulation and nitrogen fixation in different soybean va- rieties [51,52]. Synergistic effects of the co-inoculation of S. griseofla- vus P4 with B. yuanming ense MAS34 have been found in Yezin-6 grown at Kyushu University Farm, Japan. This positive interaction was observed under standard envi- ronmental conditions, using the correct varieties, and pro- per nodulated bacteria in conjunction with S. griseoflavus P4. Our experimental results confirm the findings of Aka- rapisan et al. [52] and Soe et al. [51] that S. griseoflavus P4 is an effective endophytic actinomycete, which can be used in combination with selective root nodule bacterial strains for the production of economically important le- guminous crops. Further experimental investigations of the synergistic effectiveness of selected B. yuanming ense MAS34 and S. griseoflavus P4 with different soybean cultivars are needed to determine the optimal Myanmar soybean growing environment where this MAS34 was isolated. We envisage that the Myanmar Bradyrhizobium strain and S. griseoflavus P4 will be useful as biofertiliz- ers for soybean production in the future. 5. Acknowledgements This work was supported by grants from the Ministry of Education, Culture, Supports, Sciences and Technology of Japan. REFERENCES [1] M. Aslam, S. Mirza, M. Shjah, S. M. Jayed and N. Naeemullah, “New Early Maturing and High Yielding Soybean Varieties,” Crop Production Bulletin, Vol. 4, 1995, pp. 1-11. [2] FAO, “Legume Inoculants and Their Use,” NifTAL Pro- ject, USA Food and Agriculture Organization of the United Nations, Via delle Terme di Caracalla, Rome, It- aly, 1984. [3] CSO, “Myanmar Agricultural Statistics (1992-1993 to 2004-2005),” Central Statistical Organization, Ministry of National Planning and Economic Development, Yangon, Republic of the Union of Myanmar, 2006. [4] N. Boonkerd and P. Singleton, “Production of Rhizobium Biofertilizer,” In: S. Kannaiyan, Ed., Biotechnology of Biofertilizers, Narosa Publishing House, New Delhi, 2002, pp. 122-128. [5] G. Stacey, M. Libault, L. Brechenmacher, J. Wan and G. Copyright © 2013 SciRes. AJPS  Low-Density Co-Inoculation of Myanmar Bradyrhizobium yuanmingense MAS34 and Streptomyces griseoflavus P4 to Enhance Symbiosis and Seed Yield in Soybean Varieties 1890 D. May, “Genetics and Functional Genomics of Legume Nodulation,” Current Opinion in Plant Biology, Vol. 9, No. 2, 2006, pp. 110-121. doi:10.1016/j.pbi.2006.01.005 [6] P. H. Graham, “Nitrogen Transformations,” In: M. E. Sumner, Ed., Hand Book of Soil Science, CRC Press, London, 2000, pp. 139-147. [7] G. U. Okereke, C. Onochie and E. Onyeagba, “Effective- ness of Foreign Bradyrhizobia Strains in Enhancing No- dulation, Dry Matter and Seed Yield of Soybean (Glycine max L.) Cultivars in Nigeria,” Biology and Fertility of Soils, Vol. 33, No. 1, 2001, pp. 3-9. doi:10.1007/s003740000264 [8] H. H. Tien, T. M. Hien, M. T. Son and D. Herridge, “Rhi- zobial Inoculation and N2 Fixation of Soybean and Mung- bean in the Eastern Region of South Vietnam,” Proceed- ings of a Workshop Held in Hanoi, Vietnam, 2002, pp. 29-36. [9] S. K. Mahna, “Individual Partner Report,” INCO-DEV Research Project on Soybean BNF and Mycorrhization for Improved Production in South Asia, Department of Botany, Maharshi Dayanand Saraswati University, Ajmer, 2006. [10] R. W. Weaver and L. R. Frederick, “Effects of Inoculum Rate on Competitive Nodulation of Glycine max L. Merril. II. Field Studies,” Agronomy Journal, Vol. 66, No. 2, 1974, pp. 233-236. https://www.soils.org/publications/aj/abstracts/66/2/AJ06 60020233 [11] S. M. Lohrke, C. J. Madrzak, H. Hur, A. K. Judd, J. H. Orf and M. J. Sadowsky, “Inoculum Density-Dependent Restriction of Nodulation in the Soybean-Bradyrhizobium japonicum Symbiosis,” Symbiosis, Vol. 29, No. 1, 2000, pp. 59-70. http://eurekamag.com/research/003/479/inoculum-density -dependent-restriction-nodulation-soybean-bradyrhizobiu m-japonicum-symbiosis.php [12] T. Yamakawa and Y. Saeki, “Inoculation Methods of Bradyrhizobium japonicum on Soybean in South-West Area of Japan,” In: J. E. Board, Ed., A Comprehensive Survey of International Soybean Research—Genetics, Physiology, Agronomy and Nitrogen Relationships, In- Tech, Rijeka, 2013, pp. 83-114. [13] J. Tang-um and H. Niamsup, “Chitinase Production and Antifungal Potential of Endophytic Streptomyces Strain P4,” Maejo International Journal of Science and Tech- nology, Vol. 6, No. 1, 2012, pp. 95-104. http://www.mijst.mju.ac.th/vol6/95-104.pdf [14] P. Thapanapongworakul, “Characterization of Endophytic Actinomycetes Capable of Controlling Sweet Pea Root Rot Diseases and Effects on Root Nodule Bacteria,” M.Sc. Dissertation, Chiang Mai University, Chiang Mai, 2003. [15] K. M. Soe and T. Yamakawa, “Evaluation of Effective Myanmar Bradyrhizobium Strains Isolated from Myan- mar Soybean and Effects of Coinoculation with Strepto- myces griseoflavus P4 for Nitrogen Fixation,” Soil Sci- ence and Plant Nutrition, Vol. 59, No. 3, 2013, pp. 361- 370. doi:10.1080/00380768.2013.794437 [16] S. K. Dansoc and J. D. Owiredu, “Competitiveness of Introduce and Indigenous Cowpea in Three Soil,” Soil Biology and Biochemistry, Vol. 20, No. 3, 1988, pp. 305- 310. doi:10.1016/0038-0717(88)90008-9 [17] M. Yahya-Abadi, “Evaluation of Nitrogen Fixation Po- tential and Nutrients Uptaking in Some Common Bean Symbiosis Bacteria,” Proceeding of 10th Iranian Con- gress of Crop Production and Plant Breeding, Karaj, 19-21 August 2008, pp. 1-75. [18] M. Mehrpouyan, “Nitrogen Fixation Efficiency in Native Strains Compared with Non-Native Strains of Rhizobium leguminosarum,” In: International Conference on Envi- ronment Science and Engineering IPCBEE, Vol. 8, IAC- SIT Press, Singapore, 2011. [19] J. Muramoto, I. Goto and M. Ninaki, “Rapid Analysis of Exchangeable Cation and Cation Exchange Capacity (CEC) of Soils by Shaking Extraction Method,” Japanese Soil Science and Plant Nutrition, Vol. 63, No. 2, 1992, pp. 210-215 (in Japanese). [20] D. A. Cataldo, L. E. Schrader and V. L. Youngs, “Ana- lysis by Digestion and Colrimetric Assay of Total Ni- trogen in Plant Tissues High in Nitrate,” Crop Science, Vol. 14, No. 6, 1974, pp. 854-856. doi:10.2135/cropsci1974.0011183X001400060024x [21] J. Murphy and J. P. Riley, “A Modified Single Solution for the Determination of Phosphate in Natural Waters,” Analytica Chimica Acta, Vol. 27, No. 1, 1962, pp. 31-36. doi:10.1016/S0003-2670(00)88444-5 [22] E. Truog, “The Determination of the Readily Available Phosphorus in Soils,” Agronomy Journal, Vol. 22, No. 10, 1930, pp. 874-882. doi:10.2134/agronj1930.00021962002200100008x [23] P. Somasegaran and H. J. Hoben, “Handbook for Rhizo- bia,” In: R. C. Garber, Ed., Methods in Legumes-Rhizo- bium Technology, Springer-Verlag, Inc., New York, 1994, pp. 58-64. [24] K. M. Soe, T. Yamakawa, S. Hashimoto and P. Sarr, “Phylogenetic Diversity of Indigenous Soybean Bradyr- hizobia from Different Agro-Climatic Regions in Myan- mar,” Sci enceAsia , 2013, in Press. [25] T. E. Devine and L. D. Kuykendall, “Host Genetic Con- trol of Symbiosis in Soybean (Glycine max L.),” Plant and Soil, Vol. 186, No. 1, 1996, pp. 173-187. doi:10.1007/BF00035072 [26] J. Ishizuka, Y. Suemasu and K. Mizogami, “Preference of Rj-Soybean Cultivars for Bradyrhizobium japonicum for Nodulation,” Japanese Soil Science and Plant Nutrition, Vol. 37, No. 1, 1991, pp. 15-21. http://ci.nii.ac.jp/naid/110001720006 [27] Y. Saeki, I. Akagi, H. Takaki and Y. Nagatomo, “Diver- sity of Indigenous Bradyrhizobium Strains Isolated from Three Different Rj-Soybean Cultivars in Terms of Ran- domly Amplified Polymorphic DNA and Intrinsic Anti- biotic Resistance,” Japanese Soil Science and Plant Nu- trition, Vol. 46, No. 4, 2000, pp. 917-926. http://ci.nii.ac.jp/ naid/110001717919 [28] G. Vest and B. E. Caldwell, “Rj4-A Gene Conditioning Ineffective Nodulation in Soybean,” Crop Science, Vol. 12, No. 5, 1972, pp. 692-693. Copyright © 2013 SciRes. AJPS  Low-Density Co-Inoculation of Myanmar Bradyrhizobium yuanmingense MAS34 and Streptomyces griseoflavus P4 to Enhance Symbiosis and Seed Yield in Soybean Varieties 1891 [29] L. D. Kuykendall, “Isolation and Identification of Ge- netically Marked Strains of Nitrogen-Fixing Microsym- bionts of Soybeans,” In: G. H. Elkan, Ed., Practical Sym- biotic Nitrogen Fixation Methodology, Marcel Dekker, New York, 1987, pp. 205-220. [30] M. Shimizu, Y. Nakagawa, Y. Sato, T. Furumai, Y. Iga- rashi, H. Onaka, R. Yoshida and H. Kunoh, “Studies on Endophytic Actinomycetes (I) Streptomyces sp. Isolate from Rhododendron and Its Antifungal Activity,” Journal of General Plant Pathology, Vol. 66, No. 4, 2000, pp. 360-366. doi:10.1007/PL00012978 [31] T. Ohyama, M. Ito, K. Kobayashi, S. Araki, S. Yasuyoshi, O. Sasaki, T. Yamazaki, K. Soyama, R. Tanemura, Y. Mizuno and T. Ikarashi, “Analytical Procedures of N, P, K Contents in Plant and Manure Materials Using H2SO4- H2O2 Kjeldahl Digestion Method,” Bulletin of the Faculty of Agriculture, Niigata University, Vol. 43, 1991, pp. 110-120 (in Japanese). [32] M. B. Peoples, A. W. Faizah, B. Rerkasem and D. F. Her- ridge, “Methods for Evaluating Nitrogen Fixation by No- dulated Legumes in the Field,” Australian Centre for In- ternational Agricultural Research Monograph series, Vol. 11, No. 7, 1989, pp. 39-40. [33] S. Moore and W. H. Stein, “A Modified Ninhydrin Re- agent for the Photometric Determination of Amino Acids and Realted Compounds,” The Journal of Biological Che- mistry, Vol. 211, No. 2, 1954, pp. 907-913. http://www.jbc.org/content/211/2/907.citation [34] D. A. Cataldo, M. Haroon, L. E. Schrader and V. L. Youngs, “Rapid Colorimetric Determination of Nitrate in Plant Tissue by Nitration of Salicylic Acid,” Communica- tions in Soil Science and Plant Analysis, Vol. 6, No. 1, 1975, pp. 71-80. doi:10.1080/00103627509366547 [35] E. G. Young and C. F. Conway, “On the Estimation of Allantoin by the Rimini-Schryver Reaction,” The Journal of Biological Chemistry, Vol. 142, No. 2, 1942, pp. 839- 853. http://www.jbc.org/content/142/2/839.citation [36] D. F. Herridge and M. B. Peoples, “Ureide Assay for Measuring Nitrogen Fixation by Nodulated Soybean Cali- brated by 15N Methods,” Plant Physiology, Vol. 93, No. 2, 1990, pp. 495-503. doi:10.1104/pp.93.2.495 [37] D. F. Herridge and M. B. Peoples, “Timing of Xylem Sampling for Ureide Analysis of Nitrogen Fixation,” Plant and Soil, Vol. 238, No. 1, 2002, pp. 57-67. [38] H. M. A. Purcino, P. M. Festin and G. H. Elkan, “Iden- tification of Effective Strains of Bradyrhizobium for Ara- chis pintoi,” Tropical Agriculture, Vol. 77, No. 4, 2000, pp. 226-231. [39] B. D. Pant and B. N. Prasad, “Effectiveness of Bradyr- hizobium Isolates on Seedling Growth and Nitrogen Con- tent in Soybean [Glycine max (L.) Merr.],” Botanica Ori- entalis, Vol. 4, No. 1, 2004, pp. 1-3. http://nepjol.info/index.php/BOTOR/article/view/493 [40] K. M. Soe, A. Bhromsiri and D. Karladee, “Effect of Se- lected Endophytic Actinomycetes (Steptomyces sp.) and Bradyrhizobia from Myanmar on Growth, Nodulation, Nitrogen Fixation and Yield of Different Soybean Varie- ties,” Chiang Mai University Journal of Natural Sciences, Vol. 9, No. 1, 2010, pp. 95-109. [41] S. T. Takats, “Suppression of Nodulation in Soybeans by Superoptimal Inoculation with Bradyrhizobium japoni- cum,” Physiologia Plantarum, Vol. 66, No. 4, 1986, pp. 669-673. doi:10.1111/j.1399-3054.1986.tb05597.x [42] R. S. Smith, “Legume Inoculant Formulation and Appli- cation,” Canadian Journal of Microbiology, Vol. 38, No. 6, 1992, pp. 485-492. doi:10.1139/m92-080 [43] S. Jitacksorn and M. J. Sadowsky, “Nodulation Gene Re- gulation and Quorum Sensing Control Density-Dependent Suppression and Restriction of Nodulation in the Bradyr- hizobium japonicum-Soybean Symbiosis,” Applied and Environmental Microbiology, Vol. 74, No. 12, 2008, pp. 3749-3756. doi:10.1128/AEM.02939-07 [44] J. E. Thies, P. W. Singleton and B. B. Bohlool, “Influence of Size of Indiogenous Rhizobial Populations on Esta- blishment and Symbiotic Performance of Introduced Rhi- zobia on Field-grown Legumes,” Applied and Environ- mental Microbiology, Vol. 57, No. 1, 1991, pp. 19-28. [45] T. Sato, N. Onoma, H. Fujikake, N. Ohtake, K. Sueyoshi and T. Ohyama, “Changes in Four Leghemoglobin Com- ponents in Nodules of Hypernodulating Soybean (Glycine max [L] Merr.) Mutant and Its Parent in the Early Nodule Developmental Stage,” Plant and Soil, Vol. 237, No. 1, 2001, pp. 129-135. doi:10.1023/A:1013317219871 [46] A. Souleimanov, B. Prithiviraj and D. L. Smith, “The Major Nod Factor of Bradyrhizobium japonicum Pro- motes Early Growth of Soybean and Corn,” Journal of Experimental Botany, Vol. 53, No. 376, 2002, pp. 1929- 1934. doi:10.1093/jxb/erf034 [47] H. C. Huang and R. S. Erickson, “Effect of Seed Treat- ment with Rhizobium leguminosarum on Pythium Dump- ing-Off, Seedling Height, Root Nodulation, Root Biomass, Shoot Biomass, and Seed Yield of Pea and Lentil,” Jour- nal of Phytopathology, Vol. 155, No. 1, 2006, pp. 31-37. doi:10.1111/j.1439-0434.2006.01189.x [48] S. P. Wani, O. P. Rupela and K. K. Lee, “Sustainable Agriculture in a Semiarid Tropic through Biological Ni- trogen Fixation in Grain Legume,” Plant and Soil, Vol. 174, No. 2, 1995, pp. 29-49. doi:10.1007/BF00032240 [49] D. Egamberdiyeva, D. Qarshieva and K. Davranov, “The Use of Bradyrhizobium to Enhance Growth and Yield of Soybean in Calcareous Soil in Uzbekistan,” Journal of Plant Growth Regulation, Vol. 23, No. 1, 2004, pp. 54- 57. doi:10.1007/s00344-004-0069-4 [50] V. Milic, N. Mrkovacki, M. Popovic and D. Malencic, “Nodule Efficiency of Three Soybean Genotypes Inocu- lated by Different Methods,” Rostlinna Vyroba, Vol. 48, No. 8, 2002, pp. 356-360. http://www.agriculturejournals.cz/publicFiles/53851.pdf [51] K. M. Soe, A. Bhromsiri, D. Karladee and T. Yamakawa, “Effects of Endophytic Actinomycetes and Bradyrhizo- bium japonicum Strains on Growth, Nodulation, Nitrogen Fixation and Seed Weight of Different Soybean Varie- ties,” Soil Science and Plant Nutrition, Vol. 58, No. 3, 2012, pp. 319-325. [52] A. Akarapisan, A. Bhromsiri and P. Sangmanee, “Selec- Copyright © 2013 SciRes. AJPS  Low-Density Co-Inoculation of Myanmar Bradyrhizobium yuanmingense MAS34 and Streptomyces griseoflavus P4 to Enhance Symbiosis and Seed Yield in Soybean Varieties Copyright © 2013 SciRes. AJPS 1892 tion of Suitable Isolates of Endophytic Actinomycetes and Rhizobia for Improvement of N2 Fixation and Dis- ease Control of Various Pisum sativum on the Highland Area,” Final Report for Highland Research and Devel- opment Institute, Thailand, 2008, pp. 297-306.
|