 American Journal of Plant Sciences, 2013, 4, 1799-1810 http://dx.doi.org/10.4236/ajps.2013.49221 Published Online September 2013 (http://www.scirp.org/journal/ajps) Growth of Four Varieties of Barley (Hordeum vulgare L.) in Soils Contaminated with Heavy Metals and Their Effects on Some Physiological Traits Águeda González*, M. Carmen Lobo Instituto Madrileño de Investigación y Desarrollo Rural, Agrario y Alimentación, Madrid, Spain. Email: *agueda.gonzalez@madrid.org Received April 16th, 2013; revised May 17th, 2013; accepted June 19th, 2013 Copyright © 2013 Águeda González, M. Carmen Lobo. This is an open access article distributed under the Creative Commons At- tribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is prop- erly cited. ABSTRACT To evaluate the effect of zinc (Zn), cadmium (Cd), and chromium (Cr) on growth and selected physiological traits in barley, a greenhouse trial was performed using four barley varieties that were exposed to different concentration of these metals. The parameters quantified were growth, chlorophyll content, and chlorophyll fluorescence during three phenological stages: flag leaf, anthesis, and grain filling. The metal concentrations in both the plant and soil were also quantified. We determined that the varieties studied were more tolerant to Zn and Cd than to Cr. Treatment with Zn did not negatively affect growth, and only high concentrations of Cd decreased growth by approximately 4% to 8%. Plants treated with the highest Cr concentration stopped growing at the flag leaf stage. The amount of metal that accumulated in the plant increased with increasing metal concentration, and the highest amount of accumulated metal was recorded in the root and shoot. Both the plant height and dry weight were higher in the CB502 variety plants, followed by the Reinette, Pedrezuela, and Plaisant varieties. The same trend was observed for the chlorophyll content and fluorescence, with a significant correlation between the growth parameters and chlorophyll content (p < 0.001). Thus, we determined that barley has variability in the studied traits. Keywords: Growth; Heavy Metal; Barley; Chlorophyll Content 1. Introduction Environmental quality is increasingly affected by heavy metals present in the atmosphere, water, and soil. For this reason, the interest in understanding the toxic effects of heavy metals on crop growth and physiology has in- creased in the past few years. All plants absorb heavy metals from the substrate in which they grow to different degrees. The metal concen- trations in different parts of the plant depend on intrinsic (genetic) and extrinsic (environmental) factors and vary widely between the species and types of metal. Plants can tolerate large amounts of metal in their environment using two strategies: 1) Exclusion: The metal transport is restricted and minimal, and the metal concentration in the shoot remains relatively constant even within a wide range of metal concentrations in the soil. This is the most common strategy in species that are tolerant to metals; 2) Accumulation: Metals accumulate in a non-toxic form in the upper parts of terrestrial plants for both high and low concentrations present in the soil [1-3]. The ability of ter- restrial plants to absorb contaminants from the rhizo- sphere and move them to the shoot has resulted in an increase in the number of studies on plants that can im- prove soils contaminated by heavy metals [4-7]. The use of crops for phytoremediation has the advan- tages of producing large amounts of biomass and a great adaptability to different environmental conditions. How- ever, to be effective, the plants must be tolerant to con- taminants and must also be capable of accumulating large amounts of toxic elements in their tissues [8]. If the concentration of contaminants in the crop biomass is below the critical level for cattle consumption, these crops can add important economic value to the extraction process. The phytoextraction of metals is a promising method that is applicable to soils that are mildly or mod- erately contaminated, and this is an alternative to ex-situ *Corresponding author. Copyright © 2013 SciRes. AJPS  Growth of Four Varieties of Barley (Hordeum vulgare L.) in Soils Contaminated with Heavy Metals and Their Effects on Some Physiological Traits 1800 decontamination methods, which are both expensive and environmentally damaging [2,3,9]. For phytoextraction, two groups of plants were con- sidered: hyper-accumulating species that are capable of accumulating and tolerating high levels of metals, and species that produce large amounts of biomass, which compensates for their lower metal accumulation in tis- sues. In the latter group, cereal crops are increasingly of interest for phytoextraction processing of heavy metals [10-13]. Advances in plant improvement through genetic engineering, which can modify traits including absorp- tion, transport, accumulation, and tolerance of metals, have opened new possibilities for phytoremediation [7,14, 15]. Hyper-accumulating plants are, by definition, hyper- tolerant to metals that accumulate in the shoot; however, some genetic studies suggest that accumulation and tol- erance are independent traits [16]. In reality, accumulat- ing plants must have both traits to potentially accumulate large amounts of metal [17]. A plant that is only capable of accumulating is unlikely to survive in environments with high levels of available metals. Hyper-accumulating plants are much more efficient at translocating metals from the roots to the shoot than plants that are not hyper-accumulating. This can be ex- plained by the lower sequestration of metals into root vacuoles, which is typical of non-accumulating plants, or by more efficient transport through the xylem [2]. Plant capacity for accumulating Cd, Cr, and Zn differs between genotypes [12,18-20]. The existence of variabil- ity, together with evidence that phytoextraction is a pro- mising technique applicable to moderately contaminated soils and is an alternative to ex-situ decontamination, suggests that phytoremediation can offer a viable solu- tion to metal-contaminated soils. The goals of this study were as follows: 1) Evaluate the effect of different concentrations of Zn, Cd and Cr on growth in four varieties of barley; 2) Study the relation- ship between growth and several physiological parame- ters; 3) Analyze the differences between the barley varie- ties studied. 2. Materials and Methods 2.1. Plant Materials and Metal Treatments Four varieties of barley were used. These were two two- row varieties (Pedrezuela and Reinette) and two six-row varieties (CB502 and Plaisant). In November 2010, 156 pots of 4 L were planted in the greenhouse with two seeds per pot. The substrate used contained soil and sand in a 2:1 ratio. The pots were watered with tap water until the plants reached stage 20 using Zadoks’ scale [21]. The pots were then divided into three groups of 34 pots each. Each group was watered with solutions containing dif- ferent concentrations of Zn, Cd, or Cr (VI). Four treat- ments were applied per group (T0, T1, T2, T3) with four pots per treatment. Control pots (T0) were watered with 400 ml of tap water. The metal treated pots (T1 - T3) were watered up to the final crop cycle using 300 ml of tap water + 100 ml of the corresponding metal solution prepared using ZnSO4·7H2O, CdCl2·2,5H 2O, or K2Cr 2O7 for the Zn, Cd, and Cr treatments, respectively (Table 1). 2.2. Plant Height and Dry Weight Plant height was measured at the beginning of the treat- ment (S0), which included the flag leaf stage (i.e., the S1 sampling stage (41 Zadoks)); at anthesis (i.e., S2 sam- pling stage (65 Zadoks)); and during grain filling (i.e., S3 stage (80 Zadoks)). At the end of the crop cycle, spikes from each plant were removed and weighed. Next, the plants were cut at the soil level to collect the total biomass of the aerial part. The spikes were threshed in a spike thresher (Precision Machine Co. Inc.), and the grain obtained was ground using an IKA A10 grinder for metal analysis. Once washed, the roots from each plant were dried in a stove at 80˚C for 48 hours to obtain the dry weight. 2.3. Metal Analysis The metals in the stem and root were extracted after acid digestion of ashes. The soil metals were extracted in an acid medium using a microwave extraction system (Mul- tiwave 3000, Anton Paar GmbH, Graz, Austria). The analysis of Cd, Zn, and Cr in the corresponding extracts was performed using Atomic Absorption Spectroscopy (Varian AA 240 FS, Varian, Palo Alto, CA). 2.4. Chlorophyll Content The evaluation of the chlorophyll content was performed in intact leaves using a portable device (SPAD-502). The samplings were performed at the flag leaf (S1), anthesis (S2), and grain filling (S3) stages based on the flag leaf of the main stem from each plant. Four measurements were extracted per plant, and the mean value was deter- Table 1. Metal concentration applied by treatment. Concentration (mM) Treatment Zn Cd Cr (VI) T0 0 0 0 T1 50 10 1 T2 150 20 2 T3 250 40 3 Copyright © 2013 SciRes. AJPS  Growth of Four Varieties of Barley (Hordeum vulgare L.) in Soils Contaminated with Heavy Metals and Their Effects on Some Physiological Traits 1801 mined for each leaf. 2.5. Chlorophyll Fluorescence Measurements Chlorophyll fluorescence was measured at the flag leaf (S1), anthesis (S2), and grain filling (S3) stages using an F MS2 fluorometer (Hansatech Instruments Ltd., Eng- land). Fluorescence parameters were measured in the central part of the flag leaf of the main stem from each plant after adaptation to the dark for 30 minutes. 2.6. Statistical Analysis The data were analyzed using SAS for analysis of vari- ance. The means between treatments were compared us- ing a Duncan test or with LSD values. 3. Results 3.1. Effects of Zn, Cd, and Cr on Growth 3.1.1. Pl ant Height At the beginning of each treatment, the plant heights were similar between each genotype, with a mean of 42, 33, 32, and 28 cm for the CB502, Pedrezuela, Reinette, and Plaisant varieties, respectively (Figure 1). Plants treated with solutions containing a variety of Zn and Cd concentrations continued to grow until the end of the grain filling period, with a significantly greater mean plant height during this period than plant height at the beginning of the treatments or at the flag leaf stage. The differences observed in plant height between the anthesis and grain filling stages were very small and were not significant in the four genotypes studied. The growth of the treated plants compared to the growth of the control plants demonstrated the greatest difference in plants treated with the highest metal concentrations, which cor- responded to treatment T3. In CB502 and Pedrezuela barley, the growth of the control plants was 45% and 50% respectively, similar to that of the plants treated with the highest Zn concentration. However, in plants treated with the highest Cd concentration, the growth was 41% for CB502 and 45% for Pedrezuela relative to the control plants. For the Reinette and Plaisant varieties, the mean growth of the control plants was 62%. In plants treated with the highest concentration of Zn, the growth was 58% for the two varieties. When the highest Cd con- centration was used, the growth was 54% for Reinette and 56% for Plaisant. Treatment with Cr affected plant growth more drasti- cally in all genotypes studied. The mean height of the four barley varieties treated with Cr was significantly lower than the height of plants treated with different con- centrations of Zn or Cd. As observed in Figure 1, only plants treated with the lowest concentrations of Cr con- tinued to grow until the end of the cycle. The growth of plants treated with the lowest Cr concentration was 27% for CB502, Pedrezuela, and Reinette, and 32% for Plai- sant. For the four varieties, the growth was lower than during treatments with the highest concentrations of Zn and Cd. The growth of plants treated with the intermedi- ate Cr concentration was 17% for Reinette and Pedre- zuela and 7% for Plaisant and CB502. The growth was 0% for plants treated with the highest Cr concentration for all four of the varieties studied. 3.1.2. Dry Weight The mean dry weight for the aerial plant parts was great- est for CB502, followed by Reinette, Plaisant, and Pe- drezuela. The differences between all four genotypes were statistically significant (Figure 2A). The dry weights of plants treated with Zn or Cd were similar and signifi- cantly higher than the weights of plants treated with Cr. In plants treated with different concentrations of Zn and Cd, the dry weight of control plants were very similar or even lower than the weights of the plants treated with the highest metal concentrations. Indeed, for Plaisant, the weights of the plants treated with the highest concentra- tion of Zn and Cd were 86 g and 109 g, respectively, and the weight of the control plants was 65 g. These results are expected because plants treated with Zn and Cd did not show toxicity symptoms such as leaf chlorosis until the end of the crop cycle. The leaves on plants treated with the highest concentrations of Cd dried earlier relative to the control; however, plants treated with Cr displayed the toxic effects produced by the metal across all treatments. Plants that demonstrated the most severe effect on growth were those treated with the high- est concentration of Cr. Indeed, these plants dried before completing the crop cycle. For all varieties studied, the dry weight of the plants treated with the highest Cr con- centration was lower than the weight of the controls. The percent decrease with respect to the control plants was 88%, 87%, 86%, and 79% for CB502, Pedrezuela, Rei- nette, and Plaisant, respectively. The dry weights of the roots were higher in plants treated with Zn and Cd. The dry weight varied between 12% in Reinette treated with Zn and 53% in Plaisant treated with the highest concentration of Cd (Figure 2B). The opposite occurred in plants treated with Cr. The re- duction in root dry weight was recorded in all cases and was 61%, 77%, 36%, and 40% for CB502, Pedrezuela, Reinette, and Plaisant, respectively. The correlation of the dry weight of the aerial parts and roots was highly significant (r = 0.68; p < 0.001). The correlations were also significant between the height and dry weight of the aerial parts (r = 0.85; p < 0.001) and between the height and weight of the roots (r = dry Copyright © 2013 SciRes. AJPS 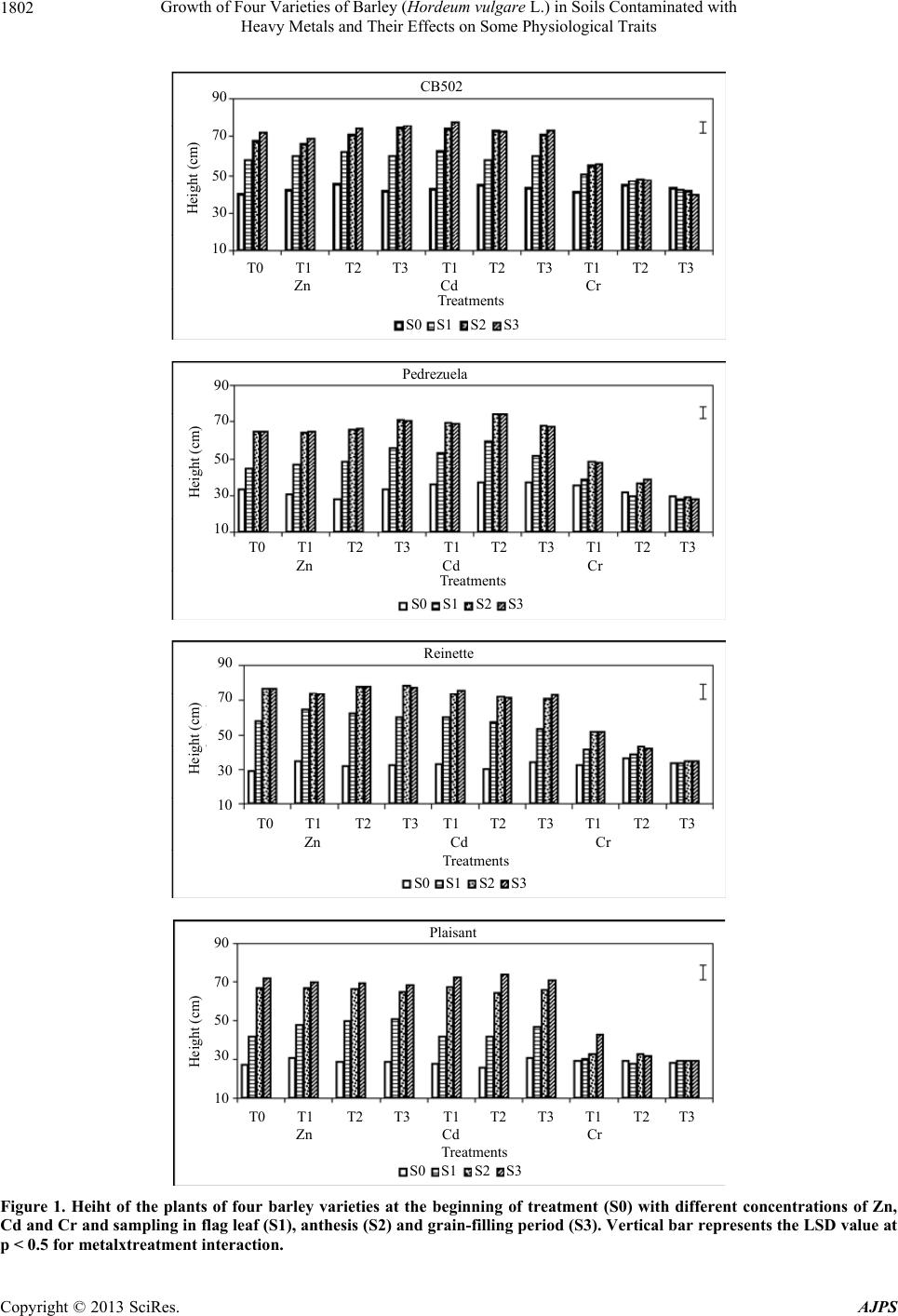 Growth of Four Varieties of Barley (Hordeum vulgare L.) in Soils Contaminated with Heavy Metals and Their Effects on Some Physiological Traits Copyright © 2013 SciRes. AJPS 1802 CB502 T0 T1 T2T3T1T2T3T1T3 T2 Zn Cd Cr Treatments S0 S1S2S3 10 30 50 70 90 Height (cm) Pedrezuela T0 T1 T2T3T1T2T3T1T3 T2 Zn Cd Cr Treatments S0 S1S2 S3 10 30 50 70 90 Height (cm) Reinette T0 T1 T2T3T1T2T3T1T3 T2 Zn CdCr Treatments S0 S1S2 S3 10 30 50 70 90 Height (cm) Plaisant T0 T1 T2T3T1T2 T3T1T3 T2 Zn Cd Cr Treatments S0 S1S2 S3 10 30 50 70 90 Height (cm) Figure 1. Heiht of the plants of four barley varieties at the beginning of treatment (S0) with different concentrations of Zn, Cd and Cr and sampling in flag leaf (S1), anthesis (S2) and grain-filling period (S3). Vertical bar represents the LSD value at p < 0.5 for metalxtreatment interaction. 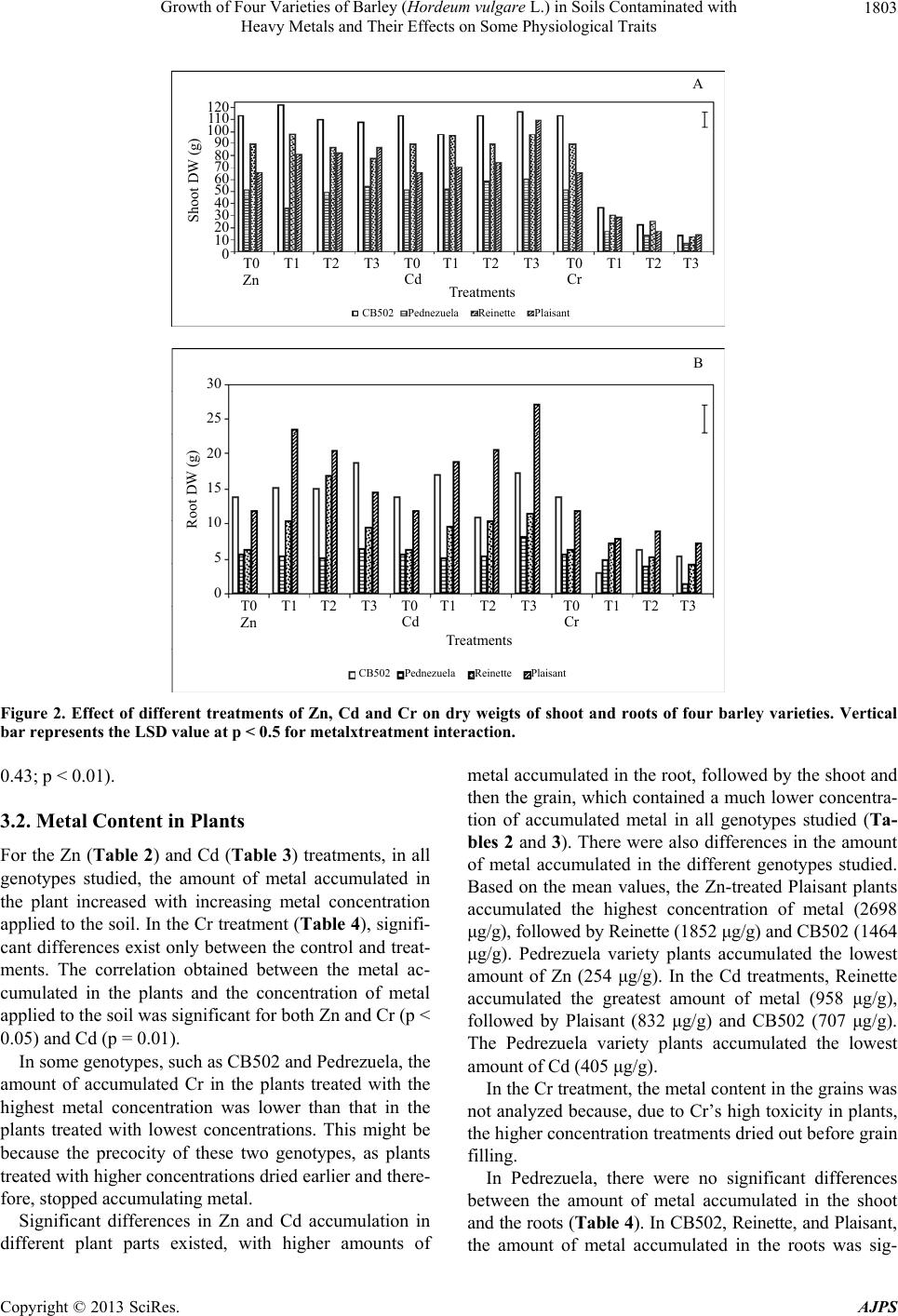 Growth of Four Varieties of Barley (Hordeum vulgare L.) in Soils Contaminated with Heavy Metals and Their Effects on Some Physiological Traits 1803 T0T1 T2 T3T1T2T3T1T3 T2 Zn Cd Cr Treatments CB502 0 Shoot DW (g) A T0T0 Pednezuela Reinette Plaisant 10 20 30 40 50 60 70 80 90 100 11 0 120 T0T1 T2 T3T1T2T3T1T3 T2 Zn CdCr Treatments CB502 0 Root DW (g) B T0T0 Pednezuela ReinettePlaisant 5 10 15 20 25 30 Figure 2. Effect of different treatments of Zn, Cd and Cr on dry weigts of shoot and roots of four barley varieties. Vertical bar represents the LSD value at p < 0.5 for metalxtreatment interaction. 0.43; p < 0.01). 3.2. Metal Content in Plants For the Zn (Table 2) and Cd (Table 3) treatments, in all genotypes studied, the amount of metal accumulated in the plant increased with increasing metal concentration applied to the soil. In the Cr treatment (Table 4), signifi- cant differences exist only between the control and treat- ments. The correlation obtained between the metal ac- cumulated in the plants and the concentration of metal applied to the soil was significant for both Zn and Cr (p < 0.05) and Cd (p = 0.01). In some genotypes, such as CB502 and Pedrezuela, the amount of accumulated Cr in the plants treated with the highest metal concentration was lower than that in the plants treated with lowest concentrations. This might be because the precocity of these two genotypes, as plants treated with higher concentrations dried earlier and there- fore, stopped accumulating metal. Significant differences in Zn and Cd accumulation in different plant parts existed, with higher amounts of metal accumulated in the root, followed by the shoot and then the grain, which contained a much lower concentra- tion of accumulated metal in all genotypes studied (Ta- bles 2 and 3). There were also differences in the amount of metal accumulated in the different genotypes studied. Based on the mean values, the Zn-treated Plaisant plants accumulated the highest concentration of metal (2698 μg/g), followed by Reinette (1852 μg/g) and CB502 (1464 μg/g). Pedrezuela variety plants accumulated the lowest amount of Zn (254 μg/g). In the Cd treatments, Reinette accumulated the greatest amount of metal (958 μg/g), followed by Plaisant (832 μg/g) and CB502 (707 μg/g). The Pedrezuela variety plants accumulated the lowest amount of Cd (405 μg/g). In the Cr treatment, the metal content in the grains was not analyzed because, due to Cr’s high toxicity in plants, the higher concentration treatments dried out before grain filling. In Pedrezuela, there were no significant differences between the amount of metal accumulated in the shoot and the roots (Table 4). In CB502, Reinette, and Plaisant, the amount of metal accumuated in the roots was sig- l Copyright © 2013 SciRes. AJPS 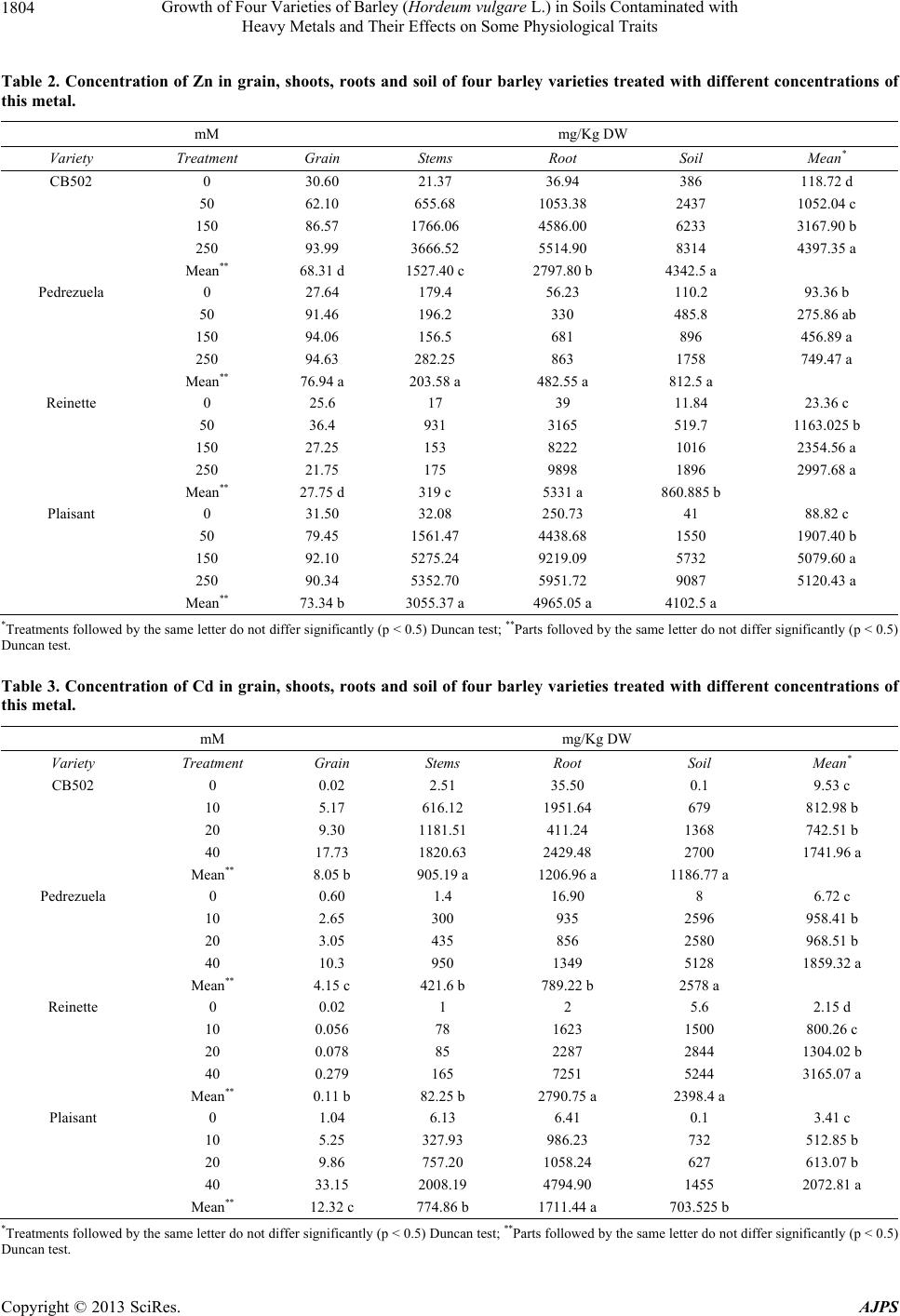 Growth of Four Varieties of Barley (Hordeum vulgare L.) in Soils Contaminated with Heavy Metals and Their Effects on Some Physiological Traits 1804 Table 2. Concentration of Zn in grain, shoots, roots and soil of four barley varieties treated with different concentrations of this metal. mM mg/Kg DW Variety Treatment Grain Stems Root Soil Mean* CB502 0 30.60 21.37 36.94 386 118.72 d 50 62.10 655.68 1053.38 2437 1052.04 c 150 86.57 1766.06 4586.00 6233 3167.90 b 250 93.99 3666.52 5514.90 8314 4397.35 a Mean** 68.31 d 1527.40 c 2797.80 b 4342.5 a Pedrezuela 0 27.64 179.4 56.23 110.2 93.36 b 50 91.46 196.2 330 485.8 275.86 ab 150 94.06 156.5 681 896 456.89 a 250 94.63 282.25 863 1758 749.47 a Mean** 76.94 a 203.58 a 482.55 a 812.5 a Reinette 0 25.6 17 39 11.84 23.36 c 50 36.4 931 3165 519.7 1163.025 b 150 27.25 153 8222 1016 2354.56 a 250 21.75 175 9898 1896 2997.68 a Mean** 27.75 d 319 c 5331 a 860.885 b Plaisant 0 31.50 32.08 250.73 41 88.82 c 50 79.45 1561.47 4438.68 1550 1907.40 b 150 92.10 5275.24 9219.09 5732 5079.60 a 250 90.34 5352.70 5951.72 9087 5120.43 a Mean** 73.34 b 3055.37 a 4965.05 a 4102.5 a *Treatments followed by the same letter do not differ significantly (p < 0.5) Duncan test; **Parts folloved by the same letter do not differ significantly (p < 0.5) Duncan test. Table 3. Concentration of Cd in grain, shoots, roots and soil of four barley varieties treated with different concentrations of this metal. mM mg/Kg DW Variety Treatment Grain Stems Root Soil Mean* CB502 0 0.02 2.51 35.50 0.1 9.53 c 10 5.17 616.12 1951.64 679 812.98 b 20 9.30 1181.51 411.24 1368 742.51 b 40 17.73 1820.63 2429.48 2700 1741.96 a Mean** 8.05 b 905.19 a 1206.96 a 1186.77 a Pedrezuela 0 0.60 1.4 16.90 8 6.72 c 10 2.65 300 935 2596 958.41 b 20 3.05 435 856 2580 968.51 b 40 10.3 950 1349 5128 1859.32 a Mean** 4.15 c 421.6 b 789.22 b 2578 a Reinette 0 0.02 1 2 5.6 2.15 d 10 0.056 78 1623 1500 800.26 c 20 0.078 85 2287 2844 1304.02 b 40 0.279 165 7251 5244 3165.07 a Mean** 0.11 b 82.25 b 2790.75 a 2398.4 a Plaisant 0 1.04 6.13 6.41 0.1 3.41 c 10 5.25 327.93 986.23 732 512.85 b 20 9.86 757.20 1058.24 627 613.07 b 40 33.15 2008.19 4794.90 1455 2072.81 a Mean** 12.32 c 774.86 b 1711.44 a 703.525 b *Treatments followed by the same letter do not differ significantly (p < 0.5) Duncan test; **Parts followed by the same letter do not differ significantly (p < 0.5) Duncan test. Copyright © 2013 SciRes. AJPS 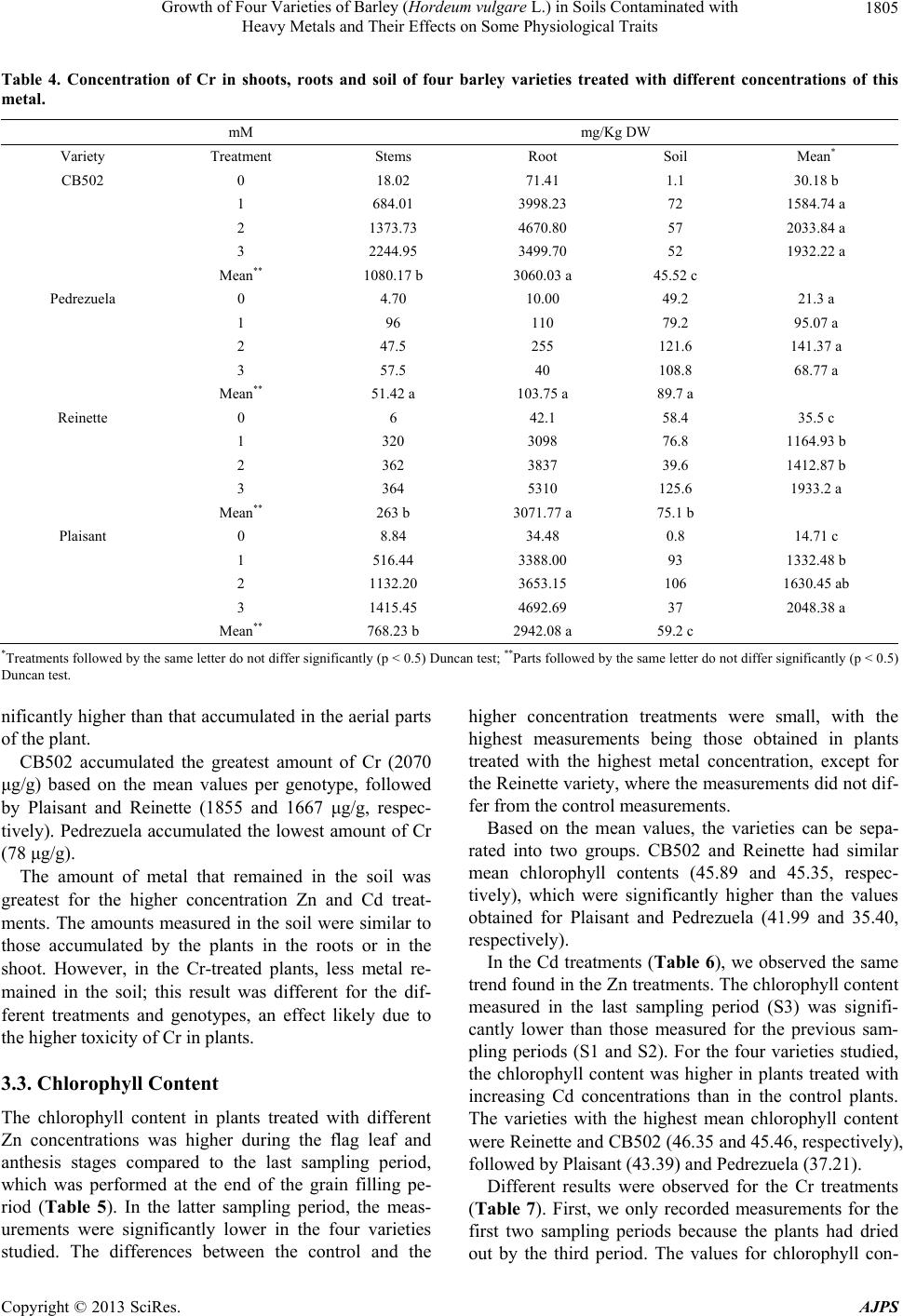 Growth of Four Varieties of Barley (Hordeum vulgare L.) in Soils Contaminated with Heavy Metals and Their Effects on Some Physiological Traits 1805 Table 4. Concentration of Cr in shoots, roots and soil of four barley varieties treated with different concentrations of this metal. mM mg/Kg DW Variety Treatment Stems Root Soil Mean* CB502 0 18.02 71.41 1.1 30.18 b 1 684.01 3998.23 72 1584.74 a 2 1373.73 4670.80 57 2033.84 a 3 2244.95 3499.70 52 1932.22 a Mean** 1080.17 b 3060.03 a 45.52 c Pedrezuela 0 4.70 10.00 49.2 21.3 a 1 96 110 79.2 95.07 a 2 47.5 255 121.6 141.37 a 3 57.5 40 108.8 68.77 a Mean** 51.42 a 103.75 a 89.7 a Reinette 0 6 42.1 58.4 35.5 c 1 320 3098 76.8 1164.93 b 2 362 3837 39.6 1412.87 b 3 364 5310 125.6 1933.2 a Mean** 263 b 3071.77 a 75.1 b Plaisant 0 8.84 34.48 0.8 14.71 c 1 516.44 3388.00 93 1332.48 b 2 1132.20 3653.15 106 1630.45 ab 3 1415.45 4692.69 37 2048.38 a Mean** 768.23 b 2942.08 a 59.2 c *Treatments followed by the same letter do not differ significantly (p < 0.5) Duncan test; **Parts followed by the same letter do not differ significantly (p < 0.5) Duncan test. nificantly higher than that accumulated in the aerial parts of the plant. CB502 accumulated the greatest amount of Cr (2070 μg/g) based on the mean values per genotype, followed by Plaisant and Reinette (1855 and 1667 μg/g, respec- tively). Pedrezuela accumulated the lowest amount of Cr (78 μg/g). The amount of metal that remained in the soil was greatest for the higher concentration Zn and Cd treat- ments. The amounts measured in the soil were similar to those accumulated by the plants in the roots or in the shoot. However, in the Cr-treated plants, less metal re- mained in the soil; this result was different for the dif- ferent treatments and genotypes, an effect likely due to the higher toxicity of Cr in plants. 3.3. Chlorophyll Content The chlorophyll content in plants treated with different Zn concentrations was higher during the flag leaf and anthesis stages compared to the last sampling period, which was performed at the end of the grain filling pe- riod (Table 5). In the latter sampling period, the meas- urements were significantly lower in the four varieties studied. The differences between the control and the higher concentration treatments were small, with the highest measurements being those obtained in plants treated with the highest metal concentration, except for the Reinette variety, where the measurements did not dif- fer from the control measurements. Based on the mean values, the varieties can be sepa- rated into two groups. CB502 and Reinette had similar mean chlorophyll contents (45.89 and 45.35, respec- tively), which were significantly higher than the values obtained for Plaisant and Pedrezuela (41.99 and 35.40, respectively). In the Cd treatments (Table 6), we observed the same trend found in the Zn treatments. The chlorophyll content measured in the last sampling period (S3) was signifi- cantly lower than those measured for the previous sam- pling periods (S1 and S2). For the four varieties studied, the chlorophyll content was higher in plants treated with increasing Cd concentrations than in the control plants. The varieties with the highest mean chlorophyll content were Reinette and CB502 (46.35 and 45.46, respectively), followed by Plaisant (43.39) and Pedrezuela (37.21). Different results were observed for the Cr treatments (Table 7). First, we only recorded measurements for the first two sampling periods because the plants had dried out by the third period. The values for chlorophyll con- Copyright © 2013 SciRes. AJPS  Growth of Four Varieties of Barley (Hordeum vulgare L.) in Soils Contaminated with Heavy Metals and Their Effects on Some Physiological Traits 1806 Table 5. Effect of different treatment of Zn on chlorophyll content of four barley varieties. Variety Treatment SPAD values Mean* mM S1 S2 S3 CB502 0 45.3245.32 35.50 42.05 c 50 46.9550.30 37.88 45.04 b 150 49.4351.58 45.35 48.79 a 250 47.6051.38 44.08 47.69 a Mean** 47.33 b49.65 a 40.70 c Pedrezuela 0 44.3240.85 15.78 33.65 b 50 48.7245.93 7.93 34.19 ab 150 48.2546.07 14.67 36.33 a 250 50.6648.10 13.49 37.41 a Mean** 47.98 a45.24 b 12.97 c Reinette 0 46.7045.31 37.70 43.24 c 50 49.9352.63 45.22 49.26 a 150 48.0048.89 40.81 45.9 b 250 45.4245.66 37.92 43 c Mean** 47.51 a48.12 a 40.41 b Plaisant 0 40.6442.68 40.28 41.2 ab 50 44.4243.88 40.57 42.95 a 150 41.0441.01 35.97 39.34 b 250 44.6345.81 42.93 44.46 a Mean** 42.69 a43.34 a 39.94 b *Treatments followed by the same letter do not differ significantly (p < 0.5) Duncan test; **Sampling followed by the same letter do not differ signifi- cantly (p < 0.5) Duncan test. Table 6. Effect of different treatments of Cd on chlorophyll content of four barley varieties. Variety Treatment SPAD values Mean* mM S1 S2 S3 CB502 0 45.32 45.32 35.50 42.05 b 10 49.77 51.23 37.15 46.05 a 20 46.88 51.32 39.15 45.78 a 40 51.25 52.47 40.15 47.95 a Mean** 48.31 a50.08 a 38.24 b Pedrezuela 0 44.32 40.85 15.78 33.65 c 10 49.82 47.92 13.87 37.2 b 20 49.52 46.84 17.44 37.93 b 40 52.17 49.51 18.47 40.05 a Mean** 48.95 a46.28 b 16.39 c Reinette 0 46.70 45.31 37.70 43.24 c 10 47.90 50.51 43.86 47.42 ab 20 46.33 49.61 41.86 45.93 b 40 49.56 51.21 45.66 48.81 a Mean** 47.62 a49.16 a 42.27 b Plaisant 0 40.64 42.68 40.28 41.2 b 10 41.52 44.78 42.37 42.89 b 20 45.68 46.90 44.08 45.55 a 40 44.31 47.26 40.19 43.92 a Mean** 43.04 b45.40 a 41.73 c *Treatments followed by the same letter do not differ significantly (p < 0.5) Duncan test; **Sampling followed by the same letter do not differ signifi- cantly (p < 0.5) Duncan test. Table 7. Effect of different treatments of Cr on chlorophyll content of four barley varieties. Variety TreatmentSPAD values SPAD valuesMean* mM S1 S2 CB502 0 45.32 45.32 45.32 b 1 55.82 55.42 55.61 a 2 51.90 28.37 40.13 c 3 21.58 10.92 16.25 d Mean** 43.65 a 35.06 b Pedrezuela0 44.32 40.85 42.58 a 1 42.20 46.47 44.33 a 2 46.45 13.08 29.76 b 3 25.62 7.94 16.78 c Mean** 39.64 a 27.08 b Reinette 0 46.70 45.31 46.10 a 1 44.58 49.63 47.11 a 2 46.37 23.44 34.90 b 3 32.23 9.78 21.01 c Mean** 42.47 a 32.04 b Plaisant 0 45.64 42.68 44.16 a 1 46.03 40.20 48.12 a 2 46.32 39.26 42.79 b 3 19.78 9.78 14.78 c Mean** 39.44 a 32.98 b *Treatments followed by the same letter do not differ significantly (p < 0.5) Duncan test; **Sampling followed by the same letter do not differ signifi- cantly (p < 0.5) Duncan test. tent obtained in the second sampling period were sig- nificantly lower than the values measured during the first period for all varieties studied. In contrast, when the Cr concentration increased, the chlorophyll content was sig- nificantly lower than in the control plants in the four va- rieties studied. It was evident that the mean values for the genotypes followed the same trend for the Zn and Cd treatments, although differences between the genotypes were smaller. The CB502 variety demonstrated the highest chlorophyll content (39.33), followed by Reinette (37.28), Plaisant (37.46), and Pedrezuela (33.36). The differences were significant only between the CB502 and Pedrezuela ge- notypes. 3.4. Chlorophyll Fluorescence In plants treated with different concentrations of Zn (Ta- ble 8), chlorophyll fluorescence was similar across all treatments. Between the sampling periods, the most im- portant differences were between the first and final sam- pling period, except for the Plaisant variety plants, where no significant differences were recorded at any of the sampling periods. The varieties with the highest mean Copyright © 2013 SciRes. AJPS  Growth of Four Varieties of Barley (Hordeum vulgare L.) in Soils Contaminated with Heavy Metals and Their Effects on Some Physiological Traits 1807 Table 8. Effect of different treatments of Zn on chlorophy ll fluorescence of four barley varieties. Variety Treatment Fv/Fm Mean mM S1 S2 S3 CB502 0 0.847 0.832 0.821 0.833 a 50 0.851 0.852 0.817 0.840 a 150 0.849 0.836 0.832 0.839 a 250 0.851 0.850 0.821 0.841 a Mean** 0.849 a0.842 a 0.823 b Pedrezuela 0 0.838 0.841 0.540 0.740 b 50 0.849 0.835 0.584 0.756 ab 150 0.843 0.834 0.650 0.775 a 250 0.845 0.838 0.653 0.779 a Mean** 0.844 a0.837 a 0.607 b Reinette 0 0.849 0.826 0.825 0.833 a 50 0.849 0.836 0.838 0.841 a 150 0.853 0.833 0.831 0.839 a 250 0.852 0.838 0.828 0.839 a Mean** 0.850 a0.833 b 0.830 b Plaisant 0 0.847 0.835 0.831 0.838 a 50 0.849 0.830 0.833 0.837 a 150 0.845 0.833 0.826 0.835 a 250 0.844 0.837 0.836 0.839 a Mean** 0.846 a0.834 a 0.831 a *Treatments followed by the same letter do not differ significantly (p < 0.5) Duncan test; **Sampling followed by the same letter do not differ signifi- cantly (p < 0.5) Duncan test. Fv/Fm values were CB502 and Reinette (0.838), fol- lowed by Plaisant (0.837) and Pedrezuela (0.763). There were no differences recorded in chlorophyll fluorescence among the different Zn concentrations tested. The Fv/Fm values were lower during the third sampling period, ex- cept for in the Plaisant variety plants, where no differ- ences between periods appeared. The CB502 and Rei- nette varieties had the highest mean Fv/Fm values (0.838), followed by Plaisant (0.836) and Pedrezuela (0.763), which had significantly lower values. In Cd treatments (Table 9), the Fv/Fm values were similar between the control plants and those treated with the lowest metal concentrations, and these values were significantly lower for plants treated with the highest metal concentration for all genotypes tested. The only ex- ceptions were in CB502 variety plants, where values for Table 9. Effect of different treatments of Cd on chlorophyll fluorescence of four barley varieties. Variety Treatment Fv/Fm Mean* mM S1 S2 S3 CB502 0 0.847 0.832 0.821 0.833 a 10 0.852 0.849 0.829 0.843 a 20 0.843 0.853 0.829 0.841 a 40 0.850 0.852 0.833 0.844 a Mean** 0.848 a 0.846 a 0.827 b Pedrezuela0 0.838 0.841 0.640 0.773 a 10 0.840 0.831 0.694 0.788 a 20 0.845 0.822 0.645 0.770 a 40 0.838 0.838 0.586 0.754 b Mean** 0.840 a 0.832 a 0.616 b Reinette 0 0.849 0.826 0.825 0.833 a 10 0.851 0.834 0.835 0.839 a 20 0.852 0.831 0.832 0.838 a 40 0.838 0.811 0.742 0.796 b Mean** 0.847 a 0.825 b 0.808 b Plaisant 0 0.847 0.835 0.831 0.837 a 10 0.842 0.840 0.840 0.840 a 20 0.842 0.845 0.831 0.842 a 40 0.841 0.839 0.767 0.815 b Mean** 0.843 a 0.839 a 0.817 b *Treatments followed by the same letter do not differ significantly (p < 0.5) Duncan test; **Sampling followed by the same letter do not differ signifi- cantly (p < 0.5) Duncan test. the high concentration treatments did not differ from the other three treatments. Across the sampling periods, the most important differences were observed between the first and the third periods. The varieties with the highest mean Fv/Fm values were CB502, Reinette, and Plaisant (0.840, 0.827, and 0.834, respectively). Pedrezuela dis- played the lowest value (0.771). The Fv/Fm values in plants treated with the highest Cd concentrations were significantly lower relative to the other treatments, ex- cept for the CB502 variety plants, in which it did not differ from the other treatments. The last sampling period recorded significantly lower values for the four varieties studied. CB501, Plaisant, and Reinette displayed the highest mean genotype values (0.840, 0.834, and 0.827, respectively), and Pedrezuela had the lowest value (0.771). Copyright © 2013 SciRes. AJPS 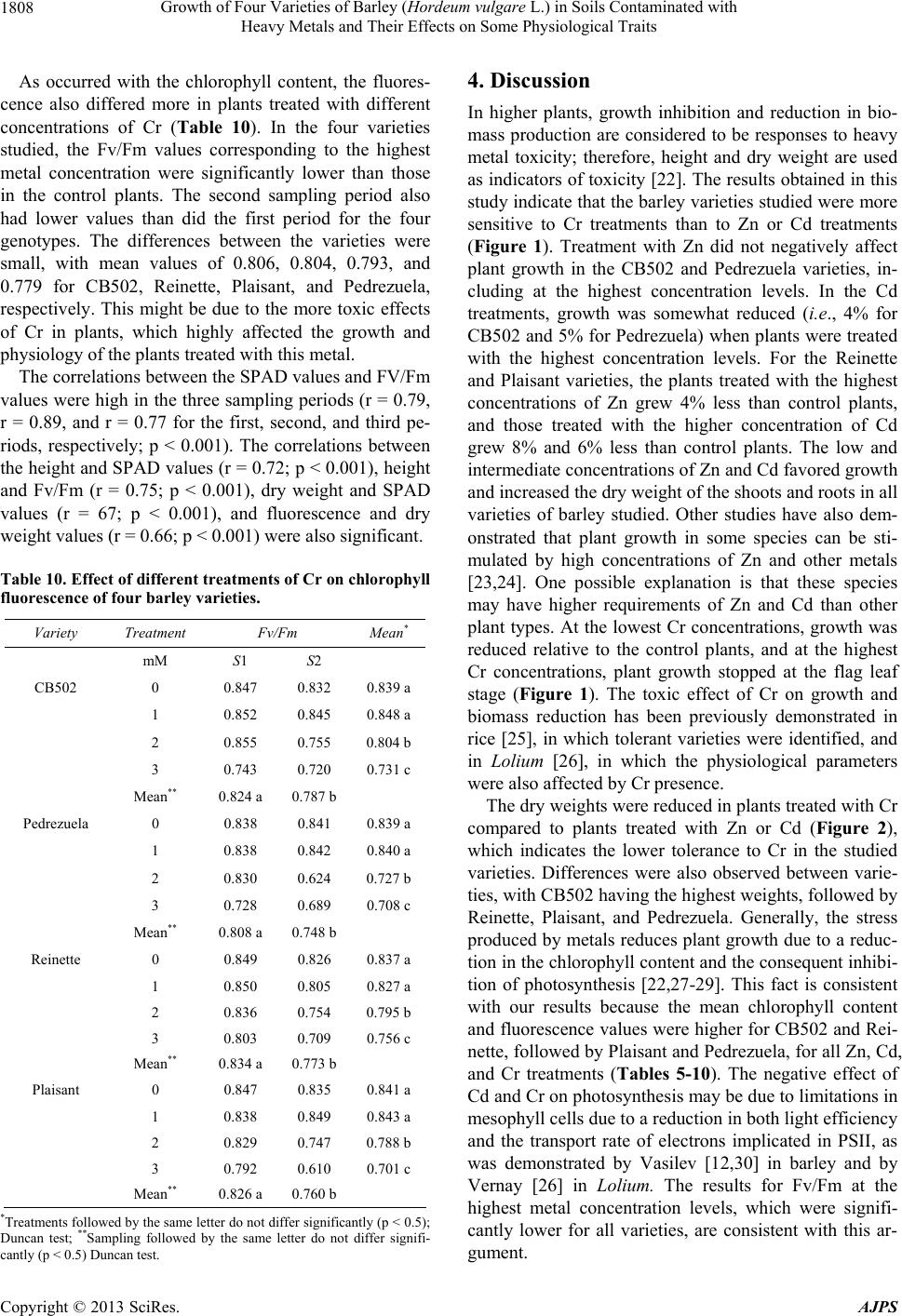 Growth of Four Varieties of Barley (Hordeum vulgare L.) in Soils Contaminated with Heavy Metals and Their Effects on Some Physiological Traits 1808 As occurred with the chlorophyll content, the fluores- cence also differed more in plants treated with different concentrations of Cr (Table 10). In the four varieties studied, the Fv/Fm values corresponding to the highest metal concentration were significantly lower than those in the control plants. The second sampling period also had lower values than did the first period for the four genotypes. The differences between the varieties were small, with mean values of 0.806, 0.804, 0.793, and 0.779 for CB502, Reinette, Plaisant, and Pedrezuela, respectively. This might be due to the more toxic effects of Cr in plants, which highly affected the growth and physiology of the plants treated with this metal. The correlations between the SPAD values and FV/Fm values were high in the three sampling periods (r = 0.79, r = 0.89, and r = 0.77 for the first, second, and third pe- riods, respectively; p < 0.001). The correlations between the height and SPAD values (r = 0.72; p < 0.001), height and Fv/Fm (r = 0.75; p < 0.001), dry weight and SPAD values (r = 67; p < 0.001), and fluorescence and dry weight values (r = 0.66; p < 0.001) were also significant. Table 10. Effect of different treatments of Cr on chlorophyll fluorescence of four barley varieties. Variety Treatment Fv/Fm Mean* mM S1 S2 CB502 0 0.847 0.832 0.839 a 1 0.852 0.845 0.848 a 2 0.855 0.755 0.804 b 3 0.743 0.720 0.731 c Mean** 0.824 a 0.787 b Pedrezuela 0 0.838 0.841 0.839 a 1 0.838 0.842 0.840 a 2 0.830 0.624 0.727 b 3 0.728 0.689 0.708 c Mean** 0.808 a 0.748 b Reinette 0 0.849 0.826 0.837 a 1 0.850 0.805 0.827 a 2 0.836 0.754 0.795 b 3 0.803 0.709 0.756 c Mean** 0.834 a 0.773 b Plaisant 0 0.847 0.835 0.841 a 1 0.838 0.849 0.843 a 2 0.829 0.747 0.788 b 3 0.792 0.610 0.701 c Mean** 0.826 a 0.760 b *Treatments followed by the same letter do not differ significantly (p < 0.5); Duncan test; **Sampling followed by the same letter do not differ signifi- cantly (p < 0.5) Duncan test. 4. Discussion In higher plants, growth inhibition and reduction in bio- mass production are considered to be responses to heavy metal toxicity; therefore, height and dry weight are used as indicators of toxicity [22]. The results obtained in this study indicate that the barley varieties studied were more sensitive to Cr treatments than to Zn or Cd treatments (Figure 1). Treatment with Zn did not negatively affect plant growth in the CB502 and Pedrezuela varieties, in- cluding at the highest concentration levels. In the Cd treatments, growth was somewhat reduced (i.e., 4% for CB502 and 5% for Pedrezuela) when plants were treated with the highest concentration levels. For the Reinette and Plaisant varieties, the plants treated with the highest concentrations of Zn grew 4% less than control plants, and those treated with the higher concentration of Cd grew 8% and 6% less than control plants. The low and intermediate concentrations of Zn and Cd favored growth and increased the dry weight of the shoots and roots in all varieties of barley studied. Other studies have also dem- onstrated that plant growth in some species can be sti- mulated by high concentrations of Zn and other metals [23,24]. One possible explanation is that these species may have higher requirements of Zn and Cd than other plant types. At the lowest Cr concentrations, growth was reduced relative to the control plants, and at the highest Cr concentrations, plant growth stopped at the flag leaf stage (Figure 1). The toxic effect of Cr on growth and biomass reduction has been previously demonstrated in rice [25], in which tolerant varieties were identified, and in Lolium [26], in which the physiological parameters were also affected by Cr presence. The dry weights were reduced in plants treated with Cr compared to plants treated with Zn or Cd (Figure 2), which indicates the lower tolerance to Cr in the studied varieties. Differences were also observed between varie- ties, with CB502 having the highest weights, followed by Reinette, Plaisant, and Pedrezuela. Generally, the stress produced by metals reduces plant growth due to a reduc- tion in the chlorophyll content and the consequent inhibi- tion of photosynthesis [22,27-29]. This fact is consistent with our results because the mean chlorophyll content and fluorescence values were higher for CB502 and Rei- nette, followed by Plaisant and Pedrezuela, for all Zn, Cd, and Cr treatments (Tables 5-10). The negative effect of Cd and Cr on photosynthesis may be due to limitations in mesophyll cells due to a reduction in both light efficiency and the transport rate of electrons implicated in PSII, as was demonstrated by Vasilev [12,30] in barley and by Vernay [26] in Lolium. The results for Fv/Fm at the highest metal concentration levels, which were signifi- cantly lower for all varieties, are consistent with this ar- gument. Copyright © 2013 SciRes. AJPS  Growth of Four Varieties of Barley (Hordeum vulgare L.) in Soils Contaminated with Heavy Metals and Their Effects on Some Physiological Traits 1809 The amount of accumulated metal in the aerial parts and the roots increased significantly with increasing me- tal concentrations in the soil (Tables 2-4). Metal accu- mulated preferentially in the root, and a significant rela- tionship has been observed between the metal concentra- tion applied to the soil and the concentration measured in the roots and stems. This suggests that the root was the first place to accumulate metal and that small amounts of metal were translocated to the aerial part of the plant. The greatest metal accumulation in roots and aerial parts has been confirmed in species as different as Hordeum vulgare [31], Triticum turgidum [19], Vicia faba [20], Nicotiana tabacum [23], Eruca sativa [32], and Prunus dulcis [28]. Following treatment with Zn or Cd, the geno- types CB502, Plaisant, and Pedrezuela accumulated ap- proximately twice the amount of metal in their roots rela- tive to their aerial parts; however, Reinette accumulated 16-fold more Zn in the roots than in the aerial parts and 34-fold more Cd. These data indicate that the first three genotypes have a mechanism of tolerance to Zn that al- lows them to accumulate metal in their tissues without affecting plant survival. Reinette seems to tolerate large amounts of Zn and Cd, hindering the translocation of these metals from the root to the aerial parts. In the Cr treatments, slightly more than double the amount of me- tal accumulated in the root relative to the aerial parts in all genotypes except in Reinette, where twice the amount of metal accumulated in the roots relative to the aerial parts, which indicates the existence of more barriers to metal transport towards the shoot in this variety. In summary, the traits studied in barley displayed va- riability, and this suggests the existence of two apparent groups. CB502 and Reinette were most tolerant to the concentrations of metals used in our trials because their growth was least affected by metals and their chlorophyll content and fluorescence values were higher relative to the Pedrezuela and Plaisant varieties. The Plaisant vari- ety plants accumulated greater amounts of Zn, Reinette accumulated greater amounts of Cd, and CB502 accu- mulated greater amounts of Cr. This is of great interest when selecting a certain variety for phytoextraction in contaminated soils because different plants will vary in their tolerance and extraction capacity based on the type of metal present in the soil. REFERENCES [1] A. J. M. Baker, S. P. McGrath, R. D. Reeves and J. A. C. Smith, “Metal Hyperaccumulator Plants: A Review of the Ecology and Physiology of a Biological Resource for Phytoremediation of Metal-Populled Soils,” In: N. Terry and G. Bañuelos, Ed., Phytoremediation of Contaminated Soil and Water, CRP Press LLC, Boca Raton, 2000, pp. 85-107. [2] S. P. McGrath, F. J. Zhao and E. Lombi, “Plant and Rhizosphere Processes Involved in Phytoremediation of Metal-Contaminated Soils,” Plant and Soil, Vol. 232, No. 1-2, 2001, pp. 207-214. doi:10.1023/A:1010358708525 [3] S. P. McGrath, F. J. Zhao and E. Lombi, “Phytoremedia- tion of Metals, Metalloids, and Radionucleides,” Ad- vances in Agronomy, Vol. 75, 2002, pp. 1-56. doi:10.1016/S0065-2113(02)75002-5 [4] R. L. Chaney, “Plant Uptake of Inorganic Waste Con- stituents,” In: J. F. Parr, P. B. Marsch and J. S. Kla, Eds., Land Treatment of Inorganic Wastes, Noyes Data, Park Ridge, 1983, pp. 50-76. [5] D. E. Salt, M. Blaylock, N. P. B. A. Kumar, V. Dushen- kov, B. D. Ensley, I. Chet and I. Raskin, “Phytoremedia- tion: A Novel Strategy for the Removal of Toxic Metals from the Environment Using Plants,” BioTechnology, Vol. 13, No. 5, 1995, pp. 468-474. doi:10.1038/nbt0595-468 [6] S. D. Cunningham and D. W. Ow, “Promises and Pros- pects of Phytoremediation,” Plant Physiology, Vol. 110, No. 3, 1996, pp. 715-719. [7] D. E. Salt, R. D. Smith and I. Raskin, “Phytoremedia- tion,” Annual Review of Plant Physiology and Plant Mo- lecular Biology, Vol. 49, No. 1, 1998, pp. 643-668. doi:10.1146/annurev.arplant.49.1.643 [8] M. A. Soriano and E. Federes, “Use of Crops for in Situ Phytoremediation of Polluted Soils Following a Toxic Flood from a Mine Spill,” Plant and Soil, Vol. 256, No. 2, 2003, pp. 253-264. doi:10.1023/A:1026155423727 [9] C. W. A. Nascimento and B. Xing, “Phytoextraction: A Review on Enhanced Metal Availability and Plant Ac- cumulation,” Scientia Agricola, Vol. 63, No. 3, 2006, pp. 299-311. doi:10.1590/S0103-90162006000300014 [10] J. M. Clark, W. A. Norvell, F. R. Clark and W. T. Buck- ley, “Concentration of Cadmium and Other Elements in the Grain of Near-Isogenic Durum Lines,” Canadian Journal of Animal Science, Vol. 82, No. 1, 2002, pp. 27- 33. [11] D. Ueno, E. Koyama, N. Yamaji and J. F. Ma, “Physio- logical, Genetic, and Molecular Characterization of a High-Cd-Accumulating Rice Cultivar, Jarjan,” Journal of Experimental Botany, Vol. 62, No. 7, 2011, pp. 2265- 2272. doi:10.1093/jxb/erq383 [12] A. Vassilev, J. Vangronsveld and I. Yordanov, “Cad- mium Phytoextraction: Present State, Biological Back- grounds and Research Needs,” Bulgarian Journal of Plant Physiology, Vol. 28, No. 3-4, 2002, pp. 68-95. [13] X. Zhang, G. Zhang, L. Guo, H. Wang, D. Zeng, G. Dong, O. Qian and D. Xue, “Identification of Quantitative Trait Loci for Cd and Zn Concentrations of Brown Rice Grown in Cd-Polluted Soils,” Euphytica, Vol. 180, No. 2, 2011, pp. 173-179. doi:10.1007/s10681-011-0346-9 [14] M. M. Lasat, “Phytoextraction of Metals from Contami- nated Soil: A Review of Plant/Soil/Metal Interaction and Assessment of Pertinent Agronomic Issues,” Journal of Hazardous Substance Research, Vol. 2, No. 5, 2000, pp. 1-25. [15] S. Kärenlampi, H. Schat, J. Vangronsveld, J. A. C. Verk- Copyright © 2013 SciRes. AJPS  Growth of Four Varieties of Barley (Hordeum vulgare L.) in Soils Contaminated with Heavy Metals and Their Effects on Some Physiological Traits Copyright © 2013 SciRes. AJPS 1810 leij, D. van der Lelie, M. Mergeay and A. I. Tervahauta, “Genetic Engineering in the Improvement of Plants for Phytoremediation of Metal Polluted Soils,” Environmen- tal Pollution, Vol. 107, 2000, pp. 225-231. doi:10.1016/S0269-7491(99)00141-4 [16] M. R. Macnair, V. Bert, S. B. Huitson, P. Saumitou-La- prade and D. Petit, “Zinc Tolerance and Hyperaccumula- tion Are Genetically Independent Characters,” Proceed- ings of the Royal Society B, Vol. 266, No. 1434, 1999, pp. 2175-2179. doi:10.1098/rspb.1999.0905 [17] N. Roosens, N. Verbruggen, P. Meerts, P. Ximénez-Em- bún and J. A. C. Smith, “Natural Variation in Cadmium Tolerance and Its Relationship to Metal Hyperaccumula- tion for Seven Populations of Thlaspi caerulescens from Western Europe,” Plant, Cell and Environment, Vol. 26, No. 10, 2003, pp. 1657-1672. doi:10.1046/j.1365-3040.2003.01084.x [18] M. J. McLaughlin, D. R. Parker and J. M. Clarke, “Metals and Micronutrients-Food Safety Issues,” Field Crops Re- search, Vol. 60, No. 1, 1999, pp. 143-163. doi:10.1016/S0378-4290(98)00137-3 [19] B. A. Adeniji, M. T. Budimir-Hussey and S. M. Macfie, “Production of Organic Acids and Adsorption of Cd on Roots of Durum Whea (Triticum turgidum L. var. Du- rum),”Acta Physiologiae Plantarum, Vol. 32, 2010, pp. 1063-1072. doi:10.1007/s11738-010-0498-6 [20] R. Cabala, L. Slováková, M. El Zohri and H. Frank, “Ac- cumulation and Translocation of Cd Metal and the Cd- Induced Production of Glutathione and Phytochelatins in Vicia faba L,” Acta Physiologiae Plantarum, Vol. 33, No. 4, 2011, pp. 1239-1248. doi:10.1007/s11738-010-0653-0 [21] J. C. Zadoks, T. T. Chang and C. F. Kozank, “A Decimal Code for the Growth Stages of Cereals,” Weed Research, Vol. 14, No. 6, 1974, pp. 415-421. doi:10.1111/j.1365-3180.1974.tb01084.x [22] D. Ci, D. Jiang, B. Wollenweber, T. Dai, Q. Jing and W. Cao, “Cadmium Stress in Wheat Seedlings: Growth, Cad- mium Accumulation and Photosynthesis,” Acta Phisiolo- giae Plantarum, Vol. 32, No. 2, 2010, pp. 365-373. doi:10.1007/s11738-009-0414-0 [23] L. L. Martins, M. P. Mourato, A. I. Cardoso, A. P. Pinto, A. M. Mota, M. L. S. Goncalves and A. de Varennes, “Oxidative Stress Induced by Cadmium in Nicotiana ta- bacum L.: Effects on Growth Parameters, Oxidative Da- mage and Antioxidant Responses in Different Plant Parts,” Acta Physiologiae Plantarum, Vol. 33, 2011, pp. 1375-1383. doi:10.1007/s11738-010-0671-y [24] Y. Yang, Ch. Sun, Y. Yao, Y. Zhang and V. Achal, “Growth and Physiological Responses of Grape (Vitis vi- nifera “Combier”) to Excess Zinc,” Acta Physiologiae Plantarum, Vol. 33, No. 4, 2011, pp. 1483-1491. doi:10.1007/s11738-010-0687-3 [25] B. Qiu, W. Zhou, D. Xue, F. Zeng, S. Ali and G. Zhang, “Identification of Cr-Tolerance Lines in a Rice (Oryza sativa L.) DH Population,” Euphytica, Vol. 174, No. 2, 2010, pp. 199-207. doi:10.1007/s10681-009-0115-1 [26] P. Vernay, C. Gauthier-Moussard and A. Hitmi, “Interac- tion of Bioaccumulation of Heavy Metal Chromium with Water Relation, Mineral Nutrition and Photosynthesis in Developed Leaves of Lolium perenne L,” Chemosphere, Vol. 68, No. 8, 2007, pp. 1563-1575. doi:10.1016/j.chemosphere.2007.02.052 [27] L. M. Sandalio, H. C. Dalurzo, M. Gómez, M. C. Ro- mero-Puertas and L. A. del Río, “Cadmium-Induced Changes in the Growth and Oxidative Metabolism of Pea Plants,” Journal of Experimental Botany, Vol. 52, 2010, pp. 2115-2126. [28] E. Nada, B. A. Ferjani, R. Ali, B. R. Bechir, M. Imed and B. Makki, “Cadmium-Induced Growth Inhibition and Al- teration of Biochemical Parameters in Almond Seedlings Grown in Solution Culture,” Acta Physiologiae Plantta- rum, Vol. 29, 2007, pp. 57-62. doi:10.1007/s11738-006-0009-y [29] R. W. dos Santos, E. C. Schmidt, R. Martins, A. Latini, M. Maraschin, P. A. Horta and Z. L. Bouzon, “Effects of Cadmium on Growth, Photosynthetic Pigments, Photo- synthetic Performance, Biochemical Parameters and Struc- ture of Chloroplasts in the Agarophyte Gracilaria domin- gensis (Rhodophyta, Gracilariales),” American Journal of Plant Sciences, Vol. 3, No. 8, 2012, pp. 1077-1084. doi:10.4236/ajps.2012.38129 [30] A. Vassilev, I. Yordanov and T. Tsonev, “Effects of Cd2+ on the Physiological State and Photosynthetic Activity of Young Barley Plants,” Photosynthetica, Vol. 34, No. 2, 1997, pp. 293-302. doi:10.1023/A:1006805010560 [31] A. González, V. Chumillas and M. C. Lobo, “Effect of Zn, Cd and Cr on Growth, Water Status and Chlorophyll Content of Barley Plants (H. vulgare L.),” Agricultural Sciences, Vol. 3, No. 4, 2012, pp. 572-581. doi:10.4236/as.2012.34069 [32] Y. Ozdener and B. K. Aydin, “The Effect of Zinc on the Growth and Physiological and Biochemical Parameters in Seedlings of Eruca sativa (L.) (Rocket),” Acta Physiolo- giae Plantarum, Vol. 32, No. 3, 2010, pp. 469-476. doi:10.1007/s11738-009-0423-z
|