 American Journal of Plant Sciences, 2013, 4, 1774-1783 http://dx.doi.org/10.4236/ajps.2013.49218 Published Online September 2013 (http://www.scirp.org/journal/ajps) Development of a SCAR Marker for Discrimination of a Thai Jasmine Rice (Oryza sativa L. cv. KDML105) Mutant, BKOS6, and Associated with Purple Color Trait in Thai Jasmine Rice-Related Varieties Nuananong Semsang1, Rattaporn Chundet2, Boonrak Phanchisri3* 1Department of Biology, Faculty of Science and Technology, Chiang Mai Rajabhat University, Chiang Mai, Thailand; 2Department of Biology, Faculty of Science, Maejo University, Chiang Mai, Thailand; 3Science and Technology Research Institute, Chiang Mai University, Chiang Mai, Thailand. Email: nsemsang@gmail.com, *phanchaisri@gmail.com Received February 11th, 2013; revised March 12th, 2013; accepted April 15th, 2013 Copyright © 2013 Nuananong Semsang et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The potential of SCAR marker for discrimination of a Thai jasmine rice (Oryza sativa L. cv. KDML 105) mutant, BKOS6, obtained from ion-beam-induced mutation, was evaluated. The improved rice variety, BKOS6, exhibited many remarkable characteristics which fitted the multiple cropping system characteristics of progressive agriculture includ- ing photoperiod insensitivity, early flowering, short in stature, and purple pigment accumulation in pericarp. The BKOS6 rice grain extract has already been proved that it exhibited higher antioxidant properties than the KDML 105 and other tested rice grain extracts. In this study, the BKOS6 specific SCAR marker was developed by HAT-RAPD analysis of rice genomic DNA. The marker was successfully used to identify BKOS6 variety and its hybrid varieties containing purple pigment accumulation in plant tissues. Moreover, it was found that this marker could be used to de- tect other purple pigmented rice varieties that genetically related to Thai jasmine rice. Recently, a wide variety of an- thocyanin-based foods are believed to provide significant potential health benefits, and become more attractive. KDML 105 is also a Thai premier fragrant rice variety which is one of the main varieties of country’s rice export. Thus this molecular marker could be useful for commercial and breeding purposes of BKOS6 mutant and other developed varie- ties from KDML 105 which contain anthocyanin accumulation. Keywords: SCAR Marker; KDML 105 Mutant; Low-Energy Ion Beam; Anthocyanin Accumulation; Purple Pigmented Rice 1. Introduction Increased grain yield and improved rice quality are ab- solutely necessary to feed the world’s population and to improve its nutrition. Thus, the recent approach for rice production including the improvement of both yield and grain quality is to supply consumer’s demand and also to increase the nutritional level for the general public. Thai jasmine rice seeds bombarded with nitrogen ions revealed one of new rice varieties, named BKOS6 rice, showing short stature (at 49 cm of the M1 plant height in culm length on the harvest day), photoperiod insensitiv- ity, early flowering and accumulation of purple pigment in various tissues [1]. This variety showed mutational stability in all characteristics when observed in M1-M8 cultivations [2]. The BKOS6 seeds are long and slender grains with dark purple/black pericarp. A previous study found the presence of the amount of anthocyanin in vari- ous tissues of BKOS6 was significantly higher than that of KDML 105 [3,4]. The increase of anthocyanin in BKOS6 is believed to provide potential health benefits due to its antioxidant properties. The study of its im- proved antioxidant properties showed the increase of to- tal phenolic content and antioxidant activities of BKOS6 rice extracts when compared with those of KDML 105 and other tested rice extracts. Moreover, it was found that there was no significant difference between the antioxi- dant properties of the cooked and uncooked BKOS6 rice *Corresponding author. Copyright © 2013 SciRes. AJPS  Development of a SCAR Marker for Discrimination of a Thai Jasmine Rice (Oryza sativa L. cv. KDML105) Mutant, BKOS6, and Associated with Purple Color Trait in Thai Jasmine Rice-Related Varieties 1775 extracts [5]. As the consumers become more health con- scious and more aware of the benefits of functional foods, diets containing bioactive compounds such as antioxi- dants have received greater attention. The development of this anthocyanin-based natural product could allow dramatically benefit to a wider population if it is avail- able in rice market. Other improved characteristics of BKOS6 plant, semi-dwarfism, photoperiod insensitivity and early-flowering, are also subjects for improvement of yield quantity. Semi-dwarfism is one of the most impor- tant traits deployed in modern rice breeding. Its semi- dwarf character results in a shortened culm with im- proved lodging resistance, allowing for the increased use of nitrogen fertilizers and a greater harvest index [6,7]. Both photoperiod insensitivity and early-flowering allow plants to start producing flowers quickly in any time of a year, and have been appeared to give high yield potential. Therefore, the further varietal improvement of BKOS6 or the production of either BKOS6 variety or its hybrid off springs could lead to an increase of both yield and grain quality so as to supply world population and also in- crease the nutritional benefit from rice consumption. As BKOS6 variety has a potential to be a commercial rice variety, a varietal identification is important for breeding processes and rights protection for newly devel- oped varieties. At present, Thailand has not joined with other developing countries such as China, Vietnam and India in hybrid rice development [8]; therefore, intellec- tual property protection on newly developed varieties has become more important. Accurate and rapid cultivar identification is especially important in plant propagation for practical breeding purposes and for proprietary rights protection. At present, the traditional methods for char- acterization and assessment of genetic variability in many plant species, based on morphological, physiological and biochemical studies, are both time consuming and af- fected by the environment. The introduction of molecular biology techniques, such as DNA based markers, pro- vides an opportunity for genetic characterization that al- lows direct comparison of different genetic material in- dependent of environmental influences [9]. A more reli- able and specific PCR-based marker known as sequence characterized amplified regions (SCARs) has been de- veloped by Paran and Michelmore [10]. The SCAR mark- ers could be identified easily by direct staining methods such as ethidium bromide and pellet painting without electrophoresis [11]. The presence or absence of the band/ color indicates variation in genomic sequence. These are better reproducible than randomly amplified polymorphic DNA (RAPD) markers. SCAR markers have been used for discrimination in wide range of different plant spe- cies/varieties such as banana [12], cotton [13], chili pep- per [11], pear [14], lychee [15], curcuma [16], longan [17], muskmelon [18] and Thai fragrant rice mutant [19]. SCAR markers also allow comparative mapping or ho- mology studies among related species, thus making it an extremely adaptable concept in the near future. The ob- jective of this work was to establish the specific SCAR marker for BKOS6 variety for its molecular identifica- tion and further use for its rights protection. 2. Materials and Methods 2.1. Plant Materials Rice varieties used in the experiment were as follows: Thai jasmine rice (Oryza sativa L. cv. KDML105), 5 varieties of KDML105 mutants induced by low-energy ion beam (BKOS6, TKOS4, HyKOS1, PKOS1, PKOS3), Patumthanee1 and 7 lines of Patumthanee1 mutants in- duced by low-energy ion beam (TPOS1, TPOS2, EPOS1, EPOS2, EPOS3, EB, EW), 4 cultivars of Thai non-glu- tinous rice (Supanburee, Chainat, Pitsanulok1, RD21), 9 cultivars of non-glutinous rice KDML105 mutants in- duced by gamma rays (RD3, RD5, RD9, RD15, RD17, RD19, RD21, RD23, RD23), KDML 105 hybrid rice (Hom-nil), 4 cultivars of colored rice cultivars (Red Hawn Rice, Red Rose Rice, Sangyod Brown Rice, Thai purple rice), 6 varieties of traditional non-glutinous rice, 6 cultivars of glutinous KDML105 mutants induced by gamma rays (RD2, RD4, RD6, RD8, RD16, RD12), 5 varieties of traditional glutinous rice (Niaw Lomkao, Niaw San-pah-tawng, Niaw Phare, Muey Nawng 62 M, Gam Pai 15), 2 varieties of wild rice and 3 varieties of japonica rice (Koshihikari, Kitaake). And hybrid rice va- rieties generated by cross-breeding were as follows: 3 lines of F1 generation of KDML105 x BKOS6 (KB1, KB2, KB3), 1 line of F1 generation of BKOS6 x KDML105 (BK1), 1 line of F1 generation of BKOS6 x TKOS4 (BT5), 1 line of TKOS4 x BKOS6 (TB1), 1 line of F1 generation of KDML105 x TKOS4 (KT1), 6 lines of F6 generation of self-pollinated KB3 (KB3-S2-1, KB3-S2-2-1, KB3-S2-2-2, KB3-M2-2, KB2-M3, KB3- T2-1-2), and 5 lines of M6 generation of re-ion bom- barded BKOS6 mutants (HyKOS17, HyKOS18, HyKOS 19/1, HyKOS19/2, HyKOS21). 2.2. Ion Bombardment and Plant Growth Conditions Two varieties of Thai Jasmine rice mutant, HyKOS1 and BKOS6, were re-ion bombarded for mutation induction. In ion bombardment condition, the rice seeds were bom- barded in vacuum condition by nitrogen ion beam (N+ + 2 N ) at energy of 60 keV with an ion fluence of 2 × 1016 ions cm–2. The bombarded seeds were grown in soil as transplant rice. The cultivations were carried out in off- Copyright © 2013 SciRes. AJPS  Development of a SCAR Marker for Discrimination of a Thai Jasmine Rice (Oryza sativa L. cv. KDML105) Mutant, BKOS6, and Associated with Purple Color Trait in Thai Jasmine Rice-Related Varieties Copyright © 2013 SciRes. AJPS 1776 season cultivation (March-July) for investigation of plant phenotypic variations. TATTAGTCATG-3’) were developed. The PCR reaction was conducted in the same conditions as described above, except 1 µM of each primer was added instead of arbi- trary primer. The amplification was carried out by 27 three-step cycles of a 45 s denaturation at 94˚C, 30 s an- nealing at 60˚C and, 30 s elongation at 72˚C, and final 5 min elongation at 72˚C in the thermal cycler. 2.3. Genomic DNA Extraction and DNA Fingerprints Generated by High Annealing Temperature-Randomly Amplification Polymorphic DNA (HAT-RAPD) Amplification 2.5. Microsatellite Marker Amplification Total DNA was isolated from rice leaves by cetyltrime- thylammonium bromide (CTAB)-based method [20]. The HAT-RAPD amplification reaction was carried out ac- cording to Anuntalabhochai et al. [21]. PCR assays con- sisted of 1x Taq buffer (75 mM Tris-HCl, 20 mM (NH4)SO4, 0.01% Tween20) with 1.5 mM MgCl2 (Fer- mentas, USA); 200 mM each of dATP, dCTP, dGTP, and dTTP; 0.5 mM of arbitrary primer; 20 ng DNA template; and 2 U of Taq DNA polymerase. The amplification was carried out by 30 three-step cycles of a 45 s denaturation at 94˚C, 30 s annealing at 45˚C and, 45 s elongation at 72˚C, and final 10 min elongation at 72˚C in the thermal cycler. Arbitrary RAPD primers (QIAGEN OPERON, USA) were randomly chosen for amplification reactions. Agarose gels with appropriately separated DNA frag- ments were photographed. Lambda DNA digested with PstI was used as a DNA size marker. Six previously developed rice microsatellite primer pairs including; RM13, RM234, RM477, RM316, RM206, RM247) were used in the analysis [22,23]. PCR reactions were done in a final volume of 20 μl containing 50 ng template DNA, 1 μl 10X PCR buffer containing 15 mM MgCl2, 0.25 mM each of the dNTPs, 0.25 μM of each primer, 1 U Taq DNA polymerase (Fermentas, USA). Amplification were carried out with the following pro- gram: Initial denaturation at 94˚C for 3 min followed by 25 cycles at 94˚C for 30 sec, 55˚C for 30 sec, and 72˚C for 1 min and a final cycle at 72˚C for 7 min. PCR prod- ucts were checked in 2% agarose gel. 3. Results 3.1. Development of BKOS6-Specific SCAR Marker from HAT-RAPD Analysis The additional DNA band was cloned into pTZ57R plasmid using InsT/AcloneTM PCR Product Cloning Kit (Fermentas, USA) according to producer-supplied meth- odology. The DNA fragment was sequenced by 1st BASE Laboratories, Malaysia. DNA fingerprints obtained by genomic DNA randomly amplified using arbitrary primers were compared be- tween BKOS6 and other 13 rice samples. By screening over 300 arbitrary primers, DNA fingerprint results showed several polymorphic bands. The OPO07 primer (5’-CAGCACTGAC-3’) provided a clear band found only in BKOS6 sample (approximately 1.1 kb in length) (Figure 1). This polymorphic band, designated BO07 fragment, was identified to produce SCAR markers for BKOS6 mutant. 2.4. Developing of SCAR Marker According to the sequence of the polymorphic band in BKOS6, named BO07, specific primers (Forward primer, BO07F1; 5’-TGTCTACGTTGGCTTCGCCATCACCG- 3’ and Reverse primer, BO07R1; 5’-ATGCTAACCATG Figure 1. HAT-RAPD amplification patterns obtained from OPO07 primer in 14 rice samples. Lane1-14 include KDML105, 5 varieties of KDML 105 mutants obtained from low-energy ion beam bombardment (BKOS6, TKOS4, HyKOS1, PKOS1, PKOS3), Patumthanee1, Supanburee, Chainat, Pitsanulok, RD21, Thai purple rice and 2 japonica cultivar rice, respectively. M is DNA marker (λ/PstI). An arrow indicates the polymorphic band found in BKOS6 sample (approximate ly 1.1 kb), named BO07 fragment.  Development of a SCAR Marker for Discrimination of a Thai Jasmine Rice (Oryza sativa L. cv. KDML105) Mutant, BKOS6, and Associated with Purple Color Trait in Thai Jasmine Rice-Related Varieties 1777 The BO07 sequence was blasted to O. sativa genomic database in GenBank (http://blast.ncbi.nlm.nih.gov/) and the sequence showed similarity to the NC_008405.2 frag- ment of O. sativa Japonica Group DNA. A pair of spe- cific primers (BO07F1 and BO07R1) was designed wi- thin the sequence for SCAR marker amplification (Fig- ure 2). 3.2. The SCAR Marker Amplification SCAR marker amplification among 55 different rice va- rieties/cultivars (Table 1) as shown in Figure 3 was car- ried out using SCAR marker primers (BO07F1 and BO07 R1). The expected band (218 bp) was found only in BKOS6 sample and a sample of purple pigmented KDML105 hybrid rice (Hom-nil). No DNA band was found in other rice samples. Microsatellite amplification was performed to discri- minate between BKOS6 and Hom-nil cultivars. Six pairs of microsatellite primers were tested. As shown in Fig- ure 4, the PCR patterns amplified from a microsatellite primer pair, RM234 (F: 5’-ACAGTATCCAAGGCCCT GG-3’, R: 5’-CACGTGAGACAAAGACGGAG-3’), showed the different patterns between BKOS6 and Hom- nil samples. 3.3. Detection of SCAR Marker in BKOS6 Hybrid Lines To test the ability of the SCAR marker in detection of BKOS6 hybrid lines, 16 rice varieties were examined using SCAR marker primers. Table 2 shows descriptions, color of several plant tissues and SCAR marker detection results of plant samples used in this experiment. The SCAR marker amplification for 16 rice varieties/lines is shown in Figure 5. The bands with expected size were found in BKOS6 sample and in all its F1 hybrids from both cross breeding of KDML105 with BKOS6 (KB1, KB2, KB3, BK1) and of TKOS4 with BKOS6 (TB1). Moreover, SCAR marker was tested among rice samples of F6 generation from self-pollinated KB3 line (F6/1- F6/6). Among these group, the expected bands were found in all F6 samples except the sample from F6/3 line. It was found that F6/3 hybrid is the only one line which has no pigment accumulation in any tissue, different from other lines which have colored tissues especially in pericarp (Table 2). Figure 2. Comparison of nucleotide sequences of BO07-2 clone from BO07 fragment and the NC_008405.2 sequence from O. sativa Japonica Group DNA. The stars are under the consensus sequences. The under lined red characters are the sequences of primers (BO07F1, BO07R1) designed for SCAR marker amplification. Copyright © 2013 SciRes. AJPS 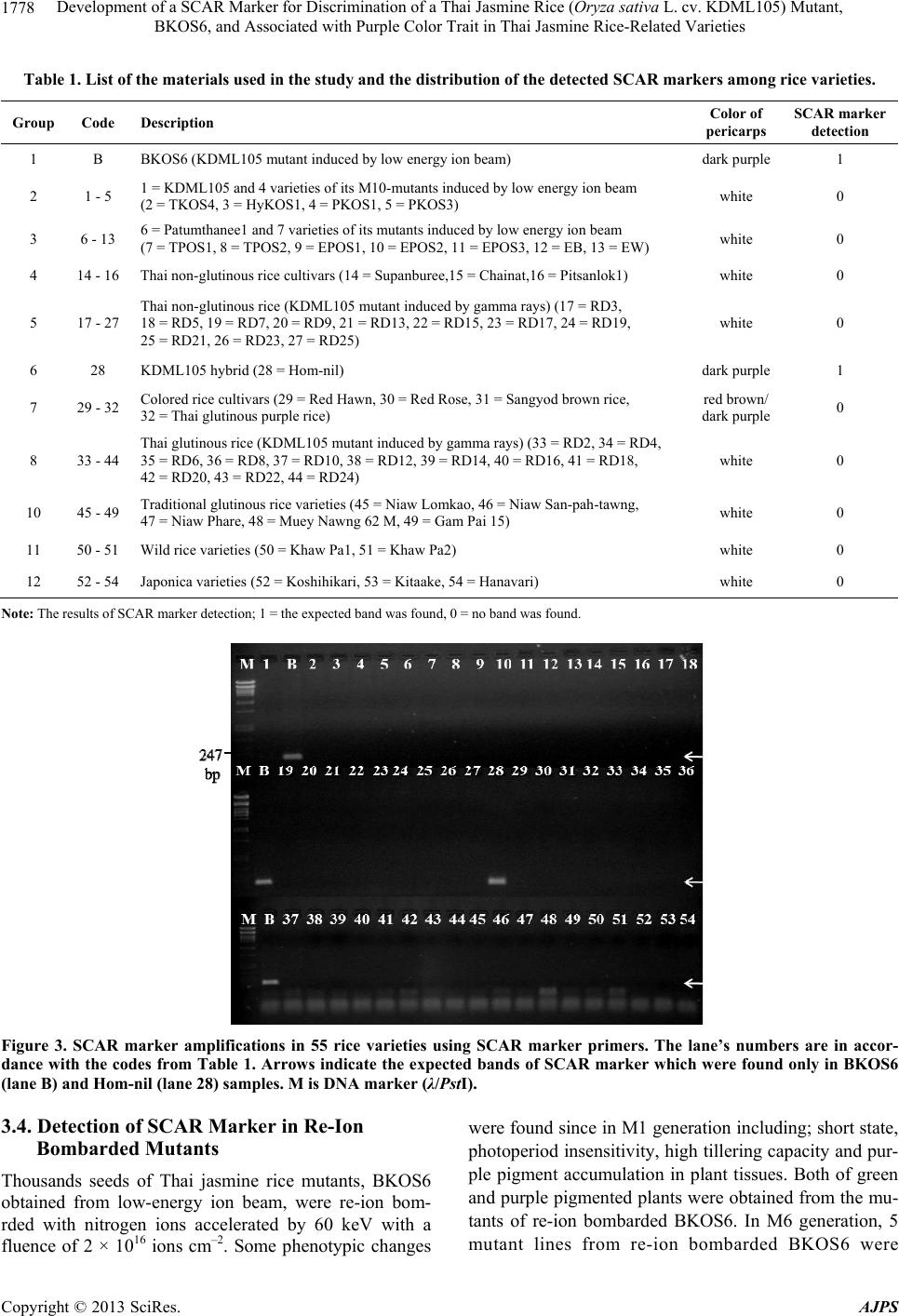 Development of a SCAR Marker for Discrimination of a Thai Jasmine Rice (Oryza sativa L. cv. KDML105) Mutant, BKOS6, and Associated with Purple Color Trait in Thai Jasmine Rice-Related Varieties 1778 Table 1. List of the materials used in the study and the distribution of the detected SCAR markers among rice varieties. Group Code Description Color of pericarps SCAR marker detection 1 B BKOS6 (KDML105 mutant induced by low energy ion beam) dark purple 1 2 1 - 5 1 = KDML105 and 4 varieties of its M10-mutants induced by low energy ion beam (2 = TKOS4, 3 = HyKOS1, 4 = PKOS1, 5 = PKOS3) white 0 3 6 - 13 6 = Patumthanee1 and 7 varieties of its mutants induced by low energy ion beam (7 = TPOS1, 8 = TPOS2, 9 = EPOS1, 10 = EPOS2, 11 = EPOS3, 12 = EB, 13 = EW) white 0 4 14 - 16 Thai non-glutinous rice cultivars (14 = Supanburee,15 = Chainat,16 = Pitsanlok1) white 0 5 17 - 27 Thai non-glutinous rice (KDML105 mutant induced by gamma rays) (17 = RD3, 18 = RD5, 19 = RD7, 20 = RD9, 21 = RD13, 22 = RD15, 23 = RD17, 24 = RD19, 25 = RD21, 26 = RD23, 27 = RD25) white 0 6 28 KDML105 hybrid (28 = Hom-nil) dark purple 1 7 29 - 32 Colored rice cultivars (29 = Red Hawn, 30 = Red Rose, 31 = Sangyod brown rice, 32 = Thai glutinous purple rice) red brown/ dark purple 0 8 33 - 44 Thai glutinous rice (KDML105 mutant induced by gamma rays) (33 = RD2, 34 = RD4, 35 = RD6, 36 = RD8, 37 = RD10, 38 = RD12, 39 = RD14, 40 = RD16, 41 = RD18, 42 = RD20, 43 = RD22, 44 = RD24) white 0 10 45 - 49 Traditional glutinous rice varieties (45 = Niaw Lomkao, 46 = Niaw San-pah-tawng, 47 = Niaw Phare, 48 = Muey Nawng 62 M, 49 = Gam Pai 15) white 0 11 50 - 51 Wild rice varieties (50 = Khaw Pa1, 51 = Khaw Pa2) white 0 12 52 - 54 Japonica varieties (52 = Koshihikari, 53 = Kitaake, 54 = Hanavari) white 0 Note: The results of SCAR marker detection; 1 = the expected band was found, 0 = no band was found. Figure 3. SCAR marker amplifications in 55 rice varieties using SCAR marker primers. The lane’s numbers are in accor- dance with the codes from Table 1. Arrows indicate the expected bands of SCAR marker which were found only in BKOS6 (lane B) and Hom-nil (lane 28) samples. M is DNA marker (λ/PstI). 3.4. Detection of SCAR Marker in Re-Ion Bombarded Mutants Thousands seeds of Thai jasmine rice mutants, BKOS6 obtained from low-energy ion beam, were re-ion bom- rded with nitrogen ions accelerated by 60 keV with a fluence of 2 × 1016 ions cm–2. Some phenotypic changes were found since in M1 generation including; short state, photoperiod insensitivity, high tillering capacity and pur- ple pigment accumulation in plant tissues. Both of green and purple pigmented plants were obtained from the mu- tants of re-ion bombarded BKOS6. In M6 generation, 5 utant lines from re-ion bombarded BKOS6 were m Copyright © 2013 SciRes. AJPS 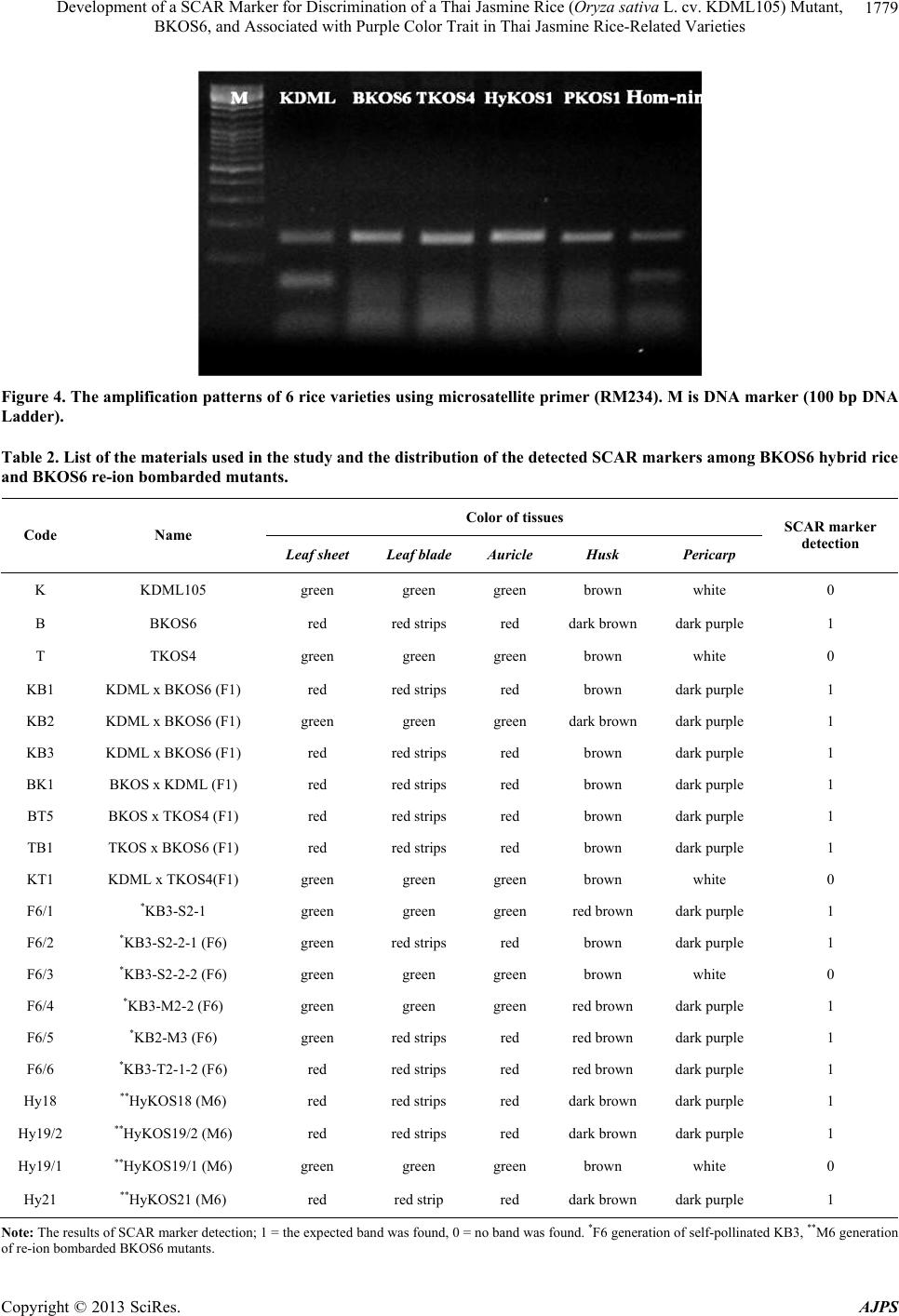 Development of a SCAR Marker for Discrimination of a Thai Jasmine Rice (Oryza sativa L. cv. KDML105) Mutant, BKOS6, and Associated with Purple Color Trait in Thai Jasmine Rice-Related Varieties 1779 Figure 4. The amplification patterns of 6 rice varieties using microsatellite primer (RM234). M is DNA marker (100 bp DNA Ladder). Table 2. List of the materials used in the study and the distribution of the detected SCAR markers among BKOS6 hybrid rice and BKOS6 re-ion bombarded mutants. Color of tissues Code Name Leaf sheet Leaf blade Auricle Husk Pericarp SCAR marker detection K KDML105 green green green brown white 0 B BKOS6 red red strips red dark brown dark purple 1 T TKOS4 green green green brown white 0 KB1 KDML x BKOS6 (F1) red red strips red brown dark purple 1 KB2 KDML x BKOS6 (F1) green green green dark brown dark purple 1 KB3 KDML x BKOS6 (F1) red red strips red brown dark purple 1 BK1 BKOS x KDML (F1) red red strips red brown dark purple 1 BT5 BKOS x TKOS4 (F1) red red strips red brown dark purple 1 TB1 TKOS x BKOS6 (F1) red red strips red brown dark purple 1 KT1 KDML x TKOS4(F1) green green green brown white 0 F6/1 *KB3-S2-1 green green green red brown dark purple 1 F6/2 *KB3-S2-2-1 (F6) green red strips red brown dark purple 1 F6/3 *KB3-S2-2-2 (F6) green green green brown white 0 F6/4 *KB3-M2-2 (F6) green green green red brown dark purple 1 F6/5 *KB2-M3 (F6) green red strips red red brown dark purple 1 F6/6 *KB3-T2-1-2 (F6) red red strips red red brown dark purple 1 Hy18 **HyKOS18 (M6) red red strips red dark brown dark purple 1 Hy19/2 **HyKOS19/2 (M6) red red strips red dark brown dark purple 1 Hy19/1 **HyKOS19/1 (M6) green green green brown white 0 Hy21 **HyKOS21 (M6) red red strip red dark brown dark purple 1 Note: The results of SCAR marker detection; 1 = the expected band was found, 0 = no band was found. *F6 generation of self-pollinated KB3, **M6 generation of re-ion bombarded BKOS6 mutants. Copyright © 2013 SciRes. AJPS  Development of a SCAR Marker for Discrimination of a Thai Jasmine Rice (Oryza sativa L. cv. KDML105) Mutant, BKOS6, and Associated with Purple Color Trait in Thai Jasmine Rice-Related Varieties 1780 Figure 5. Identification of BKOS6 mutant and its hybrid lines using SCAR marker primers. The lane’s names are in accor- dance with the materials’ code from Table 2. The expected bands found in BKOS6 sample and its hybrids containing purple colored tissues. M is DNA marker (100 bp DNA Ladder). chosen for SCAR marker detection. The expected bands were amplified in 4 samples from re-ion bombarded BKOS6 mutants (HyKOS17, HyKOS18, HyKOS19/2, HyKOS21) (Figure 6). All of these samples that could be detected by SCAR marker exhibits purple pigment accumulation in plant tissues especially in their pericarp (Table 2). 4. Discussion The heightened international competition of crop exports means that the discrimination of crop cultivars is becom- ing increasingly important to protect breeders’ rights and commercial values of original cultivars. Currently, many methods to discriminate crop cultivars at the DNA level have been reported [24-29]. Among those methods, PCR- based DNA markers are especially valuable because they are fast, easy, and reproducible. BKOS6, a new rice vari- ety created by mutation induction using low-energy ion beam bombardment, has been developed from Thai jas- mine rice (KDML 105). The appearances of BKOS6 plant and its pigmented organs were shown in Figure 7. The improved characteristics of BKOS plant include: 1) anthocyanin accumulation in various tissues, especially in pericarp, which has been proven to raise the antioxi- dant properties of rice grain [5] and might increase resis- tance to blast disease of rice plant [30]; 2) semi-dwarf character, which can be more resistance against lodging and increased responsiveness to nitrogen fertilizer [31]; 3) photoperiod-insensitivity, which can promote flowering with a slightly response to photoperiod; and 4) early flowering character, which can induce early maturing. This developed variety may fit into the multiple cropping system characteristics of progressive agriculture. Thus, a SCAR marker was attempted to be developed for BKOS6 identification. This marker detection can be used for further practical breeding purposes and proprietary- rights protection. In this study, the SCAR marker has been developed to discriminate the BKOS6 variety. In the SCAR analyses, a pair of primers was designed based on the sequences of the additional band from DNA fingerprints of BKOS6 Figure 6. Identification of re-ion bombarded BKOS6 mu- tants using SCAR primers. The lane’s names are in accor- dance with the materials’ code from Table 2. The expected bands found in BKOS6 and mutant samples containing purple colored tissues. M is DNA marker (λ/PstI). Figure 7. The appearances of BKOS6 plants. The BKOS6 plants have short stature compared to KDML105 plants (a), red strips on leaf blade (b), purplish red leaf sheet (c), pur- plish red auricle (d), purple husks (e) and dark purple pericarp of rice grains (f). sample. The sequence analysis of the additional band showed it is a flanking sequence of intergenic region which is better target than a coding region for the devel- opment of DNA markers used to discriminate among species and/or cultivars. The SCAR marker were tested among 55 different rice varieties/lines and successfully used to identify BKOS6 variety. Although it also pro- Copyright © 2013 SciRes. AJPS  Development of a SCAR Marker for Discrimination of a Thai Jasmine Rice (Oryza sativa L. cv. KDML105) Mutant, BKOS6, and Associated with Purple Color Trait in Thai Jasmine Rice-Related Varieties 1781 duced a DNA band with the same size in Hom-nil sample, the microsatellite marker (RM234) can be used to distin- guish between these two varieties. Hom-nil or Jao Hom Nil (JHN) is a hybrid cultivar developed from Chi nese purple glutinous rice and KDML 105. Hom-nil rice has several characteristics similar to BKOS6 rice such as long and slender grains with dark purple pericarp, high total phenolic content and antioxidant activity [32]. This rice cultivar has been also determined as the highest broad spectrum resistance to Thai blast pathogen popula- tion [33]. But there are some different characteristics between these two varieties such as Hom-nil plant has green leaf blades while BKOS6 had purple stripes on leaf blades and a microsatellite marker (RM234) could also be used to distinguish between these two varieties. Mi- crosatellites may be not very demanding technically, but a particularly important advantage is that microsatellite data can be easily compared among laboratories and are suitable for computer databases, which is not always the case with other markers, such as RAPD [34]. The com- bination of these results (SCAR and microsatellite mar- ker amplifications) can be practically used for BKOS6 identification and in discriminating from other rice varie- ties. It has been shown that the SCAR marker could be ap- plied for BKOS6 hybrid detection. It was successfully used to detect all F1 hybrids from cross-breeding be- tween BKOS6 and other rice varieties. It has been noted that all SCAR positive-detected F1 hybrid lines have purple pigment accumulation in plant tissues. In F6 gen- eration of self-pollinated F1 hybrid line (KB3), only samples from purple pigmented plants revealed the posi- tive results for SCAR marker. Moreover, the re-ion bom- barded mutants, obtained from re-ion bombardment of BKOS6 seeds, which exhibit purple pigment accumula- tion in their tissues, were also detectable by the SCAR marker. These results indicate that the SCAR marker might associate with purple color trait in rice lines which have been developed from KDML 105. Due to it could be detected in all purple pigmented rice lines developed from KDML 105 but not in other tested pigmented rice varieties (line 29 - 32; Red Hawn, Red Rose, Sangyod brown rice, Thai glutinous purple rice) which are not genetically related to KDML 105 (Figure 4). 5. Conclusion Due to many remarkable improved characteristics of BKOS6 variety, a specific SCAR marker was generated for its molecular identification. The developed SCAR marker was successfully used to identify BKOS6 variety and distinguish it from other rice varieties/cultivars ex- cept Hom-nil rice. However, the combination of SCAR marker and microsatellite amplification could be used to distinguish between these two varieties. The SCAR mar- ker could also be used to detect all F1hybrid lines of BKOS6 variety and the tested F6 lines which exhibited purple pigment accumulation in plant tissues especially in pericarp. Moreover, this SCAR marker could detect the purple pigmented lines obtained from re-ion bom- bardment of Thai jasmine rice mutants. Therefore, this SCAR marker could be a valuable tool for breeding pur- poses and further research of purple pigmented rice va- rieties developed from KDML 105. 6. Acknowledgements The authors thank and gratitude Associated Professor Dr. Somboon Anuntalabhochai for his guidance and advices on this work. The work was supported by the National Research Council of Thailand (NRCT), the Agricultural Research Development Agency (ARDA), the Thailand Center of Excellence in Physics, the International Atomic Energy Agency (IAEA), and Science and Technology Research Institute (STRI) of Chiang Mai University. REFERENCES [1] B. Phanchaisri, R. Chandet, L. D. Yu, T. Vilaithong, S. Jamjod and S. Anuntalabhochai, “Low-Energy Ion Beam- Induced Mutation in Thai Jasmine Rice (Oryza sativa L. cv. KDML 105),” Surface Coating Technology, Vol. 201, 2007, pp. 8024-8028. doi:10.1016/j.surfcoat.2006.02.057 [2] B. Phanchaisri, “Characterization of Khao Dawk Mali 105 Rice (Oryza sativa L. cv. KDML 105) Induced by Low-Energy Ion Beam,” Ph.D. Thesis, Chiang Mai Uni- versity, Chiang Mai, 2008. [3] S. Anuntalabhochai, R. Chandet, S. Pitakrattananukool, N. Semsang, T. Vilaithong and R. W. Cutler, “Induction of Anthocyanin Accumulation in Thai Jasmine Rice Mutant by Low-Energy Ion Beam,” BioAsia 2007: The 2nd Inter- national Conference on Rice for the Future, Bangkok, 5-9 November 2007. [4] R. Chundet, R. W. Cutler and S. Anuntalabhochai, “In- duction of Anthocyanin Accumulation in a Thai Jasmine Rice Mutant by Low-Energy Ion Beam,” International Research Journal of Plant Science, Vol. 3, No. 6, 2012, pp. 120-126. [5] N. Semsang, R. Kawaree, R. W. Cutler, R. Chundet, L. D. Yu and S. Auntalabhochai, “Improved Antioxidant Activ- ity of BKOS Thai Jasmine Rice,” Natural Product Re- search, Vol. 20, No. 12, 2012, pp. 1145-1151. doi:10.1080/14786419.2011.561207 [6] P. R. Jennings, “Plant Type as a Rice Breeding Objec- tive,” Crop Science, Vol. 4, 1964, pp. 13-15. doi:10.2135/cropsci1964.0011183X000400010005x [7] J. J. Walcott and D. R. Laing, “Some Physiological As- pects of Growth and Yield in Wheat Crops: A Compari- son of a Semi-Dwarf and Standard Height Cultivar,” Australian Journal of Experimental Agriculture and Ani- Copyright © 2013 SciRes. AJPS  Development of a SCAR Marker for Discrimination of a Thai Jasmine Rice (Oryza sativa L. cv. KDML105) Mutant, BKOS6, and Associated with Purple Color Trait in Thai Jasmine Rice-Related Varieties 1782 mal Husbandry, Vol. 16, 1976, pp. 578-587. doi:10.1071/EA9760578 [8] S. Sriwatanapongse, “Impact of Intellectual Property Right on Development and Use of Hybrid Crop Varieties in Developing Countries: Thailand Experience,” Assumption University Journal of Technology, Vol. 6, No. 3, 2003, pp. 125-128. [9] K. Weising, H. Nybom, K. Wolff and W. Meyer, “DNA Fingerprinting in Plants and Fungi,” CRC Press Inc., Boca Raton, 1995. [10] I. Paran and R. W. Michelmore, “Development of Reli- able PCR-Based Markers Linked to Downy Mildew Re- sistance Genes in Lettuce,” Theoretical and Applied Ge- netics, Vol. 85, No. 8, 1993, pp. 985-993. doi:10.1007/BF00215038 [11] I. Jang, J. H. Moon, J. B. Yoon, J. H. Yoo, T. J. Yang, Y. J. Kim and H. G. Park, “Application of RAPD and SCAR Markers for Purity Testing of F1 Hybrid Seed in Chili Pepper (Capsicum annuum),” Molecules and Cells, Vol. 18, 2004, pp. 295-299. [12] S. Ruangsuttapha, K. Eimert, M. B. Schröder, B. Silayoi, J. Denduangboripant and K. Kanchanapoom, “Molecular Phylogeny of Banana Cultivars from Thailand Based on HAT-RAPD Markers,” Genetic Resources and Crop Evo- lution, Vol. 54, No. 7, 2007, pp. 1565-1572. doi:10.1007/s10722-006-9169-2 [13] G. Wangzhen, Z. Tianzhen, S. Xinlian, Z. Y. John and J. K. Russell, “Development of SCAR Marker Linked to a Major qtl for High Fiber Strength and Its Usage in Mo- lecular-Marker Assisted Selection in Upland Cotton,” Crop Science, Vol. 43, No. 6, 2003, pp. 2252-2256. [14] G. P. Lee, C. H. Lee and C. S. Kim, “Molecular Markers Derived from RAPD, SCAR, and the Conserved 18S rDNA Sequences for Classification and Identification in Pyrus pyrifolia and P. communis,” Theoretical and Ap- plied Genetic, Vol. 108, No. 8, 2004, pp. 1487-1491. doi:10.1007/s00122-003-1582-8 [15] R. W. Cutler, R. Chundet, T. Handa and S. Anuntalabho- chai, “Development of Sequence Characterized DNA Markers Linked to Temperature Dependence for Flower Induction in Lychee (Litchi chinensis Sonn.) Cultivars,” Scientia Horticulturae, Vol. 107, No. 3, 2006, pp. 264- 270. doi:10.1016/j.scienta.2005.08.005 [16] S. Anuntalabhochai, S. Sitthiphrom, W. Thongtaksin, M. Sanguansermsri and R. W. Cutler, “Hybrid Detection and Characterization of Curcuma spp. Using Sequence Char- acterized DNA Markers,” Scientia Horticulturae, Vol. 111, No. 4, 2007, pp. 389-393. doi:10.1016/j.scienta.2006.11.008 [17] R. W. Cutler, S. Sitthiphrom, J. Marha and S. Anuntala- bhochai, “Development of Sequence-Characterized DNA Markers Linked to Temperature Insensitivity for Fruit Production in Longan (Dimocarpus longan Lour.) Culti- vars,” Journal of Agronomy and Crop Science, Vol. 193, 2007, pp. 74-78. doi:10.1111/j.1439-037X.2006.00235.x [18] L. Saengprajak and P. Saensouk, “Genetic Diversity and Species Identification of Cultivar Species in Subtribe Cu- cumerinae (Cucurbitaceae) Using RAPD and SCAR Mar- kers,” American Journal of Plant Science, Vol. 3, No. 8, 2012, pp. 1092-1097. doi:10.4236/ajps.2012.38131 [19] K. Sangwijit, P. Thangsunan, R. W. Cutler and S. Anun- talabhochai, “Development of SCAR Marker for Thai Fragrant Rice (Oryza sativa L. var. Indica cv. Pathum- thani 1) Mutants Induced by Low Energy Ion Beam,” Chiang Mai Journal of Science, Vol. 39, 2012, pp. 1-9. [20] J. J. Doyle and J. L. Doyle, “Isolation of Plant DNA from Fresh Tissue,” Focus, Vol. 12, No. 1, 1990, pp. 13-15. [21] S. Anuntalabhochai, J. Chaingda, R. Chundet and P. Apavatjrut, “Genetic Diversity within Lychee (Litchi chi- nensis Sonn.) Based on RAPD Analysis,” International Symposium on Tropical and Subtropical Fruits, Canns, 26 November-1 December 2000, p. 45. [22] S. Temnykh, W. D. Park, N. Ayres, S. Cartinhour, N. Hauck, L. Lipovich, Y. G. Cho, T. Ishii and S. R. Mc- Couch, “Mapping and Genome Organization of Microsa- tellite Sequences in Rice (Oryza sativa L.),” Theoretical and Applied Genetics, Vol. 100, No. 5, 2000, pp. 697- 712. doi:10.1007/s001220051342 [23] S. R. McCouch, X. Chen, O. Panaud, S. Temnykh, Y. Xu, Y. G. Cho, N. Huang, T. Ishii and M. Blair, “Microsatel- lite Marker Development, Mapping and Applications in Rice Genetics and Breeding,” Plant Molecular Biology, Vol. 35, No. 1, 1997, pp. 89-99. doi:10.1023/A:1005711431474 [24] S. Rajapakse, M. Hubbard, J. W. Kelly, A. G. Abbott and R. E. Ballard, “Identification of Rose Cultivars by Re- striction Fragment Length Polymorphism,” HortScience, Vol. 52, 1992, pp. 237-245. [25] S. Matsumoto and H. Fukui, “Identification of Rose Cul- tivars and Clonal Plants by Random Amplified Polymor- phic DNA,” Scientia Horticulurae, Vol. 67, No. 1, 1996, pp. 49-54. doi:10.1016/S0304-4238(96)00951-X [26] L. Goulão, L. Cabrita, C. M. Oliveira and J. M. Leitã, “Comparing RAPD and AFLP Analysis in Discrimination and Estimation of Genetic Similarities among Apple (Ma- lus domestica Borkh.) Cultivars,” Euphytica, Vol. 119, No. 3, 2001, pp. 259-270. doi:10.1023/A:1017519920447 [27] R. Bautista, R. Crespillo, F. M. Cánovas and M. G. Cla- ros, “Identification of Olive-Tree Cultivars with SCAR Markers,” Euphytica, Vol. 129, No. 1, 2003, pp. 33-41. doi:10.1023/A:1021528122049 [28] M. Kunihisa, N. Fukino and S. Matsumoto, “Development of Cleavage Amplified Polymorphic Sequence (CAPS) Markers for Identification of Strawberry Cultivars,” Eu- phytica, Vol. 134, No. 2, 2003, pp. 209-215. doi:10.1023/B:EUPH.0000003884.19248.33 [29] S. S. Kaundun, S. Matsumoto and O. Kuntze, “Develop- ment of CAPS Markers Based on Three Key Genes of the Phenylpropanoid Pathway in Tea, Camellia sinensis (L.) and Differentiation between assamica and sinensis Varie- ties,” Theoretical and Applied Genetics, Vol. 106, 2003, pp. 375-383. [30] M. Gandikota, A. de Kochko, L. L. Chen, I. Nagab- hushana, C. Fauquet and A. R. Reddy, “Development of Copyright © 2013 SciRes. AJPS  Development of a SCAR Marker for Discrimination of a Thai Jasmine Rice (Oryza sativa L. cv. KDML105) Mutant, BKOS6, and Associated with Purple Color Trait in Thai Jasmine Rice-Related Varieties Copyright © 2013 SciRes. AJPS 1783 Transgenic Rice Plants Expressing Maize Anthocyanin Genes and Increased Blast Resistance,” Molecular Breed- ing, Vol. 7, No. 1, 2001, pp. 73-83. doi:10.1023/A:1009657923408 [31] G. S. Khush, “Green Revolution: The Way Forward,” Nature Review Genetics, Vol. 2, No. 10, 2001, pp. 815- 822. doi:10.1038/35093585 [32] K. Sadabpod, K. Kangsadalampai and L. Tongyonk, “An- tioxidant Activity and Antimutagenicity of Hom Nil Rice and Black Glutinous Rice,” Journal of Health Research, Vol. 24, No. 2, 2010, pp. 49-54. [33] P. Sirithunya, S. Sriprakhon, C. Vongsaprom, T. Sree- wongchai, A. Vanavichit and T. Toojinda, “Discovery of Broad Spectrum Blast Resistance in Rice,” In: A. Va- navichit, Ed., 1st International Conference on Rice for the Future, Bangkok, 2004, p. 160. [34] S. Smith and T. Helentjaris, “DNA Fingerprinting and Plant Variety Protection,” In A. H. Paterson, Ed., Genome Mapping in Plants, Landes Company, Austin, 1996. pp. 95-110.
|