 American Journal of Plant Sciences, 2013, 4, 1744-1749 http://dx.doi.org/10.4236/ajps.2013.49214 Published Online September 2013 (http://www.scirp.org/journal/ajps) Influence of Fly Ash and Growth Regulator with Soil for Determination of Chlorophyll in Arachis hypogaea L. Shweta Sao1, Pankaj K. Sahu2 1Department of Microbiology, Dr. C. V. Raman University Kota, Bilaspur, India; 2Department of Botany, Dr. C. V. Raman Univer- sity Kota, Bilaspur, India. Email: sahu.pankaj1@gmail.com Received January 10th, 2013; revised August 10th, 2013; accepted August 23rd, 2013 Copyright © 2013 Shweta Sao, Pankaj Kumar Sahu. This is an open access article distributed under the Creative Commons Attribu- tion License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The present investigation was conducted to find out the effect of varying levels of fly ash and growth hormones on the determination of chlorophylls. The experiments were conducted in pots during 2009-2010 with Arachis hypogaea L. (groundnut) grown with different levels of fly ash concentration, and soil was used (various combinations) at Guru gha- sidas University, Bilaspur (CG.) India. In fresh leaf, chlorophylls content varies in the plain soil from 0.29 to 0.64 mg g−1, which is less for photosynthetic activities. Arachis hypogaea L. showed maximum germination percentage, in- creasing leaf area, enhancement of root & shoot length, whereas Fly ash, bio fertilizers with growth hormone showed minimum values in all parameters. Results showed that, for combination of A to E, the value of chlorophyll ranged from 0.270 mg g−1 to 0.395 mg g−1, and chlorophyll b ranged from 0.400 mg g−1 to 0.489 mg g−1, whereas fro total chlorophyll ranged from 0.67 to 0.85 mg g−1. In the present work, chlorophyll a, chlorophyll b & total chlorophyll con- tent in fresh leaf, after 45 days, were recorded as 0.395 mg g−1, 0.489 mg g−1 and 0.851 mg g−1 while in 90 days were recorded as 10.38 mg g−1, 0.48 mg g−1 and 0.86 mg g−1 respectively, in less amount combination of fly ash, soil content with application of growth hormone. Keywords: Arachis hypogaea; Growth Hormone; Soil; Chlorophyll; Fly Ash 1. Introduction Arachis hypogaea L. (Groundnut) is a small branched herb, erects or trails on the ground and bears small yellow flowers. The cylindrical reticulated two seeds pod with- in the outer shell. Increased concentration of cement dust pollutants causes invisible injuries like progressive de- cline in the physiological process such as photosynthetic ability and respiration rate of leaves. Arachis hypogaea L. can adapt to a variety of soil and climatic conditions and the use of fly ash as a liming agent in mono and dicoty- ledonous plants for better crop yields. Fly ash has tre- mendous potential as a nutrient supplement and plays a favourable role in increasing growth and yield of ground nut [1-5]. Fly ash has similar physicochemical properties with soil. It can mix homogeneously and can improve agronomic properties of soil [6]. Fly ash is the treasure of trace elements. It makes the trace element readily avail- able to the crop when mixed with soil [7,8]. Auxin in- creased respiration rates are suggestive of parallel rela- tionship of growth, respiratory activity and found to in- crease RNA synthesis in tissue of higher plants [9]. Use of fly ash ameliorates soil acidity for maximum uptake of trace elements from fly ash which acted as a reserve of trace element when mixed in soil. Fly ash helps to retain water in the soil and also helped CO2 evolution. The plant hormone Indole acetic acid and Gibberellic acid helped protein, oil synthesis and also increased respira- tion rate. Soil metabolic activities, activities of amylase invertase and protease, chlorophyll a & b, carotenoid and protein content are increased in fly ash amended soil [10- 14]. Cement dust pollutants blocked the stomata, reduction in number of annual crops and due to cement dust de- creased the productivity and concentration of chlorophyll in a number of crops reported [15,16]. The reduction in protein, starch, yield and phytomass of groundnut oc- curred due to cement dust Arachis hypogaea L. [17]. Pro- tein synthesis decreased due to the low chlorophyll and reduced leaf area surface similar findings were reported [18]. Remunerative responses of groundnut crop to fer- Copyright © 2013 SciRes. AJPS  Influence of Fly Ash and Growth Regulator with Soil for Determination of Chlorophyll in Arachis hypogaea L. 1745 tilizer application have been observed both under irri- gated and rain fed conditions in India [19]. Increase in groundnut yield due to the application of NPK was also reported [20]. 2. Material and Methods Determination of chlorophyll a, b and total chlorophyll [21], Chlorophyll is extracted in 90% acetone from 1 gm leaves of Arachis (systronics-20). Using the absorption coefficient, the amount of chlorophyll a, b and total chlo- rophyll were estimated in the leaves of Arachis hypogaea L. 1 gm of well mixed representative sample of leaves were finely cut and ground with 20 ml of 80% acetone, centrifuged (5000 rpm 5 minutes) and supernatant liquid was transferred to a 100 ml volumetric flask. The proce- dure was repeated till residue become colourless. Vol- ume was made up to 100 ml mark with 80% acetone in all the three cases individually. The amount of Chlorophyll was calculated using the formula mentioned below: Mg chlorophyll agram of leave 12.7 A615V1000W Mg chlorophyll agram of leave 12.7 A615V1000W Mg total chlorophyll gram of leaves 20.2A6458.02A 663V1000 W where, A = Absorbance at specific wave lengths, V = Final volume of chlorophyll extract in 80% acetone, W = Fresh weight of leaves extracted. Chlorophyll a and b, Carotenoids were extracted from the leaves and estimated by the method of Arnon (1949). Absorbance was measured at 645, 663 and 480 nm with a spectrophotometer (U200 1Hitachi) against 80 per cent acetone as blank. Chlorophyll content was calculated us- ing the formula proposed by Arnon (Table 1). 3. Result and Discussion In the present work chlorophyll a, chlorophyll b and total chlorophyll in fresh leaf were observed as 0.35, 0.29 and 0.64 mg g−1 respectively while after 45 days chlorophylls were estimated as 0.395 mg g−1, 0.489 mg g−1 and 0.851 mg g−1. The value of chlorophyll a was observed as 0.270 mg g−1, 0.280 mg g−1, 0.300 mg g−1, 0.377 mg g−1 and 0.395 mg g−1 in the set A, B, C, D and E respectively, whereas for chlorophyll b the value was recorded as 0.400 mg g−1, 0.454 mg g−1, 0.460 mg g−1, 0.480 mg g−1 and 0.489 mg g−1 respectively. For total chlorophyll it was 0.670 mg g−1, 0.734 mg g−1, 0.760 mg g−1, 0.848 mg g−1 and 0.851 mg g−1 respectively (Figure 1). In plain soil, chlorophyll a, chlorophyll b and total chlorophyll were present as 0.35, 0.29 and 0.64 mg g−1 in fresh leaf, respectively, which is less for photosynthetic activities. In the present work chlorophylls were present in 10.38 mg g−1, 0.48 mg g−1 and 0.86 mg g−1 fresh leaf after 90 days. For A to E treatment chlorophyll values were 0.270 mg g−1, 0.280 mg g−1, 0.300 mg g−1 and 0.377 mg g−1 fresh leaf, respectively. For chlorophyll b, values were 0.400 mg g−1 fresh leaf, 0.454 mg g−1 fresh leaf, 0.460 mg g−1 fresh leaf and 0.480 mg g−1 fresh leaf, re- spectively and total chlorophyll in combination A was found to be 0.67 mg g−1 fresh leaf and B—0.73 mg/gm fresh leaf and C—0.76 mg g−1 fresh leaf, and in combi- nation D, 0.85 mg g−1 fresh leaf (fig). Treatments with ABA and GA3 significantly increased the total chlorophyll contents in Mentha plants. Similar results were observed that in Paclo butrazol treated barley and carrot and Paclo- butrazol treated leaves were dark green due to high chlo- rophyll content in potato [22-24]. In combination of 30% FA + 70% Soil + GH, Arachis hypogaea L. showed maximum germination %, Increasing leaf area, and en- hancement of shoot length, whereas Fly ash + NPK + GH showed minimum all parameters. Gibberellic acid increased the vegetative growth and pigment concentration in maize [25]. Foliar application of GA3 improved the chlorophyll levels in salinity stressed maize plants [26]. These results suggested that plain soil could not contribute to chlorophyll formation. It was per- formed that the addition of NPK had increased chloro- phyll formation by producing nitrogen and nutrient man- agement for sustainable groundnut productivity in India [27-30]. Mg has contributed its best role in structural constitution of chlorophyll a and b, and its absence causes early derangement of chloroplast structure [31], while Fe is contributing its important role to chlorophyll synthesis [32]. Nutrient management for the sustainable productivity in India was performed that fly ash and growth hormone increased the oil content in linseed crop [33,34]. IAA and GA hormones increase the germination percentage [35] while the use of IAA and GA hormone regulates the biochemical process of the plant, which re- sults higher percentage of oleic and oleic acid content [36]. IAA and GA increase the stem and root elongation and the plant growth parameters like root length, shoot length, number of leaves, leaf area increase, and seed contents also increase. Increasing number of leaves and leaf area increases the level of the chlorophyll a & b [37]. Growth biochemical and yield responses of groundnut are due to the effect of cement dust deposition [17]. 3.1. Effect of Plant Growth Regulators on Chlorophyll Content The total chlorophyll contents of the leaves increase with the age in control and treated Mentha leaves. The maxi- mum increase was found on 90 DAP in DIZ treatments nd it was 132.91 percent over control. Among the treat- a Copyright © 2013 SciRes. AJPS 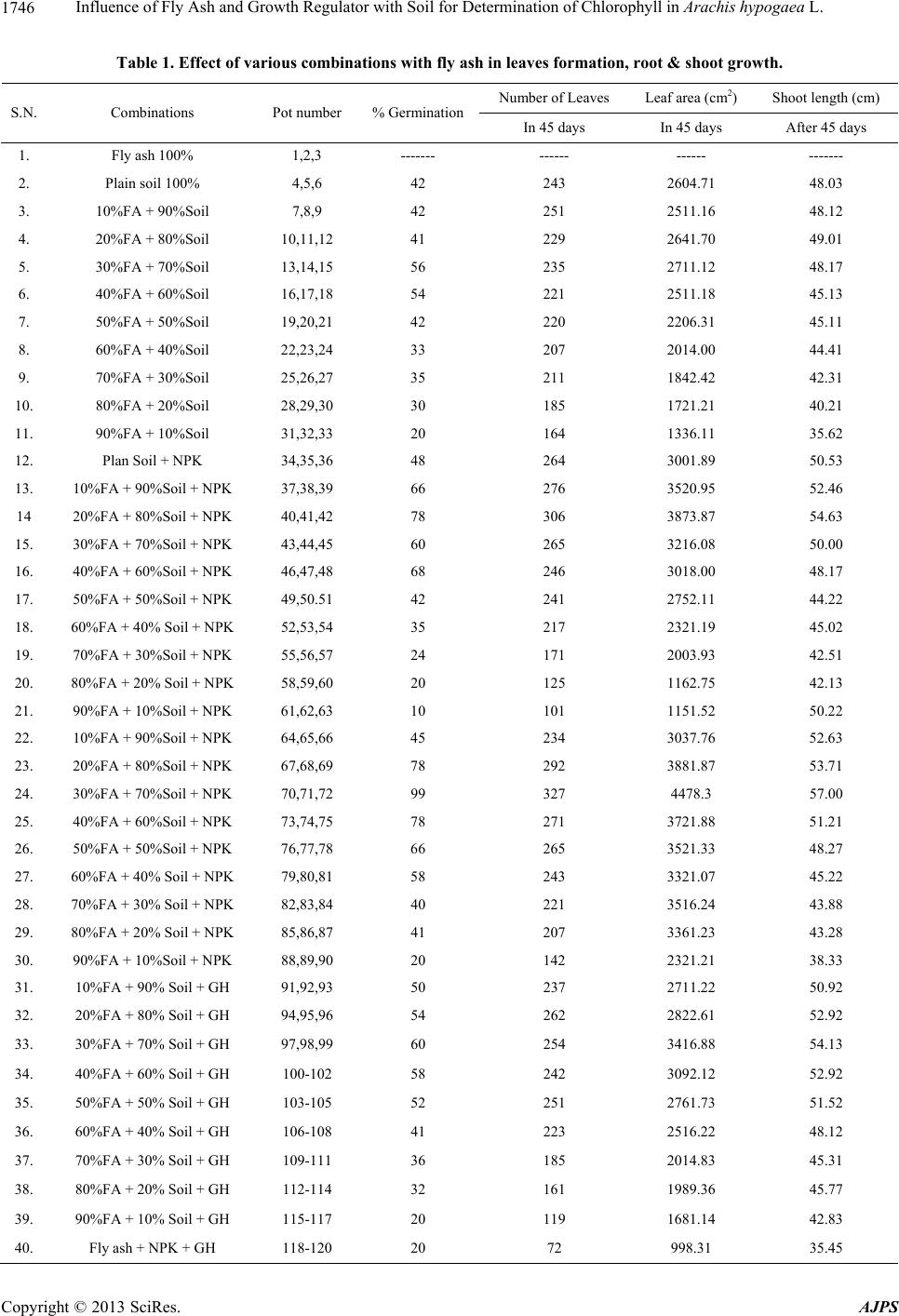 Influence of Fly Ash and Growth Regulator with Soil for Determination of Chlorophyll in Arachis hypogaea L. Copyright © 2013 SciRes. AJPS 1746 Table 1. Effect of various combinations with fly ash in leaves formation, root & shoot growth. Number of Leaves Leaf area (cm2) Shoot length (cm) S.N. Combinations Pot number % Germination In 45 days In 45 days After 45 days 1. Fly ash 100% 1,2,3 ------- ------ ------ ------- 2. Plain soil 100% 4,5,6 42 243 2604.71 48.03 3. 10%FA + 90%Soil 7,8,9 42 251 2511.16 48.12 4. 20%FA + 80%Soil 10,11,12 41 229 2641.70 49.01 5. 30%FA + 70%Soil 13,14,15 56 235 2711.12 48.17 6. 40%FA + 60%Soil 16,17,18 54 221 2511.18 45.13 7. 50%FA + 50%Soil 19,20,21 42 220 2206.31 45.11 8. 60%FA + 40%Soil 22,23,24 33 207 2014.00 44.41 9. 70%FA + 30%Soil 25,26,27 35 211 1842.42 42.31 10. 80%FA + 20%Soil 28,29,30 30 185 1721.21 40.21 11. 90%FA + 10%Soil 31,32,33 20 164 1336.11 35.62 12. Plan Soil + NPK 34,35,36 48 264 3001.89 50.53 13. 10%FA + 90%Soil + NPK 37,38,39 66 276 3520.95 52.46 14 20%FA + 80%Soil + NPK 40,41,42 78 306 3873.87 54.63 15. 30%FA + 70%Soil + NPK 43,44,45 60 265 3216.08 50.00 16. 40%FA + 60%Soil + NPK 46,47,48 68 246 3018.00 48.17 17. 50%FA + 50%Soil + NPK 49,50.51 42 241 2752.11 44.22 18. 60%FA + 40% Soil + NPK 52,53,54 35 217 2321.19 45.02 19. 70%FA + 30%Soil + NPK 55,56,57 24 171 2003.93 42.51 20. 80%FA + 20% Soil + NPK 58,59,60 20 125 1162.75 42.13 21. 90%FA + 10%Soil + NPK 61,62,63 10 101 1151.52 50.22 22. 10%FA + 90%Soil + NPK 64,65,66 45 234 3037.76 52.63 23. 20%FA + 80%Soil + NPK 67,68,69 78 292 3881.87 53.71 24. 30%FA + 70%Soil + NPK 70,71,72 99 327 4478.3 57.00 25. 40%FA + 60%Soil + NPK 73,74,75 78 271 3721.88 51.21 26. 50%FA + 50%Soil + NPK 76,77,78 66 265 3521.33 48.27 27. 60%FA + 40% Soil + NPK 79,80,81 58 243 3321.07 45.22 28. 70%FA + 30% Soil + NPK 82,83,84 40 221 3516.24 43.88 29. 80%FA + 20% Soil + NPK 85,86,87 41 207 3361.23 43.28 30. 90%FA + 10%Soil + NPK 88,89,90 20 142 2321.21 38.33 31. 10%FA + 90% Soil + GH 91,92,93 50 237 2711.22 50.92 32. 20%FA + 80% Soil + GH 94,95,96 54 262 2822.61 52.92 33. 30%FA + 70% Soil + GH 97,98,99 60 254 3416.88 54.13 34. 40%FA + 60% Soil + GH 100-102 58 242 3092.12 52.92 35. 50%FA + 50% Soil + GH 103-105 52 251 2761.73 51.52 36. 60%FA + 40% Soil + GH 106-108 41 223 2516.22 48.12 37. 70%FA + 30% Soil + GH 109-111 36 185 2014.83 45.31 38. 80%FA + 20% Soil + GH 112-114 32 161 1989.36 45.77 39. 90%FA + 10% Soil + GH 115-117 20 119 1681.14 42.83 40. Fly ash + NPK + GH 118-120 20 72 998.31 35.45 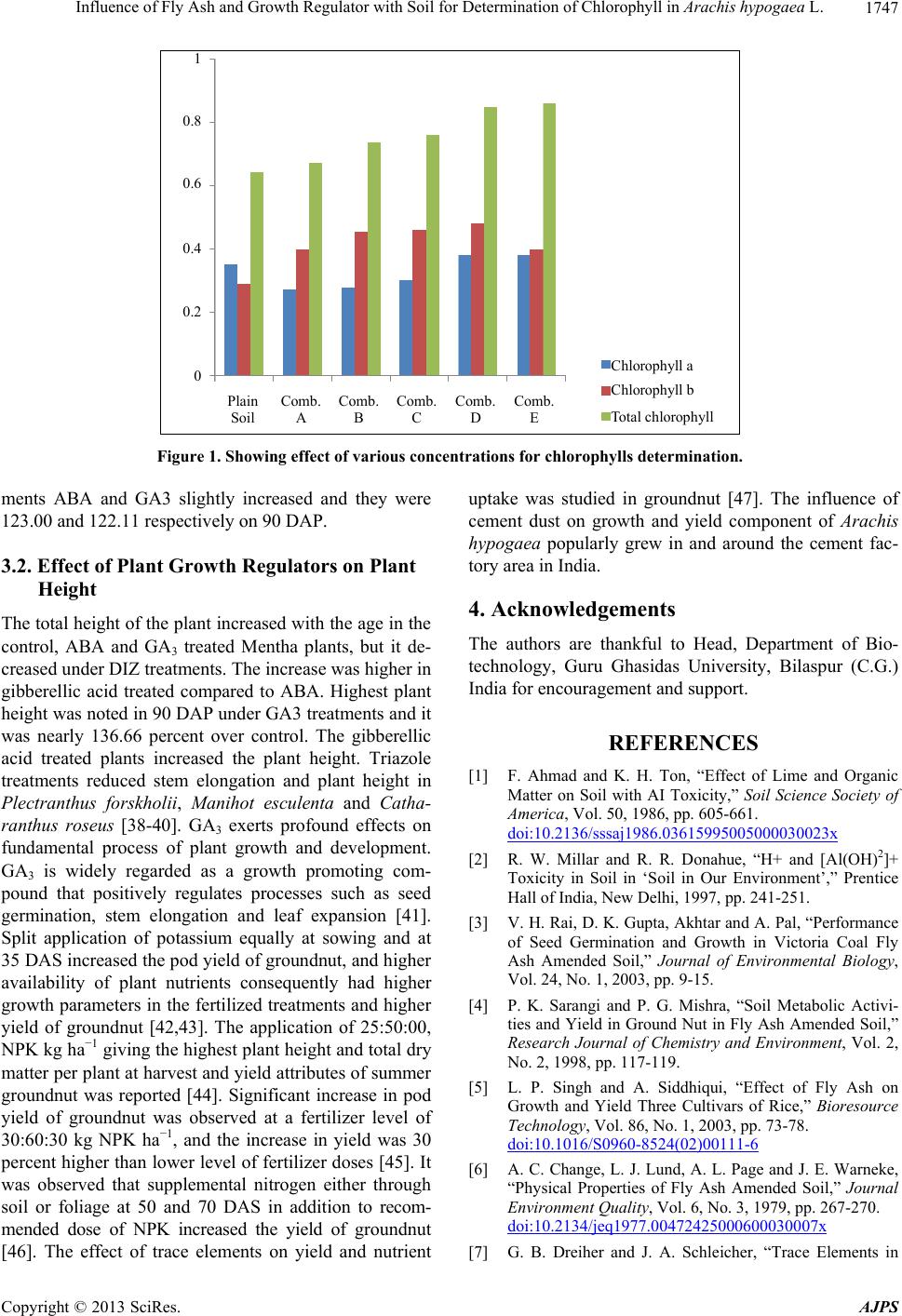 Influence of Fly Ash and Growth Regulator with Soil for Determination of Chlorophyll in Arachis hypogaea L. 1747 1 0.8 0.6 0.4 0.2 0 Plain Soil Comb. C Comb. A Comb. B Comb. D Comb. E Chlorophyll a Chlorophyll b Total chlorophyll Figure 1. Showing effect of various concentrations for chlorophylls determination. ments ABA and GA3 slightly increased and they were 123.00 and 122.11 respectively on 90 DAP. 3.2. Effect of Plant Growth Regulators on Plant Height The total height of the plant increased with the age in the control, ABA and GA3 treated Mentha plants, but it de- creased under DIZ treatments. The increase was higher in gibberellic acid treated compared to ABA. Highest plant height was noted in 90 DAP under GA3 treatments and it was nearly 136.66 percent over control. The gibberellic acid treated plants increased the plant height. Triazole treatments reduced stem elongation and plant height in Plectranthus forskholii, Manihot esculenta and Catha- ranthus roseus [38-40]. GA3 exerts profound effects on fundamental process of plant growth and development. GA3 is widely regarded as a growth promoting com- pound that positively regulates processes such as seed germination, stem elongation and leaf expansion [41]. Split application of potassium equally at sowing and at 35 DAS increased the pod yield of groundnut, and higher availability of plant nutrients consequently had higher growth parameters in the fertilized treatments and higher yield of groundnut [42,43]. The application of 25:50:00, NPK kg ha−1 giving the highest plant height and total dry matter per plant at harvest and yield attributes of summer groundnut was reported [44]. Significant increase in pod yield of groundnut was observed at a fertilizer level of 30:60:30 kg NPK ha−1, and the increase in yield was 30 percent higher than lower level of fertilizer doses [45]. It was observed that supplemental nitrogen either through soil or foliage at 50 and 70 DAS in addition to recom- mended dose of NPK increased the yield of groundnut [46]. The effect of trace elements on yield and nutrient uptake was studied in groundnut [47]. The influence of cement dust on growth and yield component of Arachis hypogaea popularly grew in and around the cement fac- tory area in India. 4. Acknowledgements The authors are thankful to Head, Department of Bio- technology, Guru Ghasidas University, Bilaspur (C.G.) India for encouragement and support. REFERENCES [1] F. Ahmad and K. H. Ton, “Effect of Lime and Organic Matter on Soil with AI Toxicity,” Soil Science Society of America, Vol. 50, 1986, pp. 605-661. doi:10.2136/sssaj1986.03615995005000030023x [2] R. W. Millar and R. R. Donahue, “H+ and [Al(OH)2]+ Toxicity in Soil in ‘Soil in Our Environment’,” Prentice Hall of India, New Delhi, 1997, pp. 241-251. [3] V. H. Rai, D. K. Gupta, Akhtar and A. Pal, “Performance of Seed Germination and Growth in Victoria Coal Fly Ash Amended Soil,” Journal of Environmental Biology, Vol. 24, No. 1, 2003, pp. 9-15. [4] P. K. Sarangi and P. G. Mishra, “Soil Metabolic Activi- ties and Yield in Ground Nut in Fly Ash Amended Soil,” Research Journal of Chemistry and Environment, Vol. 2, No. 2, 1998, pp. 117-119. [5] L. P. Singh and A. Siddhiqui, “Effect of Fly Ash on Growth and Yield Three Cultivars of Rice,” Bioresource Technology, Vol. 86, No. 1, 2003, pp. 73-78. doi:10.1016/S0960-8524(02)00111-6 [6] A. C. Change, L. J. Lund, A. L. Page and J. E. Warneke, “Physical Properties of Fly Ash Amended Soil,” Journal Environment Quality, Vol. 6, No. 3, 1979, pp. 267-270. doi:10.2134/jeq1977.00472425000600030007x [7] G. B. Dreiher and J. A. Schleicher, “Trace Elements in Copyright © 2013 SciRes. AJPS  Influence of Fly Ash and Growth Regulator with Soil for Determination of Chlorophyll in Arachis hypogaea L. 1748 Coal by Optical Emission Spectroscopy,” Advances in Chemistry, Vol. 35, 1975, p. 141. [8] C. O. Plank and D. C. Mortens, “Boron Availability as Influenced by Application of Fly Ash to Soil,” Soil Sci- ence Society of America Proceeding, Vol. 38, 1974, pp. 974-977. doi:10.2136/sssaj1974.03615995003800060038x [9] J. R. Still and W. G. Pill, “Germination, Emergence and Seedlings Growth of Tomato and Impatiens in Response to Seed Treatment with Aclobutrazol,” Horticultural Sci- ence, Vol. 38, 2003, pp. 1201-1204. [10] K. Bhandari, “Studies on the Effect of Fly Ash and Plant Hormones Treated Soil in the Increased Protein and Amino Acid Content in the Seeds Ground Nut,” Asian Journal of Chemistry, Vol. 20, 2006, p. 15. [11] M. A. Bozkurt and I. Karacal, “Quantitative Relationship between Nutrient Contents and Oil Quality of Sunflower Seed,” Journal of Food & Science Technology, Vol. 38, No. 6, 2001, pp. 635-638. [12] Y. R. Chadha, “Lime Requirement for Proper Growth of Ground Nut, Wealth of India,” CSIR Publication, New Delhi, 1998, pp. 90-102. [13] Y. R. Chadha, “Poorly Developed Seed Kernels in Ground Nut Due to Ca Deficiency. Wealth of India,” CSIR Pub- lication, New Delhi, 1998, pp. 103-109. [14] V. Goyel, M. R. Augar and D. K. Shrivastava, “Studies on the Effect of Fly Ash Treated Soil on the Increased Protein on the Effect Increased Protein Content in the Seeds of Glycine max (Soybean),” Asian Journal of Che- mistry, Vol. 14, pp. 180-182. [15] A. M. Farmer, “The Effect of Dust on Vegetation—A Re- view,” Environmental Pollution, Vol. 79, No. 1, 1993, pp. 63-75. doi:10.1016/0269-7491(93)90179-R [16] R. N. Sato, H. K. Kene, R. V. Nalamwar and R. B. Ule- male, “Effect of Cement Dust Pollution on Growth and Yield of Cotton,” Annals of Plant Physiology, Vol. 7, 1993, pp. 73-77. [17] D. Raajasubramanian, P. Sundaramoorthy, L. Baskaran, K. Sankar Ganesh, A. L. A. Chidambaram and M. Jega- Nathan, “Cement Dust Pollution on growth and Yield At- tributes of Groundnut (Arachis hypogaea L.),” Interna- tional Multidisciplinary Research Journal, Vol. 1, No.1, 2011, pp. 31-36. [18] T. Baszynski, M. Warchodowa, Z. Krupa, A. Tukendorf, M. Krol and D. Z. Wolinska, “The Effect of Magnesium Deficiency on Photochemical Activities of Rape and Buck- wheat Chloroplasts,” Zeitschrift fur Pflanzenphysiologie, Vol. 99, 1980, p. 295. [19] J. S. Kanwar, H. L. Nijhawan and S. K. Raheja, “Ground- nut Nutrition and Fertilizer Responses in India,” ICAR, New Delhi, 1983. [20] V. V. Angadi, S. V. Patil, M. N. Shilvantar and B. M. Chittapur, “Effect of NPK Levels and Split Application of N on Growth and Yield of bunch Groundnut in Vertisol under Irrigation System,” Karnataka Journal of Agricul- ture Science, Vol. 3, No. 1-2, 1980, pp. 9-14. [21] S. Sadashivam and Manickchand, “Biochemical Methods: Estimation of Fatty Acids and Oils,” New Age Interna- tional Publication Limited, New Delhi, 1996, pp. 102- 107. [22] S. Sunitha, M. R. Perras, D. E. Falk, R. Zhang, P. Pharis and R. A. Fletcher, “Relationship between Gibberellins, Height and Stress Tolerance on Barley Seedlings,” Plant Growth Regulation, Vol. 42, No. 2, 2004, pp. 125-135. doi:10.1023/B:GROW.0000017492.56792.64 [23] R. Gopi, C. A. Jaleel, R. Sairam, G. M. A. Lakshmanan, M. Gomathinayagam and R. Pannerselvam, “Differential Effects of Hexaconazole and Paclobutrazol on Biomass, Electrolyte Leakage, Lipid per Oxidation and Antioxidant Potential of Daucus carota L.,” Colloids and Surfaces B: Biointerfaces, Vol. 60, 2007, p. 180. doi:10.1016/j.colsurfb.2007.06.003 [24] T. Tekalign, S. Hammes and J. Robbertse, “Paclobutrazol Induced Leaf, Stem and Root Anatomical Modifications in Potato,” Horticulture Science, Vol. 40, No. 5, 2005, pp. 1343-1346 [25] C. Kaya, A. Levent Tuna, A. Alfredo and C. Alves, “Gib- berellic Acid Improves Water Deficit Tolerance in Maize Plants,” Acta Physiologiae Plantarum, Vol. 28, No. 4, 2006, pp. 331-337. doi:10.1007/s11738-006-0029-7 [26] A. L. Tuna, K. Cengiz, M. Dikilitas and D. Higgs, “The Combined Effect of Gibberellic Acid and Salinity on Same Antioxidant Enzyme Activities, Plant Growth Parameter and Nutritional Status in Maize Plants,” Environmental and Experimental Botany, Vol. 62, 2008, p. 19. doi:10.1016/j.envexpbot.2007.06.007 [27] J. C. Sadhna and B. M. Khan, “Nitrogen Fixation,” Jour- nal of Science Industrial Research, Vol. 36, 1977, pp. 495-533. [28] J. C. Sadhna and B. M. Khan, “Nitrogen Fixation in Ground Nut (Arachis hypogaea),” Journal of Science In- dustrial Research, Vol. 36, 1977, pp. 515-516. [29] D. L. Purich and H. J. Fromm, “Studies on Factors Influ- encing Enzyme Responses,” Journal of Biological Chem- istry, Vol. 31, 1972, pp. 247-255. [30] E. J. Hawitt and T. A. Smith, “Plant Mineral Nutrition,” English University, London, 1974, pp. 131-133. [31] J. M. Whatley, “Ultra Structural Changes in Chloroplast of Phaseolus vulgaris,” New Phytology, Vol. 70, 1971, pp. 725-742. doi:10.1111/j.1469-8137.1971.tb02573.x [32] O. T. G. Johns, “Ferro Cheletase of Spinich Chloroplast,” Journal of Biochemistry, Vol. 107, No. 1, 1968, pp. 113- 119. [33] P. Veeramani and K. Subrahmaniy, “Nutrient Manage- ment for Sustainable Groundnut Productivity in India—A Review,” International Journal of Engineering Science and Technology, Vol. 3, No. 11, 2011, pp. 41-45. [34] K. Bhandari,”Effect of Fly Ash on Growth and Yield of Linseed Crop,” Ph.D. Thesis, G.G.D. University, Bilaspur, 2004. [35] K. Rao and S. Gideron, “Use of Plant Hormones on Ger- mination,” Indian Journal of Oil Seed, Vol. 1, 1957, p. 247. [36] A. Bawaria, “Oil Analysis of Sunflower, Mustard Seed, Ground Nut, Grown in Ameliorated Acidic Soil,” Ph.D. Copyright © 2013 SciRes. AJPS  Influence of Fly Ash and Growth Regulator with Soil for Determination of Chlorophyll in Arachis hypogaea L. Copyright © 2013 SciRes. AJPS 1749 Thesis, G. G. D. University, Bilaspur C.G, 2006. [37] P. J. Davies, “The Plant Hormones: Their Nature, Occur- rence and Function,” In: P. J. Davis, Ed., Plant Hormones: Physiology, Biochemistry and Biology, Kluwer Acadamic Publisher, Drodrecht, 1995, pp. 1-12. [38] G. M. A. Lakshmanan, C. A. Jaleel, M. Gomathinayagam and R. Panneerselvam, “Changes in Antioxidant Potential and Sink Organ Dry Matter with Pigment Accumulation Induced by Hexaconazole in Plectranthus forskholii Briq,” Comptes Rendus Biologies, Vol. 330, No. 11, 2007, pp. 814-820. doi:10.1016/j.crvi.2007.08.008 [39] M. Gomathinayagam, C. A. Jaleel, G. M. A. Lakshmanan and R. Panneerselvam, “Change in Carbohydrate Me- tabolism by Triazole Growth Regulators in Cassava (Manihot esculenta Crantz); Effects on Tuber Production and Quality,” Comptes Rendus Biologies, Vol. 330, No. 9, 2007, pp. 644-655. doi:10.1016/j.crvi.2007.06.002 [40] C. A. Jaleel, R. Gopi, P. Manivannan, M. Gomathinaya- gam, P. V. Murali and R. Panneerselvam, “Soil Applied Propiconazole Alleviates the Impact of Salinity on Ca- tharanthus roseus by Improving Antioxidant Status,” Pesticide Biochemistry and Physiology, Vol. 90, No. 2, 2008, pp. 135-139. doi:10.1016/j.pestbp.2007.11.003 [41] S. M. Swain and D. P. Singh, “Tale Tales from Sly Dwarves: Novel Functions of Gibberellins Plant Devel- opment,” Trends of Plant Science, Vol. 10, No. 3, 2005, pp. 123-129. doi:10.1016/j.tplants.2005.01.007 [42] C. V. Patil, N. A. Yaledahalli and S. S. Prakash, “Inte- grated Nutrient Management for Sustainable Productivity of Groundnut in India,” National Workshop on Ground- nut Seed Technology, Raichur, 2003. [43] S. S. Mondal and S. B. Goswami, “Effect of Split Appli- cation of Potassium on Rainfed Groundnut,” Journal of Potassium Research, Vol. 7, No. 4, 1991, pp. 304-308. [44] P. Parasuraman, M. N. Budher, P. Manickasundaram and M. Nandanam, “Response of Sorghum, Finger Millet and Groundnut to the Silt Application and Combined Use of Organic Matter and Inorganic Fertilizer under Rainfed Conditions,” Indian Journal of Agronomy, Vol. 43, No. 3, 1998, pp. 528-532. [45] D. S. Thorave and M. B. Dhonde, “Morphological Indices and Yield Attributes as Influenced by Integrated Nutrient Management in Summer Groundnut,” Annals of Plant Physiology, Vol. 21, No. 2, 2007, pp. 186-188. [46] V. Kumar, B. C. Ghose, B. Ravi and S. Karmakar, “Effect of Irrigation and Fertilizer on Yield, Water-Use Effi- ciency and Nutrient Uptake of Summer Groundnut,” In- dian Journal Agronomy, Vol. 45, No. 4, 2000, pp. 756- 760. [47] T. Chitdeshwari and S. Poongathai, “Yield of Groundnut and Nutrient Uptake as Influenced by Zn, B, and S,” Ag- ricultural Science Digest, Vol. 23, 2003, pp. 263-265.
|