 Vol.5, No.9, 1025-1033 (2013) Natural Science http://dx.doi.org/10.4236/ns.2013.59127 Optimization and kinetic modeling of lipase mediated enantioselective kinetic resolution of (±)-2-octanol Jyoti B. Sontakke, Ganapati D. Yadav* Department of Chemical Engineering, Institute of Chemical Technology, Mumbai, India; *Corresponding Author: gd.yadav@ictmumbai.edu.in, gdyadav@yahoo.com Received 6 May 2013; revised 5 June 2013; accepted 15 June 2013 Copyright © 2013 Jyoti B. Sontakke, Ganapati D. Yadav. This is an open access article distributed under the Creative Commons At- tribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is prop- erly cited. ABSTRACT Chiral 2-octanol is one of the key intermediates for prep aration of liquid cry st al materials, as well as many optically active pharmaceuticals. Li- pase catalyzed kinetic resolution has proved to be an efficient technique for synthesis of enan- tiomerically enriched compounds. In the present study, optimization and kinetic modeling of ki- netic resolution of (±)-2-octanol was done by using vinyl acetate as an acyl donor in n-hep- tane as a solvent. Response surface methodol- ogy (RSM) and four-factor-five-level Centre Com- posite Rotatable Design (CCRD) were employed to evaluate the effect of various parameters su c h as speed of agitation, enzyme loading, tempera- ture and acyl donor/alcohol molar ratio on con- version, enantiomeric excess (ee), enantioselec- tivity and initial rate of reaction. Acylation of 2- oct anol with vinyl acet ate cat alyzed by Novozyme 435 follows the ternary complex mechanism (ordered bi-bi mechanism) with inhibition by 2- octanol. Keywords: Immobilized Li pas e; Novozyme 435; 2-Octanol; Response Surface Meth odology; Kinetic Modeling; Enantioselectivity 1. INTRODUCTION Enzymatic catalysis in non-aqueous media has been greatly pursued these days for the synthesis of a wide variety of pharmaceuticals, agrochemicals, perfumes, fla- vors and other fine-chemicals [1-4]. In this regard, our group has contributed extensively to mechanistic studies, kinetic modeling and separation of enantiomers, covering several industrially relevant classes of reactions such as epoxidation/oxidation [5,6], hydrolysis [7], esterification [8-10], transesterification [11-13], amidation [14] and hy- drazinolysis [15]. The synergism with microwave irra- diation in immobilized lipase catalysis [16-20] and scope of non-aqueous systems in pharmaceutical industries [21] have been embraced. Optimization of process parameters by using statistical methods has been reported in a num- ber of cases in literature and the current investigation in an effort in that direction. Kinetic resolution of chiral compounds using enzymes especially lipases has proven to be an effective technique vis-a-vis chemical methods. The main consideration for adding biotransformation in a synthetic route is the re- gio- and stereo-control that can be achieved elegantly using enzyme-catalyzed step(s) [4]. Thus, chemo-enzy- matic processes will see commercial utility in future. For instance, chiral aliphatic alcohols, which are important active pharmaceutical intermediates (API), have been ob- tained through lipase catalyzed kinetic resolution of cor- responding racemic mixtures via esterification, transesteri- fication or ester hydrolysis [21]. There are a number of ways to resolve the racemic mixtures by using enzymatic catalysis: dynamic kinetic resolution (DKR) with race- mization catalysts [22], combination of DKR with dou- ble kinetic resolution [23], deracemisation [1], and se- quential kinetic resolution [24]. Different lipases have been used for the kinetic resolution of aliphatic alcohols [23,25-30]. Various immobilization techniques for lipase immobilization have been reported; for instance, hexago- nal mesoporous silica (HMS) [12], magnetic nanoparti- cles, Diaion HP20, ultrastable-Y molecular sieve [27], SBA 15 [29], and Sol-gel method [31]. Process optimization has a great relevance in complex reaction and has been done by two ways: one-factor-at- a-time method and statistical analysis such as Response Surface Methodology (RSM). RSM is a collection of statistical and mathematical techniques useful for devel- oping, improving, and optimizing processes in which a Copyright © 2013 SciRes. OPEN ACCESS  J. B. Sontakke, G. D. Yadav / Natural Science 5 (2013) 1025-1033 1026 response of interest is influenced by several variables and the objective is to optimize this response [32]. To avoid the disadvantages of the one-factor-at-a-time method since it does not illustrate interaction effect among vari- ous factors and gives only local optima of the reaction, in this work we have used the RSM for process optimiza- tion. The Centre Composite Rotatable Design of RSM has been previously been successfully applied in food technology [33,34], microbiology [35], biotechnological [36-41] and chemical processes [42]. To the best of our knowledge, there is a dearth of literature on RSM for kinetic resolution of chiral compounds using enzymatic catalysis [40-44]. In the present study, Candida an tartica lipase B, Thermomyces lanuginosus lipase and Rhizomucor meihei lipase, were employed for the kinetic resolution of (±)- 2-octanol by using vinyl acetate as an acylating agent. Optimization of reaction parameters has been done by RSM and CCRD using four factors, each at five variables. 2. MATERIALS AND METHODS 2.1. Enzymes and Chemicals All chemicals were procured from firms of repute and used without any further purification: Novozyme 435 (Candida antarctica lipase B immobilized on a macro- porous polyacrylic resin, activity 10 PLU/g; (1 µmol propyl laurate formed/min/g-enzyme)), Lipozyme RM IM (Mucor miehei lipase immobilized on anionic resin, activity 6 BAUN (Acidolysis Unit Novo) and Lipozyme TL IM (Thermomyces lanuginosus immobilized on silica, activity 75 IUN/g) were procured as gift samples from Novo Nordisk, Denmark. (±)-2-Octanol was procured from Merck, India. Vinyl acetate and n-heptane were procured from SD Fine Chemicals Pvt. Ltd., Mumbai, India. 2.2. Experimental Setup The experimental setup consisted of a 3 cm internal diameter (ID), fully baffled mechanically agitated reactor of 50 cm3 capacity, which was equipped with four equi- spaced baffles and 1 cm diameter four bladed-pitched- turbine impeller. The entire reactor assembly was im- mersed in a thermostatic water bath which was main- tained at a desired temperature with an accuracy of ±1˚C. 2.3. Kinetic Resolution of (R,S)-2-Octanol by Immobilized Lipase The resolution was performed in the above reactor containing (±)-2-octanol, catalyst and solvent. When the set temperature was reached, vinyl acetate was added in the reactor, and agitation started. Samples were with- drawn periodically at regular time intervals, and the reso- lution process was monitored by GC. The total reaction mixture volume was 25 cm3 which was made up with n-heptane as a solvent. The total reaction time was 6 h. 2.4. Determination of Enantiomeric Excess (ee) and Enantioselectivity (E) Clear liquid samples were withdrawn periodically from the reaction mass and analyzed by using Ceres 800 plus GC instrument equipped with flame ionization detector (FID) and β-Dex 120 (30 m 0.25 mm 0.25 µm) chiral capillary column. The analytical conditions were: injec- tor temperature 220˚C; FID temperature 220˚C; oven temperature held at 65˚C for 30 min, then increased at 10˚C·min −1 to a final temperature of 130˚C, which was thereafter maintained for 10 min. The enantioselectivity (E) was calculated from the enantiomeric excess of the substrate (ees %) at a certain conversion (c, %) based on the following equations. ln 11 ln 11 s s cee Ecee (1) where, 00 1RS RS AA c AA (2) and SR s SR AA ee AA (3) where, A(R) and A(S) denote (R)-2-octanol and (S)-2-oc- tanol, respectively. 2.5. Design of Experiments and Statistical Analysis RSM was used to optimize the process of resolution of (±)-2-octanol and to study the effect of different process variables on reaction along with the interactions among them. The experimental design applied to this study was CCRD (four factors, each at five levels). Compared with one-factor-at-a-time design, which has been adopted most often in the literature, the combination of RSM and four-factor-three-level CCRD employed in this study al- lowed us to reduce the number of experiments and time. The independent variables were: speed of agitation, cata- lyst loading, reaction temperature, and ester to alcohol molar ratio. Whereas the responses (dependent variables) chosen were 1) conversion of (±)-2-octanol; 2) ee of re- maining alcohol; 3) E of the enzyme and 4) initial rate of reaction. Table 1 shows the independent variables and their levels. The responses were then analyzed using nu- merical tools provided by Design Expert, Version 6.0.10 Copyright © 2013 SciRes. OPEN ACCESS 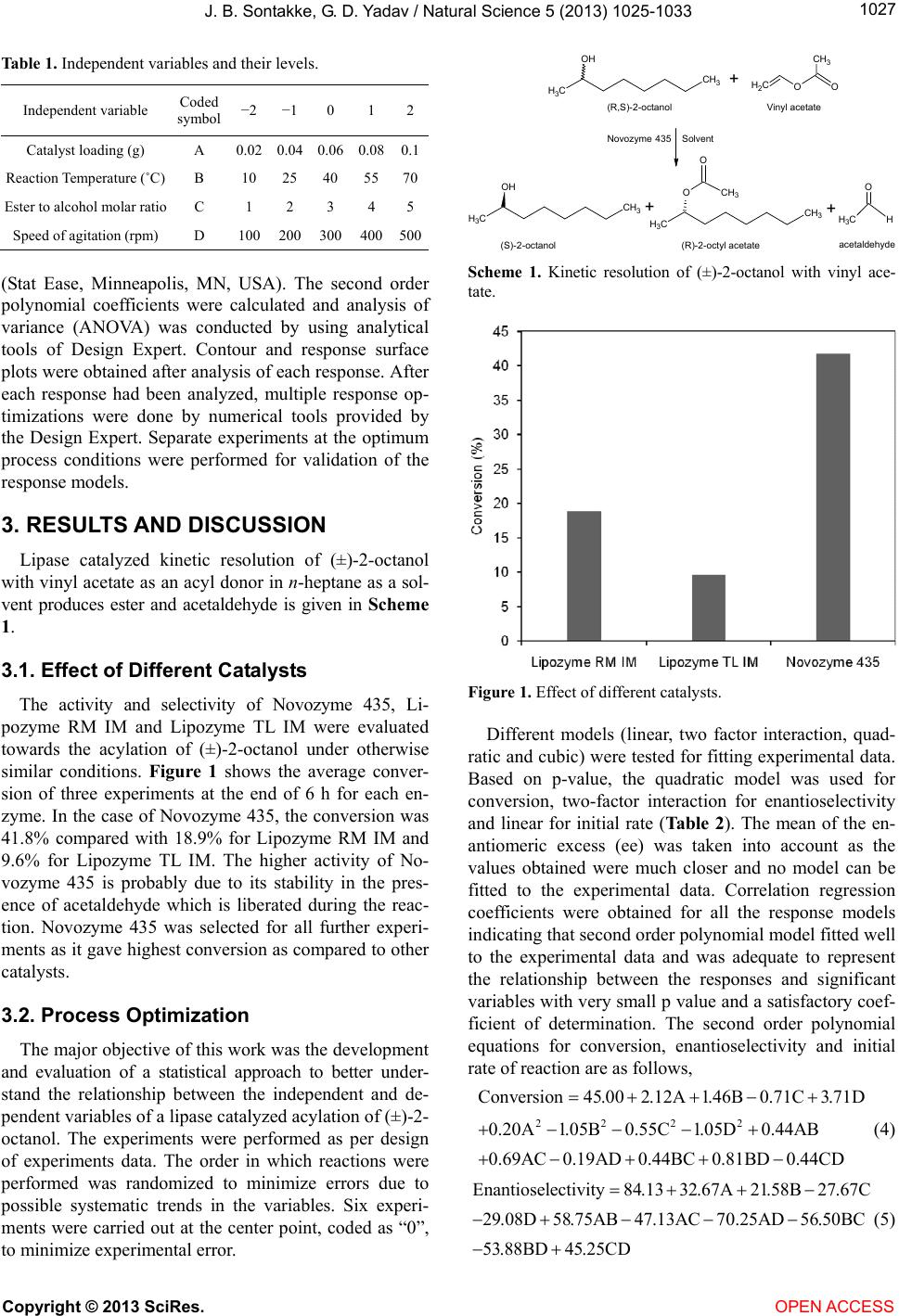 J. B. Sontakke, G. D. Yadav / Natural Science 5 (2013) 1025-1033 1027 Table 1. Independent variables and their levels. Independent variable Coded symbol −2 −1 0 1 2 Catalyst loading (g) A 0.02 0.04 0.06 0.080.1 Reaction Temperature (˚C) B 10 25 40 5570 Ester to alcohol molar ratio C 1 2 3 4 5 Speed of agitation (rpm) D 100 200 300 400500 (Stat Ease, Minneapolis, MN, USA). The second order polynomial coefficients were calculated and analysis of variance (ANOVA) was conducted by using analytical tools of Design Expert. Contour and response surface plots were obtained after analysis of each response. After each response had been analyzed, multiple response op- timizations were done by numerical tools provided by the Design Expert. Separate experiments at the optimum process conditions were performed for validation of the response models. 3. RESULTS AND DISCUSSION Lipase catalyzed kinetic resolution of (±)-2-octanol with vinyl acetate as an acyl donor in n-heptane as a sol- vent produces ester and acetaldehyde is given in Scheme 1. 3.1. Effect of Different Catalysts The activity and selectivity of Novozyme 435, Li- pozyme RM IM and Lipozyme TL IM were evaluated towards the acylation of (±)-2-octanol under otherwise similar conditions. Figure 1 shows the average conver- sion of three experiments at the end of 6 h for each en- zyme. In the case of Novozyme 435, the conversion was 41.8% compared with 18.9% for Lipozyme RM IM and 9.6% for Lipozyme TL IM. The higher activity of No- vozyme 435 is probably due to its stability in the pres- ence of acetaldehyde which is liberated during the reac- tion. Novozyme 435 was selected for all further experi- ments as it gave highest conversion as compared to other catalysts. 3.2. Process Optimization The major objective of this work was the development and evaluation of a statistical approach to better under- stand the relationship between the independent and de- pendent variables of a lipase catalyzed acylation of (±)-2- octanol. The experiments were performed as per design of experiments data. The order in which reactions were performed was randomized to minimize errors due to possible systematic trends in the variables. Six experi- ments were carried out at the center point, coded as “0”, to minimize experimental error. +CH2O CH3 O OH CH3 CH3 OH CH3 CH3 O CH3 CH3 O CH3 + (R,S)-2-octanolVinyl acetate (S)-2-octanol Novozyme 435Solvent +CH3H O acetaldehyde (R)-2-octyl acetate Scheme 1. Kinetic resolution of (±)-2-octanol with vinyl ace- tate. Figure 1. Effect of different catalysts. Different models (linear, two factor interaction, quad- ratic and cubic) were tested for fitting experimental data. Based on p-value, the quadratic model was used for conversion, two-factor interaction for enantioselectivity and linear for initial rate (Tab l e 2 ). The mean of the en- antiomeric excess (ee) was taken into account as the values obtained were much closer and no model can be fitted to the experimental data. Correlation regression coefficients were obtained for all the response models indicating that second order polynomial model fitted well to the experimental data and was adequate to represent the relationship between the responses and significant variables with very small p value and a satisfactory coef- ficient of determination. The second order polynomial equations for conversion, enantioselectivity and initial rate of reaction are as follows, 222 2 Conversion45.002.12A1.46B0.71C3.71D 0.20A1.05B0.55C 1.05D0.44AB 0.69AC0.19AD 0.44BC 0.81BD0.44CD (4) Enantioselectivity84.13 32.67A21.58B27.67C 29.08D 58.75AB47.13AC70.25AD56.50BC 53.88BD 45.25CD (5) Copyright © 2013 SciRes. OPEN ACCESS 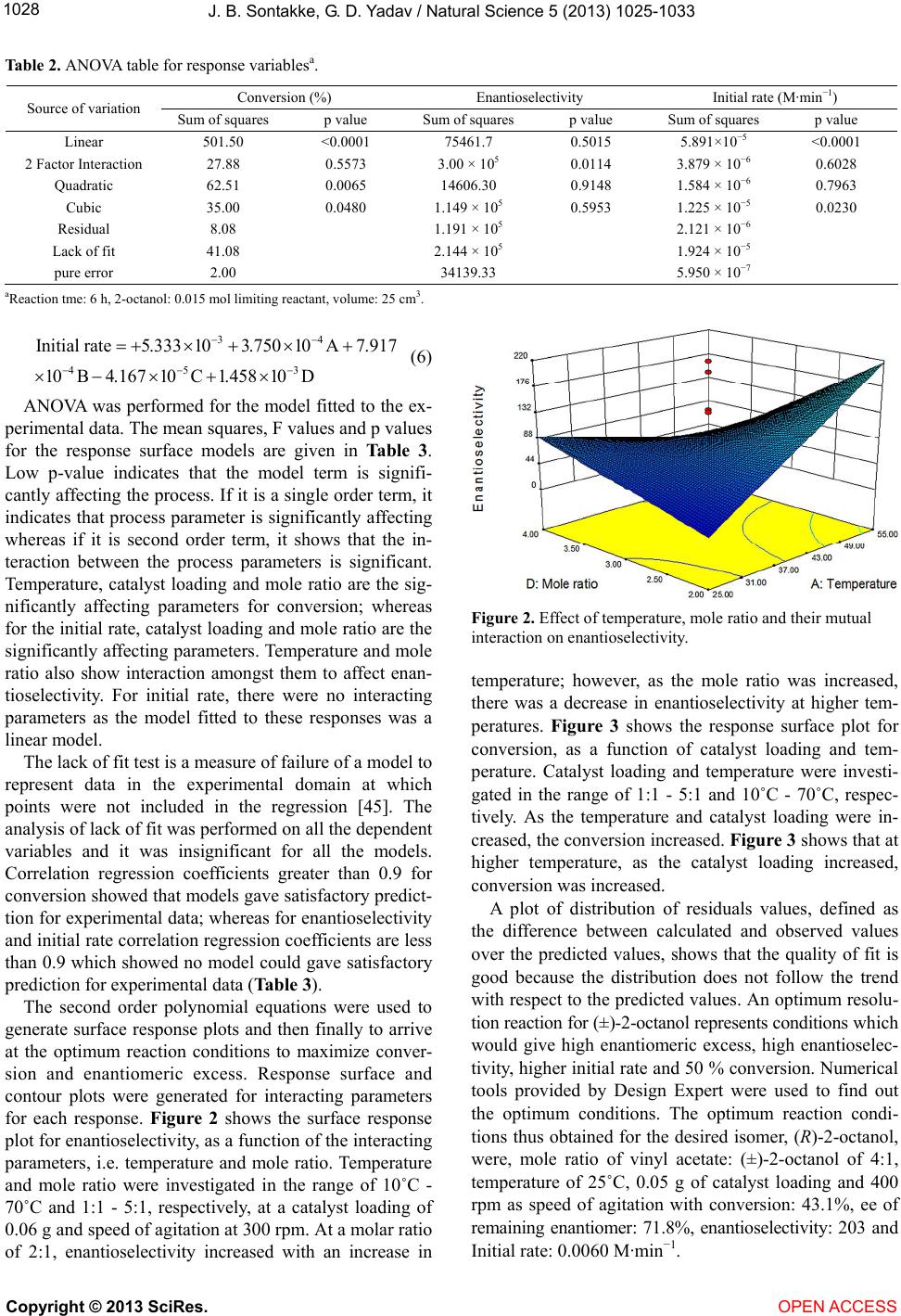 J. B. Sontakke, G. D. Yadav / Natural Science 5 (2013) 1025-1033 Copyright © 2013 SciRes. 1028 Table 2. ANOVA table for response variablesa. Conversion (%) Enantioselectivity Initial rate (M·min−1) Source of variation Sum of squares p value Sum of squares p value Sum of squares p value Linear 501.50 <0.0001 75461.7 0.5015 5.891×10−5 <0.0001 2 Factor Interaction 27.88 0.5573 3.00 × 105 0.0114 3.879 × 10−6 0.6028 Quadratic 62.51 0.0065 14606.30 0.9148 1.584 × 10−6 0.7963 Cubic 35.00 0.0480 1.149 × 105 0.5953 1.225 × 10−5 0.0230 Residual 8.08 1.191 × 105 2.121 × 10−6 Lack of fit 41.08 2.144 × 105 1.924 × 10−5 pure error 2.00 34139.33 5.950 × 10−7 aReaction tme: 6 h, 2-octanol: 0.015 mol limiting reactant, volume: 25 cm3. 34 453 Initial rate5.333103.75010A7.917 10B4.16710C1.458 10D (6) ANOVA was performed for the model fitted to the ex- perimental data. The mean squares, F values and p values for the response surface models are given in Table 3. Low p-value indicates that the model term is signifi- cantly affecting the process. If it is a single order term, it indicates that process parameter is significantly affecting whereas if it is second order term, it shows that the in- teraction between the process parameters is significant. Temperature, catalyst loading and mole ratio are the sig- nificantly affecting parameters for conversion; whereas for the initial rate, catalyst loading and mole ratio are the significantly affecting parameters. Temperature and mole ratio also show interaction amongst them to affect enan- tioselectivity. For initial rate, there were no interacting parameters as the model fitted to these responses was a linear model. Figure 2. Effect of temperature, mole ratio and their mutual interaction on enantioselectivity. temperature; however, as the mole ratio was increased, there was a decrease in enantioselectivity at higher tem- peratures. Figure 3 shows the response surface plot for conversion, as a function of catalyst loading and tem- perature. Catalyst loading and temperature were investi- gated in the range of 1:1 - 5:1 and 10˚C - 70˚C, respec- tively. As the temperature and catalyst loading were in- creased, the conversion increased. Figure 3 shows that at higher temperature, as the catalyst loading increased, conversion was increased. The lack of fit test is a measure of failure of a model to represent data in the experimental domain at which points were not included in the regression [45]. The analysis of lack of fit was performed on all the dependent variables and it was insignificant for all the models. Correlation regression coefficients greater than 0.9 for conversion showed that models gave satisfactory predict- tion for experimental data; whereas for enantioselectivity and initial rate correlation regression coefficients are less than 0.9 which showed no model could gave satisfactory prediction for experimental data (Table 3). A plot of distribution of residuals values, defined as the difference between calculated and observed values over the predicted values, shows that the quality of fit is good because the distribution does not follow the trend with respect to the predicted values. An optimum resolu- tion reaction for (±)-2-octanol represents conditions which would give high enantiomeric excess, high enantioselec- tivity, higher initial rate and 50 % conversion. Numerical tools provided by Design Expert were used to find out the optimum conditions. The optimum reaction condi- tions thus obtained for the desired isomer, (R)-2-octanol, were, mole ratio of vinyl acetate: (±)-2-octanol of 4:1, temperature of 25˚C, 0.05 g of catalyst loading and 400 rpm as speed of agitation with conversion: 43.1%, ee of remaining enantiomer: 71.8%, enantioselectivity: 203 and Initial rate: 0.0060 M·min−1. The second order polynomial equations were used to generate surface response plots and then finally to arrive at the optimum reaction conditions to maximize conver- sion and enantiomeric excess. Response surface and contour plots were generated for interacting parameters for each response. Figure 2 shows the surface response plot for enantioselectivity, as a function of the interacting parameters, i.e. temperature and mole ratio. Temperature and mole ratio were investigated in the range of 10˚C - 70˚C and 1:1 - 5:1, respectively, at a catalyst loading of 0.06 g and speed of agitation at 300 rpm. At a molar ratio of 2:1, enantioselectivity increased with an increase in OPEN ACCESS 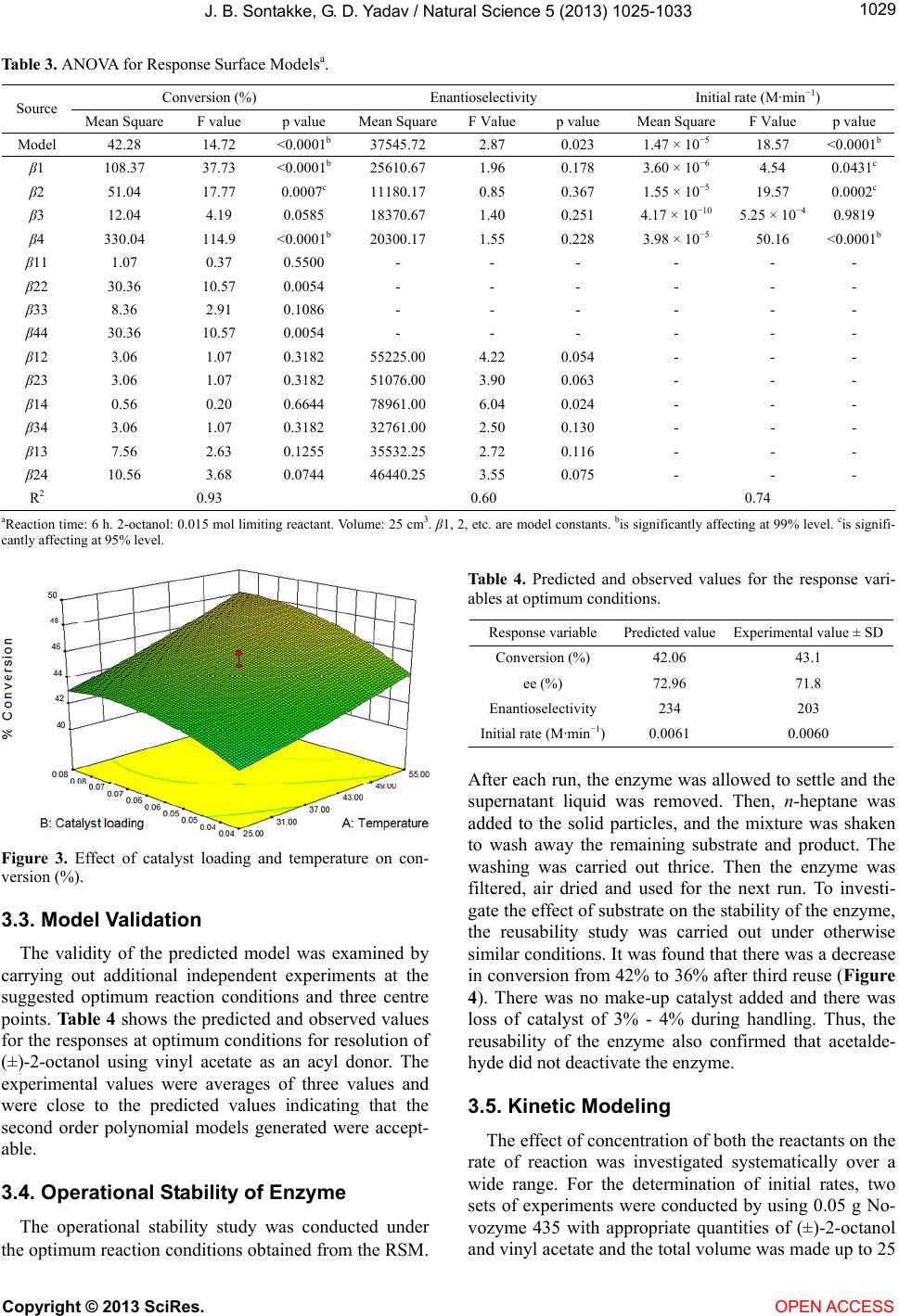 J. B. Sontakke, G. D. Yadav / Natural Science 5 (2013) 1025-1033 1029 Table 3. ANOVA for Response Surface Modelsa. Conversion (%) Enantioselectivity Initial rate (M·min−1) Source Mean Square F value p value Mean SquareF Value p value Mean Square F Value p value Model 42.28 14.72 <0.0001b 37545.72 2.87 0.023 1.47 × 10−5 18.57 <0.0001b β1 108.37 37.73 <0.0001b 25610.67 1.96 0.178 3.60 × 10−6 4.54 0.0431c β2 51.04 17.77 0.0007c 11180.17 0.85 0.367 1.55 × 10−5 19.57 0.0002c β3 12.04 4.19 0.0585 18370.67 1.40 0.251 4.17 × 10−10 5.25 × 10−4 0.9819 β4 330.04 114.9 <0.0001b 20300.17 1.55 0.228 3.98 × 10−5 50.16 <0.0001b β11 1.07 0.37 0.5500 - - - - - - β22 30.36 10.57 0.0054 - - - - - - β33 8.36 2.91 0.1086 - - - - - - β44 30.36 10.57 0.0054 - - - - - - β12 3.06 1.07 0.3182 55225.00 4.22 0.054 - - - β23 3.06 1.07 0.3182 51076.00 3.90 0.063 - - - β14 0.56 0.20 0.6644 78961.00 6.04 0.024 - - - β34 3.06 1.07 0.3182 32761.00 2.50 0.130 - - - β13 7.56 2.63 0.1255 35532.25 2.72 0.116 - - - β24 10.56 3.68 0.0744 46440.25 3.55 0.075 - - - R2 0.93 0.60 0.74 aReaction time: 6 h. 2-octanol: 0.015 mol limiting reactant. Volume: 25 cm3. β1, 2, etc. are model constants. bis significantly affecting at 99% level. cis signifi- cantly affecting at 95% level. Figure 3. Effect of catalyst loading and temperature on con- version (%). 3.3. Model Validation The validity of the predicted model was examined by carrying out additional independent experiments at the suggested optimum reaction conditions and three centre points. Table 4 shows the predicted and observed values for the responses at optimum conditions for resolution of (±)-2-octanol using vinyl acetate as an acyl donor. The experimental values were averages of three values and were close to the predicted values indicating that the second order polynomial models generated were accept- able. 3.4. Operational Stability of Enzyme The operational stability study was conducted under the optimum reaction conditions obtained from the RSM. Table 4. Predicted and observed values for the response vari- ables at optimum conditions. Response variable Predicted value Experimental value ± SD Conversion (%) 42.06 43.1 ee (%) 72.96 71.8 Enantioselectivity 234 203 Initial rate (M·min−1)0.0061 0.0060 After each run, the enzyme was allowed to settle and the supernatant liquid was removed. Then, n-heptane was added to the solid particles, and the mixture was shaken to wash away the remaining substrate and product. The washing was carried out thrice. Then the enzyme was filtered, air dried and used for the next run. To investi- gate the effect of substrate on the stability of the enzyme, the reusability study was carried out under otherwise similar conditions. It was found that there was a decrease in conversion from 42% to 36% after third reuse (Figure 4). There was no make-up catalyst added and there was loss of catalyst of 3% - 4% during handling. Thus, the reusability of the enzyme also confirmed that acetalde- hyde did not deactivate the enzyme. 3.5. Kinetic Modeling The effect of concentration of both the reactants on the rate of reaction was investigated systematically over a wide range. For the determination of initial rates, two sets of experiments were conducted by using 0.05 g No- vozyme 435 with appropriate quantities of (±)-2-octanol and vinyl acetate and the total volume was made up to 25 Copyright © 2013 SciRes. OPEN ACCESS 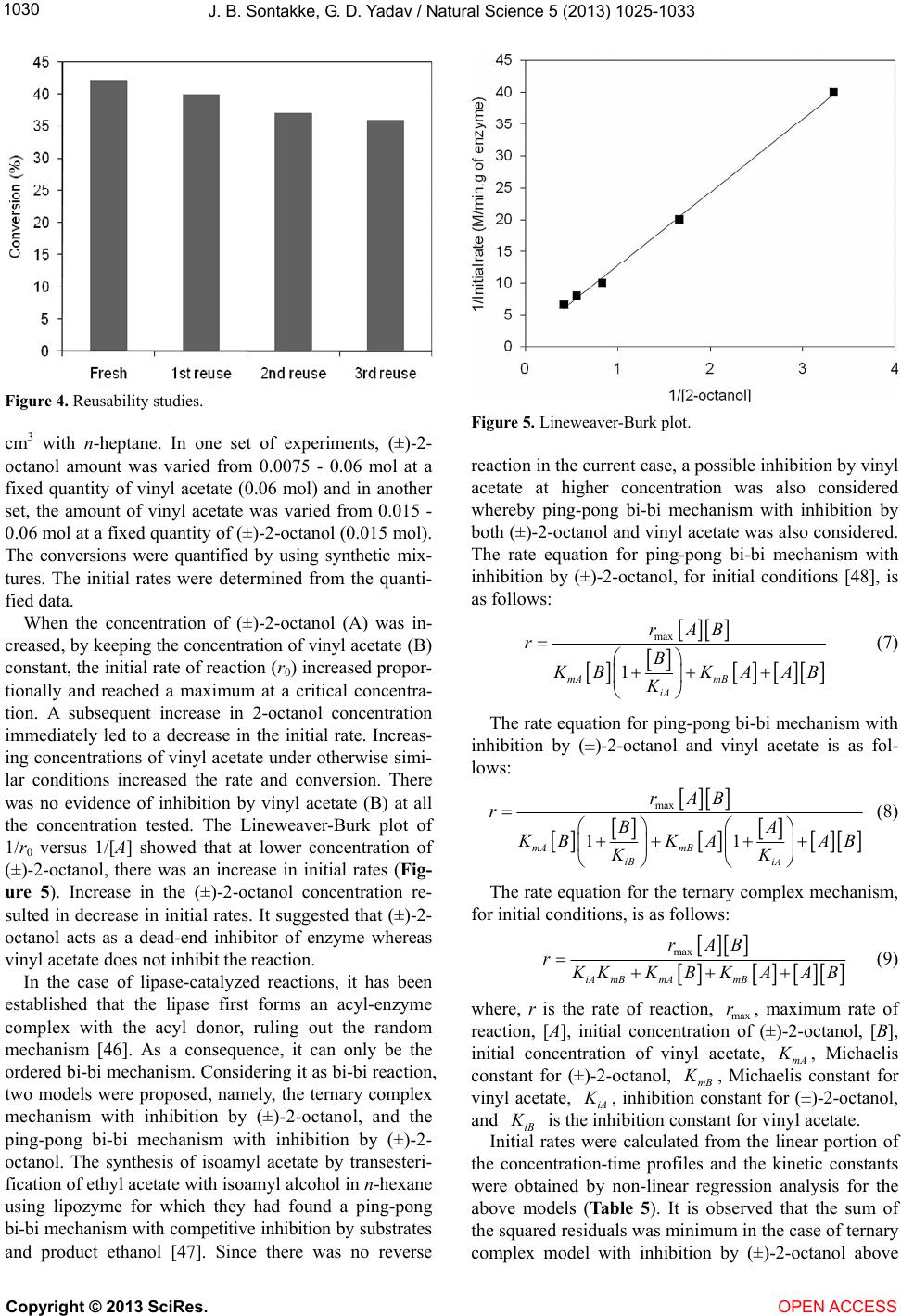 J. B. Sontakke, G. D. Yadav / Natural Science 5 (2013) 1025-1033 1030 Figure 4. Reusability studies. cm3 with n-heptane. In one set of experiments, (±)-2- octanol amount was varied from 0.0075 - 0.06 mol at a fixed quantity of vinyl acetate (0.06 mol) and in another set, the amount of vinyl acetate was varied from 0.015 - 0.06 mol at a fixed quantity of (±)-2-octanol (0.015 mol). The conversions were quantified by using synthetic mix- tures. The initial rates were determined from the quanti- fied data. When the concentration of (±)-2-octanol (A) was in- creased, by keeping the concentration of vinyl acetate (B) constant, the initial rate of reaction (r0) increased propor- tionally and reached a maximum at a critical concentra- tion. A subsequent increase in 2-octanol concentration immediately led to a decrease in the initial rate. Increas- ing concentrations of vinyl acetate under otherwise simi- lar conditions increased the rate and conversion. There was no evidence of inhibition by vinyl acetate (B) at all the concentration tested. The Lineweaver-Burk plot of 1/r0 versus 1/[A] showed that at lower concentration of (±)-2-octanol, there was an increase in initial rates (Fig- ure 5). Increase in the (±)-2-octanol concentration re- sulted in decrease in initial rates. It suggested that (±)-2- octanol acts as a dead-end inhibitor of enzyme whereas vinyl acetate does not inhibit the reaction. In the case of lipase-catalyzed reactions, it has been established that the lipase first forms an acyl-enzyme complex with the acyl donor, ruling out the random mechanism [46]. As a consequence, it can only be the ordered bi-bi mechanism. Considering it as bi-bi reaction, two models were proposed, namely, the ternary complex mechanism with inhibition by (±)-2-octanol, and the ping-pong bi-bi mechanism with inhibition by (±)-2- octanol. The synthesis of isoamyl acetate by transesteri- fication of ethyl acetate with isoamyl alcohol in n-hexane using lipozyme for which they had found a ping-pong bi-bi mechanism with competitive inhibition by substrates and product ethanol [47]. Since there was no reverse Figure 5. Lineweaver-Burk plot. reaction in the current case, a possible inhibition by vinyl acetate at higher concentration was also considered whereby ping-pong bi-bi mechanism with inhibition by both (±)-2-octanol and vinyl acetate was also considered. The rate equation for ping-pong bi-bi mechanism with inhibition by (±)-2-octanol, for initial conditions [48], is as follows: max 1 mA mB iA rAB rB BKAA K B (7) The rate equation for ping-pong bi-bi mechanism with inhibition by (±)-2-octanol and vinyl acetate is as fol- lows: max 11 mA mB iB iA rAB rBA BKAA KK B (8) The rate equation for the ternary complex mechanism, for initial conditions, is as follows: max iA mBmAmB rAB r KKBKAAB (9) where, r is the rate of reaction, max , maximum rate of reaction, [A], initial concentration of (±)-2-octanol, [B], initial concentration of vinyl acetate, mA r , Michaelis constant for (±)-2-octanol, mB , Michaelis constant for vinyl acetate, iA , inhibition constant for (±)-2-octanol, and iB is the inhibition constant for vinyl acetate. Initial rates were calculated from the linear portion of the concentration-time profiles and the kinetic constants were obtained by non-linear regression analysis for the above models (Table 5). It is observed that the sum of the squared residuals was minimum in the case of ternary complex model with inhibition by (±)-2-octanol above Copyright © 2013 SciRes. OPEN ACCESS  J. B. Sontakke, G. D. Yadav / Natural Science 5 (2013) 1025-1033 Copyright © 2013 SciRes. 1031 Table 5. Kinetic parameters for kinetic resolution of 2-octanola. Kinetic parameter Ternary complex mechanism Ping-pong mechanism with substrate inhibition Ping-pong bi-bi model with both substrate inhibition rmax (M·min−1·g·enzyme−1) 1.16 0.18 0.17 KmA (M·g·enzyme−1) 9.97 0.14 0.08 KmB (M·g·enzyme−1) 0.87 −1.09 −0.08 KiA (M·g·enzyme−1) 9.98 0.22 −66.83 SSE 2.56 × 10−11 7.75 × 10−5 1 × 109 aA: 2-octanol, B: vinyl acetate. EEB EA A EBA EPQ P A BQ E certain concentration. In the other two cases, some of the estimated parameters were found to be negative and un- realistic. It is thus concluded that the reaction sequence follows the ternary complex mechanism with inhibition by (±)-2-octanol. The sequence is as follows: According to the ordered bi-bi mechanism, the acyl donor (B) first binds with the enzyme and forms an acyl- enzyme complex (EB). The second reactant (A) then combines with (EB) to form ternary complex EBA. This ternary complex then isomerizes to another ternary com- plex, which releases the first product vinyl alcohol. This vinyl alcohol is highly unstable and therefore it irreversi- bly tautomerizes to acetaldehyde and the binary complex of (±)-2-octanol and enzyme which subsequently releases (±)-2-octyl acetate. However, at high concentrations of (±)-2-octanol the dead-end binary complex between (±)- 2-octanol and enzyme is formed instead of vinyl acetate and enzyme. The reaction mechanism may be depicted in Scheme 2. Scheme 2. Ternary complex mechanism with inhibition by A. Where E, enzyme; A, (±)-2-octanol; B, vinyl acetate; EA, enzyme-(±)-2-octanol dead-end complex; Q, acetal- dehyde; P, (±)-2-octyl acetate; EBA, ternary complex; and EPQ is the isomer of EBA. The theoretical (simu- lated) initial rates were calculated by using the parame- ters in Ta b le 5 for the ternary model and are compared against the experimental values for different (±)-2-oc- tanol concentrations (Figure 6). There is an excellent match between theory and experiment, proving the valid- ity of the ternary model. Figure 6. Parity plot. tion conditions required to obtain well-defined amount of acetate, enantiomeric excess of remaining alcohol, enan- tioselectivity of enzyme and initial rate of reaction. These models are useful to determine the optimum operating conditions for the resolution reaction using the minimal number of experiments with the consequent economical benefit. The analysis of the kinetic data showed that the acylation of (±)-2-octanol with vinyl acetate catalyzed by Novozyme 435 follows the ternary complex mechanism (ordered bi-bi mechanism) with 2-octanol inhibition pro- viding support for one of the two proposed mechanisms. The optimum reaction conditions thus obtained for the desired isomer, (R)-2-octanol, were, mole ratio of vinyl acetate: (±)-2-octanol of 4:1, temperature of 25˚C, 0.05 g of catalyst loading and 400 rpm as speed of agitation 4. CONCLUSION In the present study, three commercial lipases, Can- dida antartica lipase B (Novozyme 435), Thermomyces lanuginosus and Rhizomucor meihei, were employed for the kinetic resolution of (±)-2-octanol by using vinyl ace- tate as an acylating agent. The process of synthesis of (R)-2-octyl acetate using immobilized lipase, Novozyme 435 was optimized applying RSM with CCRD. Second order polynomial equations have been obtained for the conversion of alcohol, enantioselectivity of enzyme and initial rate of reaction. It was possible to predict the reac- OPEN ACCESS  J. B. Sontakke, G. D. Yadav / Natural Science 5 (2013) 1025-1033 1032 with conversion, 43.1%; ee, 71.8%; enantioselectivity, 203; and Initial rate, 0.0060 M·min−1. 5. ACKNOWLEDGEMENTS Authors thank Novo Nordisk, Denmark for gifts of enzymes. J.B.S. acknowledges UGC for an award of SRF. G.D.Y. acknowledges support for personal chairs from the Darbari Seth Professor and R. T. Mody Distinguished Professor Endowments, and J. C. Bose National Fellow- ship from DST-GOI. REFERENCES [1] Turner, N.J. (2003) Controlling chirality. Current Opinion in Biotechnology, 14, 401-406. doi:10.1016/S0958-1669(03)00093-4 [2] Huisman, G. and Gray, D. (2002) Towards novel proc- esses for the fine chemical and pharmaceutical industries. Current Opinion in Biotechnology, 13, 352-358. doi:10.1016/S0958-1669(02)00335-X [3] Patel, R.N. (2001) Biocatalytic synthesis of intermediates for the synthesis of chiral drug substances. Current Opin- ion in Biotechnology, 12, 587-604. doi:10.1016/S0958-1669(01)00266-X [4] Loughlin, W.A. (2000) Biotransformations in organic synthesis. Bioresource Technology, 74, 49-62. doi:10.1016/S0960-8524(99)00145-5 [5] Yadav, G.D. and Devi, K.M. (2002) Enzymatic synthesis of perlauric acid using Novozym 435. Biochemical Engi- neering Journal, 10, 93-101. doi:10.1016/S1369-703X(01)00164-4 [6] Yadav, G.D. and Borkar, I.V. (2006) Kinetic modeling of microwave assisted chemo-enzymatic epoxidation of sty- rene to styrene oxide. American Institute of Chemical En- gineers Journal, 52, 1235-1247. doi:10.1002/aic.10700 [7] Yadav, G.D. and Devi, K.M. (2004) Kinetics of hydrolysis of tetrahydrofurfuryl butyrate in a three phase system containing immobilized lipase from Candida antartica. Biochemical Engineering Journal, 17, 57-63. doi:10.1016/S1369-703X(03)00125-6 [8] Yadav, G.D. and Devi, K.M. (2004) Immobilized lipase- catalyzed esterification and transesterification reactions in non-aqueous media for synthesis of tetrahydrofurfuryl butyrate: Comparison and kinetic modelling. Chemical Engineering Science, 59, 373-383. doi:10.1016/j.ces.2003.09.034 [9] Yadav, G.D. and Lathi, P.S. (2006) Intensification of en- zymatic synthesis of propylene glycol monolaurate from 1,2-propanediol and lauric acid under microwave irradia- tion: Kinetics of forward and reverse reactions, Enzyme and Microbial Technology, 38, 814-820. doi:10.1016/j.enzmictec.2005.08.013 [10] Yadav, G.D. and Dhoot, S.B. (2009) Immobilized lipase- catalysed synthesis of cinnamyl laurate in non-aqueous media. Journal of Molecular Catalysis B: Enzymatic, 57, 34-39. doi:10.1016/j.molcatb.2008.06.013 [11] Yadav, G.D. and Jadhav, S.R. (2005) Synthesis of reus- able lipases by immobilization on hexagonal mesoporous silica and encapsulation in calcium alginate: Transesteri- fication in non-aqueous medium. Microporous and Meso- porous Materials, 86, 215-222. doi:10.1016/j.micromeso.2005.07.018 [12] Yadav, G.D. and Devendran, S. (2012) Lipase catalyzed synthesis of cinnamyl acetate via transesterification in non-aqueous medium. Process Biochemistry, 47, 496-502. doi:10.1016/j.procbio.2011.12.008 [13] Yadav, G.D. and Borkar, I.V. (2008) Kinetic modeling of immobilized lipase catalysis in synthesis of n-butyl levu- linate. Industrial and Engineering Chemistry Research, 47, 3358-3363. doi:10.1021/ie800193f [14] Yadav, G.D. and Borkar, I.V. (2009) Novelties of synthe- sis of n-butyl acetamide over immobilized lipase. Journal of Chemical Technology and Biotechnology, 84, 420-426. doi:10.1002/jctb.2056 [15] Yadav, G.D. and Borkar, I.V. (2010) Lipase-catalyzed hydrazinolysis of phenyl benzoate: Kinetic modeling ap- proach. Process Biochemistry, 45, 586-592. doi:10.1016/j.procbio.2009.12.005 [16] Yadav, G.D. and Borkar, I.V. (2009) Kinetic and mecha- nistic investigation of microwave-assisted lipase cata- lyzed synthesis of citronellyl acetate. Industrial and En- gineering Chemistry Research, 48, 7915-7922. doi:10.1021/ie800591c [17] Yadav, G.D., Dhoot, S.B. and Sajgure, A.D. (2008) In- sight into microwave irradiation and enzyme catalysis in enantioselective resolution of RS-(±)methyl mandelate. Journal of Chemical Technology and Biotechnology, 83, 1145-1153. doi:10.1002/jctb.1975 [18] Yadav, G.D. and Thorat, P.A. (2012) Microwave assisted lipase catalyzed synthesis of isoamyl myristate in sol- vent-free system. Journal of Molecular Catalysis B: En- zymatic, 83, 16-22. doi:10.1016/j.molcatb.2012.06.011 [19] Yadav, G.D. and Shinde, S.D. (2012) Synergism of micro- wave irradiation and immobilized lipase catalysis in syn- thesis of 4,8-dimethylnon-7-en-1yl (2E)-3-phenylpro- 2-enolate. International Reviews in Chemical Engineer- ing, 4, 589-596. [20] Yadav, G.D. and Pawar, S.V. (2012) Synergism between microwave irradiation and enzyme catalysis in trans-es- terification of ethyl-3-phenylpropanoate withn-butanol. Bioresource Technology, 109, 1-6. doi:10.1016/j.biortech.2012.01.030 [21] Yadav, G.D., Sajgure, A.D. and Dhoot, S.D. (2007) En- zyme catalysis in fine chemical and pharmaceutical in- dustries. In: Bhattacharya, S.K., Ed., Enzyme Mixtures and Complex Biosynthesis, Landes Biosciences, Austin. [22] Riermeier, T.H., Gross, P., Monsees, A., Hoff, M. and Trauthwein, H. (2005) Dynamic kinetic resolution of secondary alcohols with a readily available ruthenium- based racemization catalyst. Tetrahedron Letters, 46, 3403- 3406. doi:10.1016/j.tetlet.2005.03.074 [23] Cong, F.D., Wang, Y.H., Ma, C.Y., Yub, H.F., Han, S.P., Tao, J. and Cao, S.G. (2005) A way for resolution of (R, S)-2-octanol by combining dynamic kinetic resolution with double kinetic resolution. Enzyme and Microbial Technology, 36, 595-599. doi:10.1016/j.enzmictec.2004.12.009 Copyright © 2013 SciRes. OPEN ACCESS  J. B. Sontakke, G. D. Yadav / Natural Science 5 (2013) 1025-1033 Copyright © 2013 SciRes. OPEN ACCESS 1033 [24] Yu, D., Chen, P., Wang, L., Gu, Q., Li, Y., Wang, Z. and Cao, S. (2007) A chemo-enzymatic process for sequential kinetic resolution of (R,S)-2-octanol under microwave ir- radiation. Process Biochemistry, 42, 1312-1318. doi:10.1016/j.procbio.2007.06.011 [25] Wang, Y., Wang, R., Li, Q., Zhanga, Z. and Feng, Y. (2009) Kinetic resolution of rac-alkyl alcohols via lipase-cata- lyzed enantioselective acylation using succinic anhydride as acylating agent. Journal of Molecular Catalysis B: En- zymatic, 56, 142-145. doi:10.1016/j.molcatb.2008.02.002 [26] Xun, E.N., Lv, X.L., Kang, W., Wang, J.X., Zhang, H., Wang, L. and Wang, Z. (2012) Immobilization of pseu- domonas fluorescens lipase onto magnetic nanoparticles for resolution of 2-octanol. Applied Biochemistry Biotech- nology, 168, 697-707. doi:10.1007/s12010-012-9810-9 [27] Ren, L., Xu, T., He, R., Jiang, Z., Zhou, H. and Wei, P. (2013) A green resolution-separation process for aliphatic secondary alcohols. Tetrahedron: Asymmetry, 24, 249- 253. doi:10.1016/j.tetasy.2013.01.018 [28] Zhao, L.F. and Zheng, L.Y. (2011) Resolution of 2-octa- nol via immobilized Pseudomonas sp. lipase in organic medium. Biocatalysis and Biotransforamtion, 29, 47-53. doi:10.3109/10242422.2010.551189 [29] Yu, D., Ma, D., Wang, Z., Wang, Y., Pan, Y. and Fang, X. (2012) Microwave-assisted enzymatic resolution of (R,S)- 2-octanol in ionic liquid. Process Biochemistry, 47, 479- 484. doi:10.1016/j.procbio.2011.12.007 [30] Wang, Y., Li, Q., Zhang, Z., Ma, J. and Feng, Y. (2009) Solvent effects on the enantioselectivity of the thermo- philic lipase QLM in the resolution of (R,S)-2-octanol and (R,S)-2-pentanol. Journal of Molecular Catalysis B: En- zymatic, 56, 146-150. doi:10.1016/j.molcatb.2008.01.010 [31] Ursoiu, A., Ungurean, M., Paul, C. and Peter, F. (2010) Optimization of 2-octanol kinetic resolution by selection of solgel immobilization precursors and reaction parame- ters. Journal of Biotechnology, 150S, S1-S576. doi:10.1016/j.jbiotec.2010.09.493 [32] Bas, D. and Boyaci, I.H. (2007) Modeling and optimiza- tion I: Usability of response surface methodology. Jour- nal of Food Engineering, 78, 836-845. doi:10.1016/j.jfoodeng.2005.11.024 [33] Soto-Cruz, O., Saucedo-Castañeda, G., Pablos-Hach, J.L., Gutiérrez-Rojas, M. and Favela-Torres, E. (1999) Effect of substrate composition on the mycelial growth of Pleu- rotus ostreatus. An analysis by mixture and response sur- face Methodologies Process Biochemistry, 35, 127-133. doi:10.1016/S0032-9592(99)00043-6 [34] Diniz, F.M. and Martin, A.M. (1996) Use of response surface methodology to describe the combined effects of temperature and E:S ratio on the hydrolysis of dogfish (Squalus acanthias) muscle. International Journal of Food Science and Technology, 31, 419-426. doi:10.1046/j.1365-2621.1996.00351.x [35] Guinard, J.X., Zoumas-Morse, C., Mori, L., Panyam, D. and Kilara, A. (1996) Effect of sugar and fat on the ac- ceptability of vanilla ice cream. Journal of Dairy Science and Technology, 79, 1922-1927. doi:10.3168/jds.S0022-0302(96)76561-X [36] Hwang, S. and Hancen, C.L. (1997) Modeling and opti- mization in anaerobic bioconversion of complex sub- strates to acetic and butyric acids. Biotechnology and Bioengineering, 54, 451-460. doi:10.1002/(SICI)1097-0290(19970605)54:5<451::AID- BIT5>3.0.CO;2-D [37] Rastogi, N.K., Rajesh, G. and Shamala, T.R. (1998) Op- timization of enzymatic degradation of coconut residue. Journal of Science Food Agriculture, 76, 129-134. doi:10.1002/(SICI)1097-0010(199801)76:1<129::AID-JS FA909>3.0.CO;2-C [38] Gokhale, S.V. and Lele, S.S. (2012) Optimization of convective dehydration of Beta vulgaris for color reten- tion. Food and Bioprocess Technology, 5, 868-878. [39] Mahajan, P.M., Gokhale, S.V. and Lele, S.S. (2010) Pro- duction of nattokinase using Bacillus natto NRRL 3666: Media optimization, scale up and kinetic modeling. Food Science Biotechnolology, 19, 1593-1603. doi:10.1007/s10068-010-0226-4 [40] Sontakke, J.B. and Yadav, G.D. (2011) Optimization and kinetic modeling of lipase catalyzed enantioselective N-acetylation of (±)-1-phenylethylamine under micro- waves irradiation. Journal of Chemi cal Technology and Biotechnology, 86, 739-748. doi:10.1002/jctb.2582 [41] Sontakke, J.B. and Yadav, G.D. (2011) Kinetic modeling and statistical optimization of lipase catalyzed enantiose- lective resolution of (R,S)-2-pentanol. Industry Engi- neering and Chemistry Research, 50, 12975-12983. doi:10.1021/ie2012032 [42] Yadav, G.D. and Sontakke, J.B. (2011) Optimization of chiral resolution of (R,S)-1-phenylethanol by statistical methods. International Journal of Chemical Reactor En- gineering, 9, A77, 1-15. [43] Dai, D.Z. and Xia, L.M. (2006) Resolution of (R,S)-2- octanol by Penicillium expansum PED-03 lipase immobi- lized on modified ultrastable-Y molecular sieve in mi- croaqueous media. Process Bioc hemistry, 41, 1455-1460. doi:10.1016/j.procbio.2006.01.015 [44] Zhang, D.H., Bai, S., Ren, M.Y. and Sun, Y. (2008) Opti- mization of lipase-catalyzed enantioselective esterifica- tion of (±)-menthol in ionic liquid. Food Chemistry, 109, 72-80. doi:10.1016/j.foodchem.2007.12.020 [45] Montgomery, D.C. (1984) Design and analysis of ex- periments. 2nd edition, John Wiley and Sons, New York. [46] Faber, K. and Riva, S. (1992) Enzyme-catalyzed ire- versible acyl transfer. Synthesis, 10, 895-910. doi:10.1055/s-1992-26255 [47] Rizzi, M., Stylos, P. and Reuss, M. (1992) A kinetic study of immobilized lipase catalysing the synthesis of isoamyl acetate by transesterification in n-hexane. Enzyme Micro- bial Technology, 14, 709-714. doi:10.1016/0141-0229(92)90110-A [48] Segel, I.H. (1975) Enzyme kinetics. Wiley/Interscience, New York.
|