Paper Menu >>
Journal Menu >>
 Pharmacology & Pharmacy, 2011, 2, 17-30 doi:10.4236/pp.2011.21003 Published Online January 2011 (http://www.SciRP.org/journal/pp) Copyright © 2011 SciRes. PP 17 Pharmacodynamics of CNTO 530 and Darbepoetin-α in Human TNF-α Transgenic Mice, a Murine Model of Anemia of Chronic Disease Ram Achuthanandam1, Dorie Makropoulos1, Laura Johns1, Amy Volk1, Kerry Brosnan1, Jin Lu1, Wojciech Krzyzanski2, Peter J. Bugelski1 1Centocor R&D, Radnor, USA; 2Department of Pharmaceutics, State University of New York, Buffalo, USA. Email: pbugelsk@its.jnj.com Received October 22nd, 2010; revised November 16th, 2010; accepted December 13th, 2010. ABSTRACT CNTO 530 and darbepoetin- are long lived erythropoietin receptor agonists (ERAs). Clinically, anemia of chronic disease (ACD) is associated with increased expression of tumor necrosis factor- (TNF- ) and mice transgenic for human TNF- develop ACD. The purpose of this investigation was to compare the effects of these agents in a murine model of ACD. Human TNF- expressing (Tg197) mice were administered a single subcutaneous dose of CNTO 530 or darbepoetin- and the pharmacodynamic response in bone marrow spleen and peripheral blood evaluated. RBC life span and reticulocyte age distribution were also evaluated. CNTO 530 induced a dose responsive increase in reticulo- cytes, RBCs and Hgb in both wild type and Tg197 mice. Although the reticulocyte response was similar to wild types, the RBC and Hgb response to CNTO 530 in Tg197 mice was blunted. There was no statistically significant difference in RBC life span with either compound. Darbepoetin-α caused a greater peak in % dead Pro/basophilic erythroblasts, greater peak EMH in the spleen and a greater increase in reticulocyte maturation time. In contrast, despite a similar peak increase, CNTO 530 caused a more sustained response of reticulocyte, EMH, RBC and Hgb, consistent with in- creased exposure. In conclusion, CNTO 530 and darbepoetin- increased RBC and hemoglobin in a murine model of ACD. Compared to darbepoetin- , CNTO 530 had a more sustained effect, consistent with increased exposure. Keywords: Fusion Protein, Recombinant, Flow Cytometry, Erythropoiesis 1. Introduction Anemia of chronic disease (ACD) is an important cause of co-morbidity in rheumatoid arthritis (RA), a systemic autoimmune disease afflicting 1-2% of the population [1,2]. Tumor necrosis factor- (TNF-) has been shown to play a central role in disease progression in RA (see review by Sfikakis and Kollias [3]) and has been impli- cated in the pathogenesis of ACD. In ACD patients there is increased local production of TNF- in the bone mar- row [4] and TNF- is thought to contribute to ACD by decreasing red blood cell (RBC) survival, causing dys- functional erythropoiesis and impairing mobilization of iron stores [5,6]. Darbepoetin- and CNTO 530 are long-lived erythro- poietin receptor agonists. CNTO 530 is a 58 kD glyco- protein MIMETIBODY™ construct that has been shown to cause a long-lived stimulation of erythropoiesis in mice and rats [7,8]. CNTO 530 has no sequence homol- ogy with erythropoietin (EPO). Rather, CNTO 530 in- cludes two EMP1 sequences, a 20-amino acid peptide that binds to EPO receptors (EPO-R), on a human IgG4 Fc framework and expresses EPO-like bioactivity [9,10] The purpose of this study was to determine if darbepo- etin- and CNTO 530 had beneficial effects in a murine model of ACD. 2. Materials and Methods 2.1. Reagents and Monoclonal Antibodies Darbepoetin- (Aranesp®, Amgen, Inc. Thousand Oaks, CA) was purchased commercially and CNTO 530 was supplied by Centocor R & D (Radnor, PA). Phosphate buffered saline (PBS) without Ca++ and Mg++, fetal calf serum (FCS), PE-anti-Ter-119, APC-streptavidin; PE- streptavidin; FITC-annexin V, isotype-matched IgG con-  Pharmacodynamics of CNTO 530 and Darbepoetin-α in Human TNF-α Transgenic Mice, a Murine Model of Anemia of Chronic Disease Copyright © 2011 SciRes. PP 18 trols and the viability/DNA probe 7-amino-actinomycin D (7-AAD) were purchased from Invitrogen (Carlsbad, CA). EZ-Link NHS biotin was purchased from Pierce (Rockford, IL). 2.2. Mice Heterozygous, 9 wk old Tg197 mice and CBA/C57Bl/6 F1 (CBA F1) control mice were supplied by Ace Labo- ratories (Boyertown, PA). Tg197 mice carry a human TNF-/β globin transgene on a C57Bl/6 background [11]. For these experiments, homozygous TNF- transgenic males were bred to CBA females to provide heterozy- gous mice. All mice were maintained in the pathogen- free animal facility at Centocor, Radnor PA. The Cento- cor Institutional Animal Care and Use Committee ap- proved all procedures. 2.3. Hematology and Histology Blood was collected from mice anesthetized with a CO2 mixture via open chest cardiac puncture into EDTA coated microtubes. Analyses were performed on whole blood using an Advia 120 blood analyzer (Siemens, Tarrytown, NY). Spleens were collected, fixed in 10% neutral buffered formalin and processed for paraffin sec- tions, sectioned and stained with Giemsa using routine methods. Extramedullary hematopoiesis (EMH) was evaluated morphometrically. Three 4x microscope fields from each mouse at each time point were digitally im- aged. Polychromic/orthochromic erythroblasts (P/O EB) were identified by their size and staining characteristics and the percent area measured using ImagePro Plus V 5.1 (Media Cybernetics, Inc. Bethesda, MD). Mean val- ues were calculated for each mouse. Data are shown as group mean ± standard deviation. 2.4. Bone Marrow Cell Preparation and Analysis Flow cytometric analysis was performed as described previously [12,13]. Briefly, femoral bone marrow was suspended in PBS without Ca++ or Mg++ supplemented with 2% heat-inactivated fetal calf serum (FCS) and 0.1% NaN3. Following Fc block with anti-murine CD16/ CD32, cells were incubated with anti-Ter-119 and anti- CD71 to discriminate erythroblasts from non-erythroid cells and pro/basophilic erythroblasts (Pro/BasoEB) from poly/orthochromic erythroblasts (Poly/OrthoEB). Apop- totic and dead cells were enumerated with annexin V and 7-aminoactinomycin-D (7AAD) as per manufacturer’s instructions kit (BD Biosciences, San Jose, CA). Samples were analyzed on a FACSCalibur flow cy- tometer (BD Biosciences, San Jose, CA). Data analysis was performed with CellQuest ProTM (BD Biosciences, San Jose, CA) and FlowJo (Treestar, Seattle, WA). Data for at least 10,000 cells/sample were collected. A nested analysis strategy was used to enumerate cell populations of interest: Debris and aggregates were excluded using FSC and SSC; apoptotoic (7AAD negative/annexin V+) and dead cells (7AAD+) and Pro/Basophilic (Pro/Ba- soEB, Ter119+/CD71 bright) and Poly/Orthochomic Erythroblasts (Poly/OrthoEB, Ter119+/CD71 dim) de- limited using a Boolean “AND” operator. 2.5. Pharmacokinetics Normal female C57Bl/6 mice received a single sc dose of 0.3 mg/kg of CNTO 530 or darbepoetin-. Mice were euthanized (3-6 mice per time point) and blood samples collected by cardiac puncture into EDTA-coated micro- tubes. A total of 63 mice were used in this analysis. The plasma concentration of CNTO 530 was measured as described previously [12]. The plasma concentration of darbepoetin- was measured in mouse plasma using a double-antibody sandwich ELISA (R&D Systems, Inc., Minneapolis, MN) adapted for use with darbepoetin- and murine plasma as described previously for recombi- nant human EPO [7]. 2.6. RBC Lifespan An in vivo biotinylation technique was used to determine RBC survival [14,15]. On Day 1, groups of Tg197 mice (5-6/group) received a single subcutaneous (sc) dose of saline, CNTO 530 or darbepoetin- (0.3 mg/kg). On Days 2, 3 and 4, the mice received EZ-Link Sulfo-NHS biotin (1 mg/mouse, intravenous in 200 L PBS). Blood samples (25 L) were collected from the retro-orbital sinus (EDTA anticoagulant) on 1, 3, 6, 10, and 17 days after the last injection of biotin reagent. Blood (5 L) was mixed with 1 mL of 5 g/mL of streptavidin-PE in PBS, and incubated for 30 min. After a wash, the cells were resuspended in 200 uL of PBS and analyzed by flow cytometry. Data for mean % biotinylated RBC was plotted as survival fraction vs. time. The initial slope 0 S of such a survival curve determines the mean RBC life- span TRBC according to the equation [16]: 0 1 RBC TS Although derived under homeostatic conditions, this relationship has been shown to be accurate for a non- stationary applications [17]. To determine the initial slope the RBC survival curve for each animal was fitted with the liner-exponential model [18,19]: 00 0 1, , 1, . t t StTS eiftT SF tSe iftT  Pharmacodynamics of CNTO 530 and Darbepoetin-α in Human TNF-α Transgenic Mice, a Murine Model of Anemia of Chronic Disease Copyright © 2011 SciRes. PP 19 where is the first-order rate constant of RBC random destruction, T denotes the RBC lifespan due to senes- cence, and 0 1 is the weight factor between these two processes of the RBC removal. The parameters T, , and S0 were estimated, and S0 was calculated as follows: 0 1 1 ST The nonlinear regression was performed by WinNonlin 5.0 (Pharsight, Mountain View, CA). 2.7. Reticulocyte Age Distribution Oxazine 750 has been used as a marker of reticulocyte age. The fluorescent intensity signal s is correlated to the levels of RNA present in the reticulocyte. If the reticulo- cyte RNA degrades at a first-order rate, one can establish a relationship between the reticulocyte age and s, know- ing that s is proportional to the RNA content [20]. To determine the absolute reticulocyte age, the signal from the bone marrow reticulocytes is necessary. Thus, the signal s0 at which a reticulocyte becomes a mature RBC has been used as a reference, and an absolute reticulocyte age/maturity has been replaced by a relative time to be- come RBC (RT) according to the following equation: 1/2 0 ln ln 2 RNA t s RT s where t1/2RNA is the half-life of the RNA degradation in reticulocytes. RT densities were calculated at times t = 1, 2, 4, 10, and 21 days after test article administration. The RT density r(RT) function is defined as [20]: #, ,a ofreticulocytes ofRTaaa rta Unit Volume ofblood where, Δa is an infinitesimally small age increment. The RT densities were approximated by the product of abso- lute reticulocyte count at time t and the frequency histo- gram for RT at time t: *, ,RETthistt RT rtRTRT where, ΔRT is the length of the histogram bin. The pro- duction rate of mature RBC at time t was calculated as: 0 max , RET RT kt rtRT and the maximal value of the RT density was used to approximate the reticulocyte release rate from the bone marrow to blood: 0 max , RET RT kt rtRT The mean RT was calculated from the histogram hist (t, RT). The median of r(RT) was interpreted as the median cell age (mid age of reticulocytes). Since the half-life of the RNA degradation in Tg197 transgenic mice has not been determined, RT was expressed in t1/2RNA units. The ADVIA 120 FCS files were converted to ASCII files containing reticulocyte oxazine 750 absorption intensity data using FCS Express v. 3 (De Novo Software). The RT densities, and other reticulocyte related parameters were obtained using MATLAB 7.6 (MathWorks, Natick, MA). 2.8. Statistical Analysis Data were expressed as means +/– standard deviation. Significant differences between experimental groups were determined by ANOVA (Bonferroni’s correction). P values less than 0.05 were considered significant. 3. Results 3.1. Dose Response of CNTO 530 in Tg197 Mice As shown in Table 1, Tg197 mice have a mild compen- sated, hypochromic aqnemia. To establish the dose re- sponse for CNTO 530, female Tg197 mice (4/group) received a single subcutaneous (sc) dose of CNTO 530 at 0.03, 0.1 or 0.3 mg/kg. Control Tg197 mice received a single sc dose of PBS. As shown in Figures 1(a)-(c), CNTO 530 caused a dose responsive increase in reticu- locytes, RBC and Hgb in Tg197 mice. Based on these results, a dose of 0.3 mg/kg was chosen for the remaining experiments. 3.2. Comparative Effects of CNTO 530 in Normal and Tg197 Mice To compare the response of normal (CBF1) and Tg197 mice to CNTO 530, 9 week-old female mice (3/group) received a single sc dose of 0.3 mg/kg. Control mice re- ceived a single sc dose of PBS. As shown in Figure 2(a), the reticulocyte response to CNTO 530 in CBF1 and Table 1. Combined control values for normal and Tg197 mice. Parameter CBF1 Tg197 Reticulocytes 210 ± 27 256 ± 65* Total RBC 9.7 ± 0.3 9.6 ± 0.7 MCV 54.2 ± 0.5 53.1 ± 1.8 MCH 16.4 ± 0.7 15.5 ± 0.9* Hgb 15.8 ± 0.4 14.8 ± 0.9* *Significantly different from CBF1 (t-test).  Pharmacodynamics of CNTO 530 and Darbepoetin-α in Human TNF-α Transgenic Mice, a Murine Model of Anemia of Chronic Disease Copyright © 2011 SciRes. PP 20 (a) (b) (c) Figure 1. Dose response for CNTO 530 in Tg197 mice. (a), (b) and (c) show reticulocyte, RBC and HgB responses to CNTO 530 as a function of time. *statistically significant difference from control.  Pharmacodynamics of CNTO 530 and Darbepoetin-α in Human TNF-α Transgenic Mice, a Murine Model of Anemia of Chronic Disease Copyright © 2011 SciRes. PP 21 (a) (b) (c) Figure 2. Comparative pharmacodynamics of CNTO 530 in normal CBF1 and Tg197 mice. (a) reticulocyte response. (b) RBC response. (c) Hgb response. *statistically significant difference from untreated control. **statistically significant difference from Tg197 mice.  Pharmacodynamics of CNTO 530 and Darbepoetin-α in Human TNF-α Transgenic Mice, a Murine Model of Anemia of Chronic Disease Copyright © 2011 SciRes. PP 22 Tg197 mice was similar. However, as shown in Figures 2(b) and 2(c), the RBC and Hgb response was attenuated, suggesting that Tg197 mice are resistant to CNTO 530. 3.3. Comparative Pharmacokinetics of CNTO 530 and Darbepoetin- Female C57Bl/6 mice (5-6/group) received a single sc dose of CNTO 530 or darbepoetin- (0.3 mg/kg) and blood samples collected from 2 hrs to 10 days after dos- ing. Plasma concentrations are shown in Figure 3 and the pharmacokinetic parameters in Table 2. Compared to darbepoetin-, there was a statistically significant in- crease in the area under the curve (AUC) and terminal half-life for CNTO 530. 3.4. Pharmacodynamics of CNTO 530 and Darbepoetin- in Tg197 Mice Female Tg197 mice (4/group) received a single sc dose of CNTO 530 or darbepoetin- (0.3 mg/kg) and blood, bone marrow and spleens were collected 1, 2, 4, 10 and Figure 3. Comparative pharmacokinetics of CNTO 530 and darbepoetin- in normal mice. Table 2. Pharmacokinetics of CNTO 530 and darbepoetin- following a single subcutaneous dose in mice. Parameter CNTO 530 (mean ± SD) Darbepoetin- (mean ± SD) Cmax (ug/mL) 2.2 ± 0.5 2.9 ± 0.5 Tmax (hr) 20 ± 8 19 ± 5 AUCt (ughr/mL) 152 ± 28* 80 ± 10 AUC (ughr/mL) 160 ± 20* 95 ± 11 t1/2 (hr) 41 ± 9* 14 ± 4 *Statistically greater than darbepoetin-. 21 days after dosing. Controls received a single sc dose of PBS. As shown in Figure 4(a), CNTO 530 and dar- bepoetin- caused a similar peak reticulocyte response. However, the reticulocyte response to CNTO 530 was longer-lived. CNTO 530 and darbepoetin- also caused an increase in RBC (Figure 4(b)) and Hgb (Figure 4(c)). Despite a similar peak effect on RBC and Hgb, the ef- fects on CNTO 530 were significantly longer-lived than the effects of darbepoetin-. Representative scatter plots showing CD71 vs. Ter119 and annexin-V vs. 7-AAD for bone marrow from Tg197 mice are shown in Figures 5(a) and (b). As shown in Figure 5(c), with both test articles the relative percent- ages of Pro/BasoEB initially fell and then recovered to control values by Day 10 and then again fell below con- trol values on Day 21. Similarly, Poly/OrthoEB percent- ages also initially fell below control values and then re- covered for darbepoetin- or rose above control values for CNTO 530 by Day 10 (Figure 5(d)). Poly/OrthoEB were elevated for both test articles on Day 21. The results of the analysis of apoptosis and cell death in the bone marrow are shown in Figures 5(e) and (f). Both test arti- cles transiently increased cell death in Pro/BasoEB. In contrast, neither test article had a significant effect on apoptosis of Pro/BasoEB until Day 21 when apoptosis was decreased. Neither test article had an effect on apop- tosis or cell death in Poly/OrthoEB at any time point evaluated (Data not shown). In the spleen, animals treated with CNTO 530 or dar- bepoetin- showed an expansion of extramedullary he- matopoiesis (EMH) (Figure 6). On Day 1, erythroid pre- cursors were the predominant cell types and were found in clusters in the red pulp. In these clusters, some cells could be identified as Pro/BasoEB, however many cells appeared to be more primitive, with large pale nuclei that contained multiple nucleoli. This early progenitor popu- lation was the predominant cell type in roughly half of the animals. By Day 2, the primitive population and the Pro/BasoEB had expanded to a high degree and mitotic figures could be observed in the early progenitors. By Day 4, EMH peaked in both groups of treated animals. At this time, there was a shift from the primitive cells toward Pro/BasoEB. Additionally, the number of Poly/ OrthoEB and mature RBC had expanded to nearly the degree of the earlier precursors. There also was also an increase in apoptotic bodies in both groups of treated animals compared with control animals. Although the effects of CNTO 530 and darbepoetin- were similar on Days 1 and 2, by Day 4 the degree of EMH was greater in animals treated with darbepoetin- relative to those treated with CNTO 530. By Day 10, there was a striking difference between CNTO 530 and darbepoetin--treated  Pharmacodynamics of CNTO 530 and Darbepoetin-α in Human TNF-α Transgenic Mice, a Murine Model of Anemia of Chronic Disease Copyright © 2011 SciRes. PP 23 (a) (b) (c) Figure 4. Comparative pharmacodynamics of CNTO 530 and darbepoetin in Tg197 mice. (a), (b) and (c) show reticulocyte, RBC and HgB responses to CNTO 530 as a function of time. *statistically significant difference from untreated control. **significantly different from control and darbepoetin-.  Pharmacodynamics of CNTO 530 and Darbepoetin-α in Human TNF-α Transgenic Mice, a Murine Model of Anemia of Chronic Disease Copyright © 2011 SciRes. PP 24 (a) (b) (c) (d) (e) (f) Figure 5. Comparative effects of CNTO 530 and darbepoetin- in the bone marrow of Tg197 mice. (a) representative scatter plot showing flow cytometric discrimination of erythroid precursors. Proerythroblasts and basophilic erythroblasts (Pro/BasoEB) are identified as Ter119 high/CD71+. Polychromic and orthochromic erythroblasts (Poly/OrthoEB) are identi- fied as Ter199 hi/CD71-. (b) representative scatter plot showing flow cytometric discrimination of live, apoptotic and dead erythroid precursors. Live cells are identified as AnnexinV-/7AAD-, apoptotic cells as AnnexinV+/7AAD- and dead cells as 7AAD+. (c) comparative pharmacodynamic effect of CNTO 530 and darbepoetin- on Pro/BasoEB. (d) comparative phar- macodynamic effect of CNTO 530 and darbepoetin- on Poly/OrthoEB. (e) and (f) comparative pharmacodynamic effects on % apoptotic and % dead Pro/BasoEB. *statistically significant difference from untreated control. **significantly different from control and darbepoetin-.  Pharmacodynamics of CNTO 530 and Darbepoetin-α in Human TNF-α Transgenic Mice, a Murine Model of Anemia of Chronic Disease Copyright © 2011 SciRes. PP 25 (a) (b) (c) (d) (e) (f) (g) Figure 6. Comparative pharmacodynamic effects of CNTO 530 and darbepoetin- on extramedullary erythropoiesis (EMH). Photomicrographs of the red pulp of formalin fixed, paraffin embedded, Giemsa stained sections of the spleen from Tg197 mice. (a) control Day 4, (b) CNTO 530 Day 4, (c) darbepoetin- Day 4, (d) CNTO 530 Day 10, (e) darbepoetin- Day 10 and (f) CNTO 530 Day 21. Basophilic erythroblasts are recognized as the large deeply blue stained nuclei. Bar = 100 m. (g) morphometric analysis of EMH. CNTO 530 caused a longer-lived increase in expansion of P/OEB in the spleen. animals. While there remained significant EMH in ani- mals treated with CNTO 530, EMH was reduced in the darbepoetin- group. In the CNTO 530 treated animals, early stage progenitors were still present, however the overall EMH had shifted to later stage Poly/OrthoEB and there were widespread areas of mature RBCs. Scattered apoptotic bodies could be observed in this group. In the darbepoetin--treated animals, the progenitor population was mixed, with sporadic progenitors at varying stages present at low frequency and there were abundant mature  Pharmacodynamics of CNTO 530 and Darbepoetin-α in Human TNF-α Transgenic Mice, a Murine Model of Anemia of Chronic Disease Copyright © 2011 SciRes. PP 26 RBC. By Day 21, the control animals were also showing moderate EMH, probably reflecting a response to disease progression. At this time, there were no notable differ- ences between the CNTO 530 and darbepoetin- treated animals. In peripheral blood, increases in reticulocytes are ob- served by Day 1for both compounds, and by Day 4 there is a ~6 fold increase in circulating reticulocytes. After Day 4, the levels of reticulocytes reflect the pharma- cokinetic properties of each of the molecules as shown in Figure 4. The increase in reticulocytes is followed by a delayed increase in RBCs observed at Day 4. Again, the RBC profiles reflect the pharmacokinetic properties with increased RBC levels observed up till Day 21 in CNTO 530 treated group. 3.5. Effects of CNTO 530 and Darbepoetin- on RBC Life Span As shown in Figure 7(g), the surviving fraction of RBC was significantly lower than control for both treatment groups on Day 1 and significantly higher than control for the CNTO 530 group on Day 17. There was no statisti- cally significant effect of either test article on mean RBC lifespan: control = 22 ± 6 days; CNTO 530 = 24 ± 2 days and darbepoetin- = 19 ± 1 days. 3.6. Effects of CNTO 530 and Darbepoetin- on Reticulocyte and RBC Production Rates and Reticulocyte Age Distribution The reticulocyte rates of release to the blood are pre- sented in Figure 7(d). For treated mice kRET gradually increased up to Day 2, to rapidly increase within the next two days, and reached a peak on Day 4. Following the peak, the production rates declined to reach the values below the control group on Day 21. The kRET for mice that received PBS was at the same level of about 0.34 ± 0.08 1012cells/L/t1/2RNA. Darbepoetin- increased kRET higher than CNTO 530 (2.07 ± 0.16 vs. 1.71 ± 0.36 1012cells/L/t1/2RNA) but the difference was not statistically significant. This effect of CNTO 530 on kRET lasted longer than darbepoetin-. Both darbepoetin- and CNTO 530 resulted in kRET values that were significantly less than the control group (0.21 ± 0.04 and 0.16 ± 0.05 1012cells/L/t1/2RNA, respectively) at Day 21. The time courses of the mean time for reticulocyte to become mature RBC (RT) for the three groups of mice are shown in Figure 7(e). For control mice, the mean RT(mRT) values remained at the same level of 4 t1/2RNA. The mRT values for both CNTO 530 and darbepoetin- groups reached the peaks of 7.4 ± 0.6 t1/2RNA and 9.3 ± 0.9 t1/2RNA, respectively, on Day 2 which were significantly different. The time courses of the mRT following the peak were different for two groups. The RT values for mice treated with darbepoetin- declined more rapidly and reach a nadir of 2.9 ± 0.6 t1/2RNA on Day 10 and reached a plateau at this value, whereas the mean for the CNTO 530 group gradually declined to reach 3.5 ± 0.4 t1/2RNA on Day 21. The mRT on Day 21 for both treatment groups was significantly smaller than the mRT value for the control group. The mature RBC production rate vs. time plot is shown in Figure 7(f). The kRBC values for the PBS group were relatively constant 0.16 ± 0.03 1012cells/L/t1/2RNA. The time courses of kRBC for treated animals exhibited a 2 day lag prior to a rapid increase followed by a gradual decline. The peak of 0.46 ± 0.08 1012cells/L/t1/2RNA for the darbepoetin- group occurred on Day 4 whereas the peak of 0.46 ± 0.04 1012cells/L/t1/2RNA for the CNTO 530 group was on Day 10. The peak values were not significantly different. This effect of CNTO 530 on kRBC lasted longer than darbepoetin-. The kRBC values on Day 21 for the treated animals were significantly lower than for the PBS group. 4. Discussion In our studies in huTNF- transgenic mice, it was ob- served that CNTO 530 and darbepoetin- caused a dose responsive stimulus of erythropoiesis. Moreover, CNTO 530 had a superior pharmacokinetic and pharmacody- namic profile compared to darbepoetin- in this model where the mice were relatively resistant to CNTO 530 (blunted response) compared to normal mice at the same dose level. The model of ACD used in these experiments, Tg197 mice, are transgenic for human TNF- and develop a progressive polyarthritis starting at 4-5 weeks of age [1]. Arthritis is well established by 10 weeks of age and ane- mia is correlated with disease progression and huTNF- concentrations in the serum, despite the presence of ade- quate iron stores in the spleen and normal levels of en- dogenous erythropoietin [2]. That TNF- can induce anemia in mice has been reported previously. Single in- traperitoneal doses of murine TNF- have been reported to cause a decrease in hematocrit [3] and transplantation of CHO cells transfected with a human TNF- gene caused anemia within 3 weeks [4]. Similarly, mice trans- genic for human TNF- developed a microcytic anemia at 16 weeks of age [5]. When compared to normal mice, Tg197 mice were relatively resistant to CNTO 530. In contrast to our pre- vious results in normal mice where a single sc dose of epoetin- or CNTO 530 caused an expansion of Pro/ BasoEB in bone marrow [6,7], in Tg197 mice CNTO 530 and darbepoetin- caused a decrease in Pro/BasoEB. As 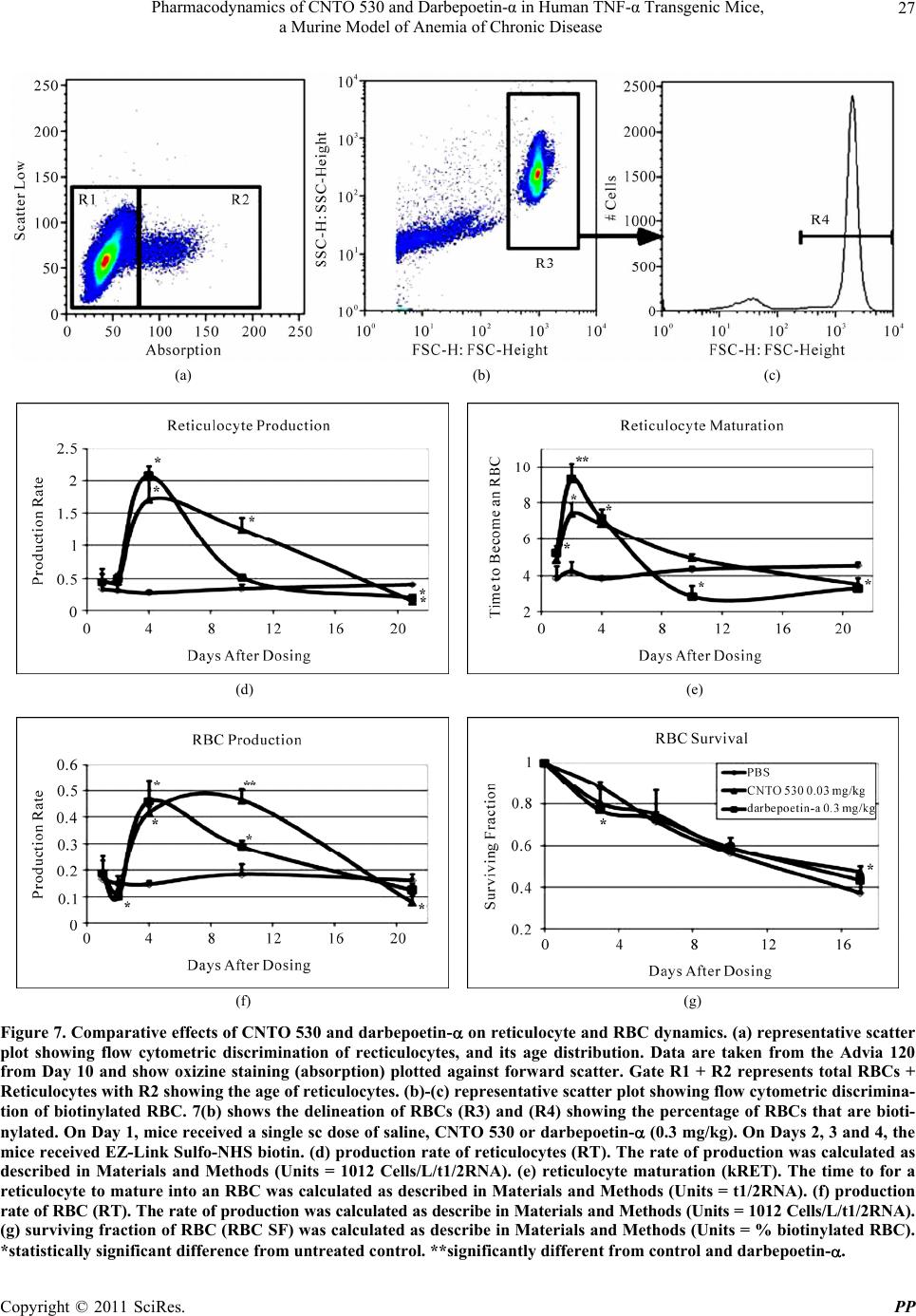 Pharmacodynamics of CNTO 530 and Darbepoetin-α in Human TNF-α Transgenic Mice, a Murine Model of Anemia of Chronic Disease Copyright © 2011 SciRes. PP 27 (a) (b) (c) (d) (e) (f) (g) Figure 7. Comparative effects of CNTO 530 and darbepoetin- on reticulocyte and RBC dynamics. (a) representative scatter plot showing flow cytometric discrimination of recticulocytes, and its age distribution. Data are taken from the Advia 120 from Day 10 and show oxizine staining (absorption) plotted against forward scatter. Gate R1 + R2 represents total RBCs + Reticulocytes with R2 showing the age of reticulocytes. (b)-(c) representative scatter plot showing flow cytometric discrimina- tion of biotinylated RBC. 7(b) shows the delineation of RBCs (R3) and (R4) showing the percentage of RBCs that are bioti- nylated. On Day 1, mice received a single sc dose of saline, CNTO 530 or darbepoetin- (0.3 mg/kg). On Days 2, 3 and 4, the mice received EZ-Link Sulfo-NHS biotin. (d) production rate of reticulocytes (RT). The rate of production was calculated as described in Materials and Methods (Units = 1012 Cells/L/t1/2RNA). (e) reticulocyte maturation (kRET). The time to for a reticulocyte to mature into an RBC was calculated as described in Materials and Methods (Units = t1/2RNA). (f) production rate of RBC (RT). The rate of production was calculated as describe in Materials and Methods (Units = 1012 Cells/L/t1/2RNA). (g) surviving fraction of RBC (RBC SF) was calculated as describe in Materials and Methods (Units = % biotinylated RBC). *statistically significant difference from untreated control. **significantly different from control and darbepoetin-.  Pharmacodynamics of CNTO 530 and Darbepoetin-α in Human TNF-α Transgenic Mice, a Murine Model of Anemia of Chronic Disease Copyright © 2011 SciRes. PP 28 reported previously, a single intraperitoneal dose of murine TNF- caused a decrease in the number of col- ony forming units in the spleen and bone marrow in mice [3]. Similarly, in human ACD there is a decrease in the number of erythroblasts and increased apoptosis in the bone marrow [8] and a relative resistance to EPO [9]. As the murine p75 TNF-receptor is selective for murine TNF- and fails to mediate signaling from human TNF- [10], resistance to CNTO 530 may be mediated through the p55 TNF-receptor. Previously, darbepoetin- has been shown to be active in a model of ACD in rats [11]. In our study, we have found that compared to darbepoetin-, CNTO 530 has a superior pharmacodynamic effect on RBC and Hgb in a murine model of ACD. In an attempt to elucidate the mechanism of the pharmacodynamics we have evaluated the effects of CNTO 530 and darbepoetin- on the bone marrow, EMH, reticulocyte production and maturation and RBC life span. The superior pharmacodynamic ef- fects of CNTO 530 are unlikely to be due to differential effects on the bone marrow. Neither test article caused an expansion of medullary hematopoiesis. Similarly, both test articles caused an increase in cell death in Pro/Ba- soEB that followed a roughly similar time course. More- over, neither test article had an effect on apoptosis in Pro/BasoEB nor an effect on apoptosis/cell death in Poly/ OrthoEB. Apoptosis and cell death have been shown previously to play a critical role in controlling erythro- poiesis and it is well established that deprivation of EPO-R stimulation leads to apoptosis of erythroid precursors [12, 13] However, as we have reported previously in normal mice [6,7], a single sc dose of epoetin- or CNTO 530 caused an increase in cell death in erythroblasts. Simi- larly, Chang et al. [14] reported increased apoptosis/cell death in erythroblasts in mice receiving three doses of recombinant murine EPO. Increased apoptosis/cell death was also reported by Robinson et al. [15] in erythroblasts in mice following hemorrhagic shock, despite a strong endogenous EPO response. Taken together, these find- ings suggest that apoptosis and cell death play a role in erythroid homeostasis when EPO-R mediated stimulation of erythropoiesis is either high or low. Our data also suggest that the superior pharmacody- namics of CNTO 530 cannot be attributed to a differen- tial effect on EMH. Both test articles caused an expan- sion of EMH, albeit with somewhat different kinetics. Repeated dosing with epoetin- has been shown to in- crease EMH in mice and rats [16,17] Similarly, Kapa et al. [18] found that the response to exogenous EPO after hypoxia was more pronounced in the spleen than in the bone marrow. This is consistent with the expansion of EMH in mice receiving either CNTO 530 or darbepo- etin- and suggests that in Tg197 mice as has been de- scribed previously in normal mice [17], the spleen pro- vides a better microenvironment for EPO mediated ex- pansion of erythropoiesis than the bone marrow. Both darbepoetin- and CNTO 530 affected reticulo- cyte maturation and RBC production profiles. Though, there was not a statistically significant difference in the number of reticulocytes in the periphery at Day 1 and 2, a difference in reticulocyte maturation was observed at these time points. Both test articles induced release of immature reticulocytes into the periphery, without changing the reticulocyte production. Moreover, a de- crease in RBC production rate is concomitantly observed with the increase in immature reticulocytes. This sug- gests that not only are immature reticulocytes released into the periphery, but both compounds delay the matu- ration of the reticulocytes to RBCs. However, the com- pounds did not affect the RBC life span with treatment, making it unclear how the delayed maturation process of reticulocytes aided in production of better RBCs. When we compare the two erythropoietin receptor agonists, they have similar mode of action in this model of ACD. Having ruled out fundamentally different ef- fects on most aspects of erythropoiesis, we are left with differences in the duration of the stimulation of erythro- poiesis between the two compounds. Pharmacokinetic analysis showed that the terminal t1/2 and AUC for CNTO 530 was significantly greater than darbepoetin-, resulting in a longer duration of exposure to CNTO 530. This longer duration of exposure was reflected in the prolonged increase in RT, kRET, kRBC and reticulocytosis seen with CNTO 530 and suggests that the differential effects of CNTO 530 and darbepoetin- on RBC and Hgb may simply reflect longer lasting effects on the final stages of erythropoiesis. 5. Acknowledgements The authors thank Renold Capocasale for generating the flow cytometry data. All authors (except WK) are em- ployees of Centocor R&D. CNTO 530 was discovered by Centocor R&D. REFERENCES [1] G. S. Alarcon, “Epidemiology of Rheumatoid Arthritis,” Rheumatologic Disease Clinics of North America, Vol. 21, No. 3, 1995, pp. 589-604. [2] K. C. Das and M. A. Sattar, “Serum and Red Cell Ferritin Content in the Evaluation of Iron Status in Rheumatoid Arthritis,” Scandinavian Journal of Rheumatology, Vol. 18, 1989, pp. 399-405. doi:10.3109/03009748909102102 [3] P. P. Sfikakis and G. Kollias, “Tumor Necrosis Factor Biology in Experimental and Clinical Arthritis,” Current  Pharmacodynamics of CNTO 530 and Darbepoetin-α in Human TNF-α Transgenic Mice, a Murine Model of Anemia of Chronic Disease Copyright © 2011 SciRes. PP 29 Opinion in Rheumatology, Vol. 2003, No. 15, 2005, pp. 380-386. [4] M. Jongen-Lavrencic, H. R. Peeters, A. Wognum, G. Vreugdenhil, F. C. Breedveld and A. J. Swaak, “Elevated Levels of Inflammatory Cytokines in Bone Marrow of Patients with Rheumatoid Arthritis and Anemia of Chronic Disease,” Journal of Rheumatology, Vol. 24, No. 8, 1997, pp. 1504-1509. [5] R. T. Means, Jr., “Recent Developments in the Anemia of Chronic Disease,” Current Hematology Reports, Vol. 2, No. 2, 2003, pp. 116-121. [6] L. L. Moldawer, M. A. Marano, H. Wei, et al., “Cachec- tin/Tumor Necrosis Factor-Alpha Alters Red Blood Cell Kinetics and Induces Anemia in vivo,” FASEB Journal, Vol. 3, No. 5, 1989, pp.1637-1643. [7] P. J. Bugelski, T. Nesspor, A. Volk, et al., “Pharmacody- namics of Recombinant Human Erythropoietin in Murine Bone Marrow,” Pharmaceutics Research, Vol. 25, No. 2, 2008, pp. 369-378. doi:10.1007/s11095-007-9372-7 [8] P. Sathyanarayana, E. Houde, D. Marshall, et al., “CNTO 530 Functions as a Potent EPO Mimetic via Unique Sus- tained Effects on Bone Marrow Proerythroblast Pools,” Blood, Vol. 113, No. 20, 2009, pp. 4955-4962. doi:10.1182/blood-2008-08-172320 [9] D. L. Johnson, F. X. Farrell, F. P. Barbone, et al., “Identi- fication of a 13 Amino Acid Peptide Mimetic of Erythro- poietin and Description of Amino Acids Critical for the Mimetic Activity of EMP1,” Biochemistry, Vol. 37, No. 11, 1998, pp. 3699-3710. doi:10.1021/bi971956y [10] O. Livnah, D. L. Johnson, E. A. Stura, et al., “An An- tagonist Peptide-EPO Receptor Complex Suggests that Receptor Dimerization is not Sufficient for Activation,” Nature Structural Biology, Vol. 5, No. 11, 1998, pp. 993- 1004. doi:10.1038/2965 [11] J. Keffer, L. Probert, H. Cazlaris, et al., “Transgenic Mice Expressing Human Tumour Necrosis Factor: A Predictive Genetic Model of Arthritis,” EMBO Journal, Vol. 10, No. 13, 1991, pp. 4025-4031. [12] P. J. Bugelski, R. J. Capocasale, D. Makropoulos, et al., “CNTO 530: Molecular Pharmacology in Human UT- 7EPO Cells and Pharmacokinetics and Pharmacodynam- ics in Mice,” Journal of Biotechnology, Vol. 134, No. 1-2, 2008, pp. 171-180. doi:10.1016/j.jbiotec.2007.12.005. [13] R. Achuthanandam, J. Quinn, R. J. Capocasale, P. J. Bugelski, L. Hrebien and M. Kam, “Sequential Univariate Gating Approach to Study the Effects of Erythropoietin in Murine Bone Marrow,” Cytometry A, Vol. 73, No. 8, 2008, pp. 702-714. doi:10.1002/cyto.a.20584 [14] G. Hoffmann-Fezer, C. Trastl, W. Beisker, et al. “Pre- clinical Evaluation of Biotin Labeling for Red Cell Sur- vival Testing,” Annals of Hematology, Vol. 74, No. 5, 1997, pp. 231-238. doi:10.1007/s002770050290 [15] T. Suzuki and G. L. Dale, “Biotinylated Erythrocytes: In Vivo Survival and In Vitro Recovery,” Blood, Vol. 70, No. 3, 1987, pp. 791-795. [16] A. C. Dornhorst, “The Interpretation of Red Cell Survival Curves,” Blood, Vol. 6, No. 12, 1951, pp. 1284-1292. [17] H. Breny, “Non-Stationary Models,” In: J. M. Paulus, Ed., Platelet Kinetics: Radioisotopic, Cytological, Mathe- matical and Clinical Aspects, North-Holland, Amsterdam, The Netherlands, 1971. [18] D. M. Mock, G. L. Lankford, J. A. Widness, L. F. Bur- meister, D. Kahn and R. G. Strauss, “Measurement of Red Cell Survival Using Biotin-Labeled Red Cells: Vali- dation Against 51Cr-Labeled Red Cells,” Transfusion, Vol. 39, No. 2, 1999, pp. 156-162. doi:10.1046/j.1537-2995.1999.39299154729.x [19] M. Kotilainen, “Linear and Exponential Components of Platelet Turnover: A Computer Analysis,” In: J. M. Pau- lus, Ed., Platelet Kinetics: Radioisotopic, Cytological, Mathematical and Clinical Aspects, North-Holland, Am- sterdam, The Netherlands, 1971. [20] P. Wiczling and W. Krzyzanski, “Flow Cytometric As- sessment of Homeostatic Aging of Reticulocytes in Rats,” Experimental Hematology, Vol. 36, No. 2, 2008, pp. 119-127. doi:10.1016/j.exphem.2007.09.002 [21] R. J. Capocasale, D. A. Makropoulos, R. Achuthanandam, et al., “Myelodysplasia and Anemia of Chronic Disease in Human Tumor Necrosis Factor-Alpha Transgenic Mice,” Cytometry A, Vol. 73, No. 2, 2008, pp. 148-159. doi:10.1002/cyto.a.20512 [22] C. S. Johnson, C. A. Cook and P. Furmanski, “In vivo Suppression of Erythropoiesis by Tumor Necrosis Fac- tor-Alpha (TNF-alpha): Reversal with Exogenous Erythro- poietin (EPO),” Experimental Hematology, Vol. 18, No. 2, 1990, pp. 109-113. [23] R. A. Johnson, T. A. Waddelow, J. Caro, A. Oliff and G. D. Roodman, “Chronic Exposure to Tumor Necrosis Factor in vivo Preferentially Inhibits Erythropoiesis in Nude Mice,” Blood, Vol. 74, No. 1, 1989, pp. 130-138. [24] H. Glosli, O. P. Veiby, H. Fjerdingstad, et al., “Effects of hTNFalpha Expression in T Cells on Haematopoiesis in Transgenic Mice,” European Journal of Haematology, Vol. 63, No. 1, 1999, pp. 50-60. doi:10.1111/j.1600-0609.1999.tb01850.x [25] H. A. Papadaki, H. D. Kritikos, V. Valatas, D. T. Boum- pas and G. D. Eliopoulos, “Anemia of Chronic Disease in Rheumatoid Arthritis is Associated with Increased Apop- tosis of Bone Marrow Erythroid Cells: Improvement Fol- lowing Anti-Tumor Necrosis Factor-Alpha Antibody Therapy,” Blood, Vol. 100, No. 2, 2002, pp. 474-482. doi:10.1182/blood-2002-01-0136 [26] G. Vreugdenhil, A. W. Wognum, H. G. van Eijk and A. J. Swaak, “Anaemia in Rheumatoid Arthritis: The Role of Iron, Vitamin B12, and Folic Acid Deficiency, and Erythropoietin Responsiveness,” Annals of Rheumatic Disease, Vol. 49, No. 2, 1990, pp. 93-98. doi:10.1136/ard.49.2.93 [27] M. Lewis, L. A. Tartaglia, A. Lee, et al., “Cloning and Expression of cDNAs for Two Distinct Murine Tumor Necrosis Factor Receptors Demonstrate One Receptor is  Pharmacodynamics of CNTO 530 and Darbepoetin-α in Human TNF-α Transgenic Mice, a Murine Model of Anemia of Chronic Disease Copyright © 2011 SciRes. PP 30 Species Specific,” Proceeding of the National Academy of Science (USA), Vol. 88, No. 7, 1991, pp. 2830-2834. doi:10.1073/pnas.88.7.2830 [28] M. A. Coccia, K. Cooke, G. Stoney, et al., “Novel Erythropoiesis Stimulating Protein (Darbepoetin Alfa) Alleviates Anemia Associated with Chronic Inflamma- tory Disease in a Rodent Model,” Experimental Hema- tology, Vol. 29, No. 10, 2001, pp. 1201-1209. doi:10.1016/S0 301 - 472X(01)00723-8 [29] U. Testa, “Apoptotic Mechanisms in the Control of Erythropoiesis,” Leukemia, Vol. 18, No. 7, 2004, 1176- 1199. doi:10.1038/sj.leu.2403383 [30] M. J. Weiss and C. O. dos Santos, “Chaperoning Erythro- poiesis,” Blood, Vol. 113, No. 10, 2009, pp. 2136-2144. doi:10.1182/blood-2008-09-115238 [31] K. H. Chang, M. Tam and M. M. Stevenson, “Inappropri- ately Low Reticulocytosis in Severe Malarial Anemia Correlates with Suppression in the Development of Late Erythroid Precursors,” Blood, Vol. 103, No. 10, 2004, pp. 3727-3735. doi:10.1182/blood-2003-08-2887 [32] Y. Robinson, A. Matenov, S. K. Tschoke, et al., “Im- paired Erythropoiesis after Haemorrhagic Shock in Mice is Associated with Erythroid Progenitor Apoptosis in vivo,” Acta Anaesthesiologica Scandinavia, Vol. 52, No. 5, 2008, pp. 605-613. doi:10.1111/j.1399-6576.2008.01656.x [33] M. Kato, Y. Kato and Y. Sugiyama, “Mechanism of the Upregulation of Erythropoietin-Induced Uptake Clear- ance by the Spleen,” American Journal of Physiology, Vol. 276, No. 5, 1999, pp. E887-E895. [34] W. Nijhof, H. Goris, B, Dontje, J. Dresz and M. Loeffler, “Optimal Erythroid Cell Production during Erythropoietin Treatment of Mice Occurs by Exploiting the Splenic Mi- croenvironment,” Experimental Hematology, Vol. 21, No. 4, 1993, pp. 496-501. [35] D. Kapa, L. Biljanovic-Paunovic, P. Milenkovic and V. Pavlovic-Kentera, “Effect of Suppression and Stimulation of Erythropoiesis on CFU-E in Mouse Spleen,” Acta Haematologica, Vol. 72, No. 5, 1984, pp. 295-302. doi:10.1159/000206405 |

