 International Journal of Organic Chemistry, 2013, 3, 185-189 http://dx.doi.org/10.4236/ijoc.2013.33023 Published Online September 2013 (http://www.scirp.org/journal/ijoc) Salen-Cu(II) Complex Catalyzed N-Arylation of Imidazoles under Mild Conditions Yan Liu, Qin Zhang, Xiaowei Ma, Ping Liu*, Jianwei Xie, Bin Dai, Zhiyong Liu School of Chemistry and Chemical Engineering/Key Laboratory for Green Processing of Chemical Engineering of Xinjiang Bingtuan, Shihezi University, Shihezi, China Email: *liuping1979112@yahoo.com.cn Received June 26, 2013; revised July 28, 2013; accepted August 8, 2013 Copyright © 2013 Yan Liu et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Three inexpensive and air-/moisture-stable Salen-Cu complexes 1-3 were evaluated to be a novel class of catalysts for the N-arylation of imidazoles with aryl halides. A v ariety of aryl iodides, bromides underwent the coupling with imida- zoles, prom ot ed by the complex 3, in moderate to excellent yields without the protection by an inert gas. Keywords: Salen-Cu Complex; N-Arylation; Imidazole; Catalyze 1. Introduction N-Aryl imidazoles and its derivatives are prevalent buil- ding blocks of numerous drugs, natural products and en- ergetic materials, [1-5] and have been exploited as im- portant precursors in N-heterocyclic carbene chemistry. [6,7] Therefore, their preparation has been attracted much attention. In recent years, the transition-metal pal- ladium catalyzed N-arylation of imidazoles has made remarkable achievements, and shows relative mild reac- tion conditions, broad substrate scope and excellent func- tional-group tolerance [8-10]. However, in comparison with the use of costly palladium, it is desirable to develop more effective copper catalytic systems for N-arylation of imidazoles. The breakthroughs in this area, which were achieved by two research groups of Buchwald [11,12] and Taillefer, [13] respectively, have typically been driven by the implementation of new class of ligands and only catalytic amounts of copper metal under mild conditions. Following these pioneering works, sev- eral classes of mono-, bidentate, and polydentate chela- tors have thereby been developed to expedite the reaction rates and substantially lower the reaction temperature of Cu-based C-N coupling reaction [14-27]. In spite of the significant progress made in the aforementioned trans- formation, more efficient, air stable and cheaper ligands or metal-complexes for facilitating these coupling reac- tions under relatively milder conditions are still in de- mand. Recently, our group had developed a series of effective catalysts, pyrrolecarbaldiminato-Cu complexes for C-N coupling reaction [28], and we previously reported Su- zuki-Miyaura reaction catalyzed by Salen and half-salen palladium(II) complexes [29]. Although Salen-Pd com- plexes show low catalytic activity in the C-C coupling reaction, we reasonable assumed that Salen-Cu com- plexes might be a class of effective catalysts for the C-N coupling reaction. Herein, we wish to report Salen-Cu complexes as catalysts for the N-arylation of imidazoles and its derivatives. This system contains several advan- tages as follows: 1) the complexes were easily synthe- sized from cheap starting materials, and stable in air and moisture; 2) the reaction condition was relatively milder and did not require the protection by an inert atmos- phere; 3) the complexes worked well for aryl iodides, and bromides with moderate to excellent yields. 2. Results and Discussion Initially, the catalytic activity of the complexes 1-3 evaluated by using the C-N coupling of 4-iodotoluene with imidazole as a model reaction in the presence of NaOH at 120˚C for 12 h in DMSO (Scheme 1). As ex- pected, the three Salen-Cu complexes all exhibited high catalytic activity for this process, and which gave the desired product in 92% - 94% isolated yields (Table 1, entries 1-3). The coupling reaction did not occur in the absence of any catalyst (Table 1, entry 4). Subsequently, we select the complex 3 as catalyst to further investigate the effects of the other reaction conditions on the *Corresponding a uthor. C opyright © 2013 SciRes. IJOC 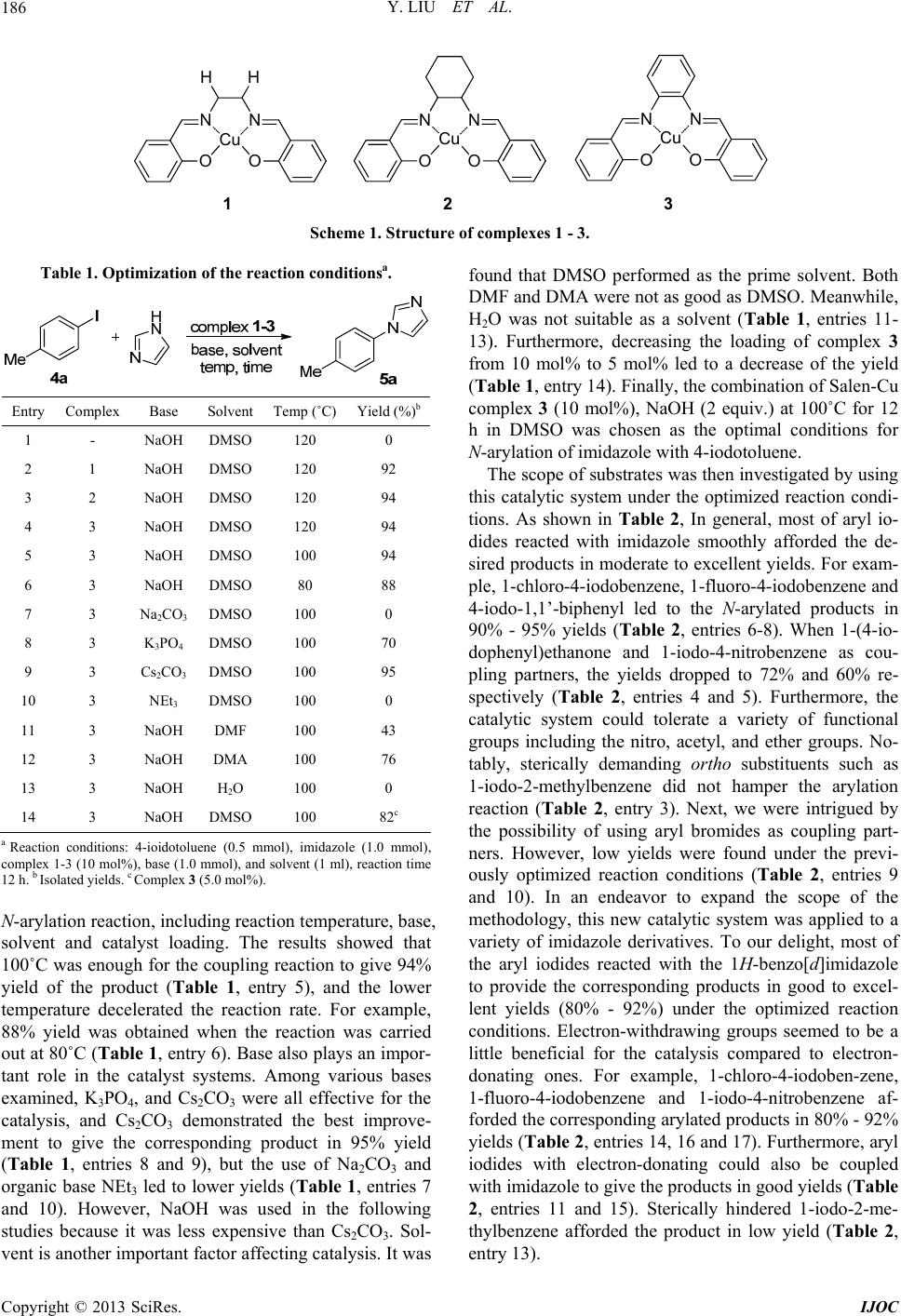 Y. LIU ET AL. 186 NN O O Cu 21 H H NN OO Cu NN O O Cu 3 Scheme 1. Structure of complexes 1 - 3. Table 1. Optimization of the reaction conditionsa. Entry Complex Base Solvent Temp (˚C) Yield (%)b 1 - NaOH DMSO 120 0 2 1 NaOH DMSO 120 92 3 2 NaOH DMSO 120 94 4 3 NaOH DMSO 120 94 5 3 NaOH DMSO 100 94 6 3 NaOH DMSO 80 88 7 3 Na2CO3 DMSO 100 0 8 3 K3PO4 DMSO 100 70 9 3 Cs2CO3 DMSO 100 95 10 3 NEt3 DMSO 100 0 11 3 NaOH DMF 100 43 12 3 NaOH DMA 100 76 13 3 NaOH H2O 100 0 14 3 NaOH DMSO 100 82c a Reaction conditions: 4-ioidotoluene (0.5 mmol), imidazole (1.0 mmol), complex 1-3 (10 mol%), base (1.0 mmol), and solvent (1 ml), reaction time 12 h. b Isolated yields. c Complex 3 (5.0 mol%). N-arylation reaction, including reaction temperature, base, solvent and catalyst loading. The results showed that 100˚C was enough for the coupling reaction to give 94% yield of the product (Table 1, entry 5), and the lower temperature decelerated the reaction rate. For example, 88% yield was obtained when the reaction was carried out at 80˚C (Tabl e 1, entry 6). Base also plays an impor- tant role in the catalyst systems. Among various bases examined, K3PO4, and Cs2CO3 were all effective for the catalysis, and Cs2CO3 demonstrated the best improve- ment to give the corresponding product in 95% yield (Table 1, entries 8 and 9), but the use of Na2CO3 and organic base NEt3 led to low er yields (Tab le 1, entries 7 and 10). However, NaOH was used in the following studies because it was less expensive than Cs2CO3. Sol- vent is another important factor affecting catalysis. It was found that DMSO performed as the prime solvent. Both DMF and DMA were not as good as DMSO. Meanwhile, H2O was not suitable as a solvent (Table 1, entries 11- 13). Furthermore, decreasing the loading of complex 3 from 10 mol% to 5 mol% led to a decrease of the yield (Table 1, entry 14). Finally, the combination of Salen-Cu complex 3 (10 mol%), NaOH (2 equiv.) at 100˚C for 12 h in DMSO was chosen as the optimal conditions for N-arylation of imidazole with 4-iodotoluene. The scope of substrates was then investigated by using this catalytic system under the optimized reaction condi- tions. As shown in Table 2, In general, most of aryl io- dides reacted with imidazole smoothly afforded the de- sired products in moderate to excellent yields. For exam- ple, 1-chlor o-4-iodoben zene, 1-fluoro -4-iodoben zene and 4-iodo-1,1’-biphenyl led to the N-arylated products in 90% - 95% yields (Table 2, entries 6-8). When 1-(4-io- dophenyl)ethanone and 1-iodo-4-nitrobenzene as cou- pling partners, the yields dropped to 72% and 60% re- spectively (Table 2, entries 4 and 5). Furthermore, the catalytic system could tolerate a variety of functional groups including the nitro, acetyl, and ether groups. No- tably, sterically demanding ortho substituents such as 1-iodo-2-methylbenzene did not hamper the arylation reaction (Table 2, entry 3). Next, we were intrigued by the possibility of using aryl bromides as coupling part- ners. However, low yields were found under the previ- ously optimized reaction conditions (Table 2, entries 9 and 10). In an endeavor to expand the scope of the methodology, this new catalytic system was applied to a variety of imidazole derivatives. To our delight, most of the aryl iodides reacted with the 1H-benzo[ d]imidazole to provide the corresponding products in good to excel- lent yields (80% - 92%) under the optimized reaction conditions. Electron-withdrawing groups seemed to be a little beneficial for the catalysis compared to electron- donating ones. For example, 1-chloro-4-iodoben-zene, 1-fluoro-4-iodobenzene and 1-iodo-4-nitrobenzene af- forded the co rrespond ing arylated produ cts in 80% - 92% yields (Table 2 , entries 14, 16 and 17). Furthermore, aryl iodides with electron-donating could also be coupled with imidazole to give the products in good yields (Table 2, entries 11 and 15). Sterically hindered 1-iodo-2-me- thylbenzene afforded the product in low yield (Table 2, entry 13). Copyright © 2013 SciRes. IJOC  Y. LIU ET AL. 187 Table 2. N-Arylation of imidazole with aryl halides cataly- zed by complex 3a. Entry ArX R, X HeT-NH ProductYield (%)b 1 H, I (4b) imidazole (5a) 6b 98 2 4-OEt, I (4c) 5a 6c 83 3 2-Me, I (4d) 5a 6d 59 4 4-COMe, I (4e) 5a 6e 72 5 4-NO2, I (4f) 5a 6f 60 6 4-Ph, I (4g) 5a 6g 95 7 4-Cl, I (4h) 5a 6h 90 8 4-F, I (4i) 5a 6i 98 9 4-Me, Br (4j) 5a 6a 19 10 4-NO2, Br (4k) 5a 6f 49 11 Me, I (4a) 1H-benzo[d]imidazole (5b) 6j 87 12 H, I (4b) 5b 6k 83 13 2-Me, I (4d) 5b 6l 20c 14 4-NO2, I (4f) 5b 6m 89 15 4-Ph, I (4g) 5b 6n 82 16 4-Cl, I (4h) 5b 6o 80 17 4-F, I (4i) 5b 6p 92 18 4-F, Br (4l) 5b 6p 21 a Reaction conditions: aryl halides (0.5 mmol), imidazoles (1.0 mmol), complex 3 (10 mol%), NaOH (1.0 mmol), and DMSO (1 ml), 100˚C, 12 h. b Isolated yields. 3. Conclusion In summary, we have developed a novel and general catalytic method for N-arylation of imidazoles promoted by Salen-Cu(II) complex 3. The system is efficient for the coupling of imidazoles and its derivatives with ArX (X = I, Br) to give moderation to excellent yields. The easy availability of the catalyst, mild reaction conditions, experimental simplicity, and broad substrate scope are the features of the catalytic method presented in the cur- rent paper. Further application of these Salen-Cu(II) complexes catalyzed organic reaction is currently ongo- ing in our laboratory. 4. Experimental 4.1. Materials and Instruments All reactions were carried out under air using magnetic stirring unless otherwise noted. 1H NMR spectral data were recorded on a Bruker DPX-400 spectrometer using TMS as internal standard and CDCl3 as solvent. Mass spectra were recorded on GC-MS (Agilent 7890A/5975C) instrument under EI model. All other reagents were of analytical grade quality purchased commercially and used. 4.2. Synthesis of Complexes 1-3 Cu(OAc)2·H2O (0.012 mol, 2.40 g) was added to a solu- tion of substituted ethane-1,2-diamine (0.01 mol) and 2-hydroxybenzaldehyde (0.02 mol) in 35 ml methanol. The mixture was stirred at 60˚C for 5 h and then filtered. The precipitate was washed with dichloromethane. The solid product was collected and dried under vacuum to afford the de sired complex 1-3. Complex 1 [30,32]: yield 65%. Anal. Calcd. for C16H14CuN2O2, %: C, 58.26; H, 4.28; N, 8.49; Found, %: C, 57.59; H, 4.38; N, 8.37. Complex 2 [31,33,34]: yield 70%. Anal. Calcd. for C20H20CuN2O2, %: C, 62.57; H, 5.25; N, 7.30; Found, %: C, 62.72; H, 5.26; N, 7.49. Complex 3 [34-37]: yield 72%. Anal. Calcd. for C20H14CuN2O2, %: C, 63.57; H, 3.73; N, 7.41; Found, %: C, 63.45; H, 3.81; N, 7.45. 4.3. General Procedure for N-Arylation of Imidazole with 4-Iodotoluene To a 10 ml of sealed tube was added complex 3 (37.8 mg, 0.05 mmol), 4-iodotoluene (109 mg, 0.5 mmol), imida- zole (68 mg, 1.0 mmol), NaOH (40 mg, 1.0 mmol), and DMSO (1 ml). The reaction mixture was reacted at 100˚C in a preheated oil bath for 12 h. The reaction mix- ture was cooled to r.t., diluted with 10 mL H2O, and then the mixture was extracted with ethyl acetate (3 × 20 mL). The combined organic phases was washed with water and brine, dried over anhydrous Na2SO4, and concen- trated in vacuo. The residue was purified by flash column chromatograph on silica gel (ethyl acetate/petroleum ether, 2:1 to pure ethyl acetate) to afford the target prod- uct (75 mg, 95% yield). 1-p-Tolyl-1H-imidazole (6a) [38-40], 1H NMR (400 MHz, CDCl3): δ 7.81 (s, 1H), 7.27 (s, 4H), 7.24 (t, J = 1.2 Hz, 1H), 7.19 (s, 1H), 2.40 (s, 3H). GC-MS (EI): m/z = 158 [M]+. 5. Acknowledgements We gratefully acknowledge financial support of this work by the National Basic Research Program of China (973 Program: 2012CB722603), the National Natural Science Foundation of Ch ina (No. 21103114), the Min is- try of Education Innovation Team (No. IRT1161), and Start-Up Foundation for Young Scientists of Shihezi Copyright © 2013 SciRes. IJOC  Y. LIU ET AL. 188 University (RCZX201012, RC ZX201 014, RCZ X201015). REFERENCES [1] C. Jacobs, M. Frotscher, G. Dannhardt and R. W. Hart- mann, “1-Imidazolyl(alkyl)-Substituted Di- and Tetrahy- droquinolines and Analogues: Syntheses and Evaluation of Dual Inhibitors of Thromboxane A2 Synthase and Aro- matase,” Journal of Medicinal Chemistry, Vol. 43, No. 9, 2000, pp. 1841-1851. doi:10.1021/jm991180u [2] J. Zhong, “Muscarine, Imidazole, Oxazole and Thiazole Alkaloids,” Natural Product Reports, Vol. 22, No. 2, 2005, pp. 196-229. doi:10.1039/b316104h [3] C. Kison and T. Opatz, “Modular Synthesis of Tetrasub- stituted Imidazoles and Trisubstituted Oxazoles by Aldi- mine Cross-Coupling,” Chemistry—A European Journal, Vol. 15, No. 4, 2009, pp. 843-845. doi:10.1002/chem.200802175 [4] H. Gao and J. M. Shreeve, “Azole-Based Energetic Salts,” Chemical Reviews, Vol. 111, No. 11, 2011, pp. 7377- 7436. doi:10.1021/cr200039c [5] S. Fujishima, R. Yasui, T. Miki, A. Ojida and I. Hamachi, “Ligand-Directed Acyl Imidazole Chemistry for Labeling of Membrane-Bound Proteins on Live Cells,” Journal of the American Chemical Society, Vol. 134, No. 9, 2012, pp. 3961-3964. doi:10.1021/ja2108855 [6] D. Enders, O. Niemeier and A. Henseler, “Organocataly- sis by N-Heterocyclic Carbenes,” Chemical Reviews, Vol. 107, No. 2, 2007, pp. 5606-5655. doi:10.1021/cr068372z [7] L. Benhamou, E. Chardon, G. Lavigne, S. Bellemin- Laponnaz and V. César, “Synthetic Routes to N-Hetero- cyclic Carbene Precursors,” Chemical Reviews, Vol. 111, No. 4, 2011, pp. 2705-2733. doi:10.1021/cr100328e [8] F. Monnier and M. Taillefer, “Catalytic C-C C-N, and C-O Ullmann-Type Coupling Reactions,” Angewandte Chemie International Edition, Vol. 48, No. 38, 2009, pp. 6954-6971.doi:10.1002/anie.200804497 [9] D. S. Surry and S. L. Buchwald, “Dialkylbiaryl Pho- sphines in Pd-Catalyzed Amination: A User’s Guide,” Chemical Science, Vol. 2, No. 1, 2011, pp. 27-50. [10] D. Maiti, B. P. Fors, J. L. Henderson, Y. Nakamura and S. L. Buchwald, “Palladium-Catalyzed Coupling of Func- tionalized Primary and Secondary Amines with Aryl and Heteroaryl Halides: Two Ligands Suffice in Most Cases,” Chemical Science, Vol. 2, No. 1, 2011, pp. 57-68. doi:10.1039/c0sc00330a [11] S. L. Buchwald, A. Klapars, J. C. Antilla, G. E. Job, M. Wolter, F. Y. Kwong, G. Nordmann and E. J. Hennessy, “Copper-Catalyzed Formation of Carbon-Heteroatom and Carbon-Carbon Bonds,” US 2001 0286286-WO02/085838. [12] A. Klapars, J. C. Antilla, X. Huang and S. L. Buchwald, “A General And Efficient Copper Catalyst for the Amida- tion of Aryl Halides and the N-Arylation of Nitrogen Heterocycle,” Journal of the American Chemical Society, Vol. 123, No. 31, 2001, pp. 7727-7729. doi:10.1021/ja016226z [13] M. Taillefer, H.-J. Cristau, P. P. Cellier, J.-F. Spindler and A. Ouali, “Method for Forming a Carbon-Carbon or Carbon-Heteroatom Linkage,” Fr 2001, 16547-WO035 3225. [14] A. Klapars, X. Huang and S. L. Buchwald, “A General and Efficient Copper Catalyst for the Amidation of Aryl Halides,” Journal of the American Chemical Society, Vol. 124, No. 25, 2002, pp. 7421-7428. doi:10.1021/ja0260465 [15] Z. Lu and R. Twieg, “Copper-Catalyzed Aryl Amination in Aqueous Media with 2-Dimethylaminoethanol Li- gand,” Tetrahedron Lettersers, Vol. 46, No. 17, 2005, pp. 2997-3001. doi:10.1016/j.tetlet.2005.03.027 [16] H. Rao, H. Fu, Y. Jiang and Y. Zhao, “Copper-Cata- lyzed Arylation of Amines Using Diphenyl Pyrrolidine-2- Phosphonate as the New Ligand,” The Journal of Organic Chemistry, Vol. 70, No. 20, 2005, pp. 8107-8109. doi:10.1021/jo051221w [17] H. Zhang, Q. Cai and D. Ma, “Amino Acid Promoted CuI-Catalyzed C-N Bond Formation between Aryl Hal- ides and Amines or N-Containing Heterocycles,” The Journal of Organic Chemistry, Vol. 70, No. 13, 2005, pp. 5164-5173. doi:10.1021/jo0504464 [18] Y. Chen and H. Chen, “1,1,1-Tris(hydroxymethyl)ethane as A New, Efficient, and Versatile Tripod Ligand for Copper-Catalyzed Cross-Coupling Reactions of Aryl Io- dides with Amides, Thiols, and Phenols,” Organic Letters, Vol. 8, No. 24, 2006, pp. 5609-5612. doi:10.1021/ol062339h [19] M. Yang and F. Liu, “Diamine Ligands in Copper-Ca- talyzed Reactions, An Ullmann Coupling of Aryl Iodides and Amines Using An Air-Stable Diazaphospholane Li- gand,” The Journal of Organic Chemistry, Vol. 72, No. 23, 2007, pp. 8969-8971. doi:10.1021/jo0712291 [20] P. Suresh and K. Pitchumani, “Per-6-amino-β-cyclodex- trin as An Efficient Supramolecular Ligand and Host for Cu(I)-Catalyzed N-Arylation of Imidazole with Aryl Bromides,” The Journal of Organic Chemistry, Vol. 73, No. 22, 2008, pp. 9121-9124. doi:10.1021/jo801811w [21] D. Wang and K. Ding, “2-Pyridinyl β-Ketones as New Ligands for Room-Temperature CuI-Catalysed C-N Cou- pling Reactions,” Chemical Communications, No. 14, 2009, pp. 1891-1893. doi:10.1039/b821212k [22] H. Zhao, H. Fu and R. Qiao, “Copper-Catalyzed Direct Amination of Ortho-Functionalized Haloarenes with So- dium Azide as the Amino Source,” The Journal of Or- ganic Chemistry, Vol. 75, No. 10, 2010, pp. 3311-3316. doi:10.1021/jo100345t [23] K. G. Thakur, K. S. Srinivas, K. Chiranjeevi and G. Sekar, “D-Glucosamine as An Efficient Ligand for the Copper- Catalyzed Selective Synthesis of Anilines from Aryl Ha- lides and NaN3,” Green Chemistry, Vol. 13, No. 9, 2011, pp. 2326-2329. doi:10.1039/c1gc15469a [24] D. Wang, F. Zhang, D. Kuang, J. Yu and J. Li, “A Highly Efficient Cu-Catalyst System for N-Arylation of Azoles in Water,” Green Chemistry, Vol. 14, No. 5, 2012, pp. 1268-1271. doi:10.1039/c2gc35077g [25] Z. Q. Wu, Z. Q. Jiang, D. Wu, H. F. Xiang and X. G. Zhou, “A Simple and Efficient Catalytic System for Cou- pling Aryl Halides with Aqueous Ammonia in Water,” Copyright © 2013 SciRes. IJOC  Y. LIU ET AL. Copyright © 2013 SciRes. IJOC 189 European Journal of Organic Chemistry, Vol. 2010, No. 10, 2010, pp. 1854-1857. doi:10.1002/ejoc.201000060 [26] Z. Q. Wu, L. Zhou, Z. Q. Jiang, D. Wu, Z. K. Li and X. G. Zhou, “Sulfonato-Cu(salen) Complex Catalyzed N-Aryla- tion of Aliphatic Amines with Aryl Halides in Water,” European Journal of Organic Chemistry, Vol. 2010, No. 26, 2010, pp. 4971-4975. doi:10.1002/ejoc.201000840 [27] Y. Wang, Z. Wu, L. X. Wang, Z. K. Li and X. G.Zhou, “A Simple and Efficient Catalytic System for N-Arylation of Imidazoles in Water,” Chemistry—A European Jour- nal, Vol. 15, No. 36, 2009, pp. 8971-8974. doi:10.1002/chem.200901232 [28] Y. L. Jiao, N. N. Yan, J. W. Xie, X. W. Ma, P. Liu and B. Dai, “A Simple and Efficient Copper(II) Complex as a Catalyst for N-Arylation of Imidazoles,” Chinese Journal of Chemistry, Vol. 31, No. 2, 2013, pp. 267-270. doi:10.1002/cjoc.201201121 [29] P. Liu, X.-J. Feng and R. He, “Salen and Half-Salen Pal- ladium(Ii) Complexes: Synthesis, Characteriztion and Ca- talytic Activity toward Suzuki—Miyaura Reaction,” Tet- rahedron, Vol. 66, No. 3, 2010, pp. 631-636. doi:10.1016/j.tet.2009.11.072 [30] N. I. Giricheva, G. V. Girichev, N. P. Kuzmina, Y. S. Medvedeva, A. Y. Rogachev, “Structure of the Cu(Salen) Molecule, CuO2N2C16H14, According to Gas-Phase Elec- tron Diffraction Data and Quantum Chemical Calcula- tions,” Journal of Structural Chemistry, Vol. 50, No. 1, 2009, pp. 52-59. doi:10.1007/s10947-009-0007-1 [31] Y. N. Belokon, R. G. Davies, J. A. Fuentes and M. North, “The Influence of Imine Structure, Catalyst Structure and Reaction Conditions on the Enantioselectivity of the Al- kylation of Alanine Methyl Ester Imines Catalyzed by Cu(ch-salen),” Tetrahedron Letters, Vol. 42, No. 45, 2001, pp. 8093-8096. doi:10.1016/S0040-4039(01)01718-X [32] Y. L. Wen, W. Huang, B. Wang, J. C. Fan, Z. H. Gao and L. H. Yin, “Synthesis of Salicylaldehyde Schiff Base Modified Cu Nanocrystals by Thermal Treatment in Liq- uid Paraffin,” Applied Surface Science, Vol. 258, No. 2, 2011, pp. 946-949. doi:10.1016/j.apsusc.2011.09.033 [33] Y. N. Belokon, M. North, T. D. Churkina, N. S. Ikonnik- ova and V. I. Maleev, “Chiral Salen-Metal Complexes as Novel Catalysts for the Asymmetric Synthesis of A-Ami- no Acids under Phase Transfer Catalysis Conditions,” Tetrahedron, Vol. 57, No. 13, 2001, pp. 2491-2498. doi:10.1016/S0040-4020(01)00072-2 [34] S. Sabarinathan, G. Vasuki, P. S. Rao, “Chiral Cu(II) Salen Complexes Catalyzed Aerobic Oxidative Biaryl Coupling Probing the Reaction by EPR,” Chemistry—A European Journal, Vol. 1, No. 4, 2010, pp. 360-367. doi:10.5155/eurjchem.1.4.360-367.221 [35] M. G. B. Drew, J. F. Godsell, S. Roy, G. Mukhopadhyay and D. Maity, “Synthesis and Characterization of Cu(II) Complexes of Tetradentate and Tridentate Symmetrical Schiff Base Ligands Involving O-Phenelenediamine, Sali- cylaldehyde and Diacetylmonoxime,” Transition Metal Chemistry, Vol. 35, No. 2, 2010, pp. 197-204. doi:10.1007/s11243-009-9314-9 [36] M. M. Abd-Elzaher, “Synthesis and Spectroscopic Char- acterization of Some Tetadentate Schiff Bases and Their Nickel, Copper and Zinc Complexes,” Synthesis and Re- activity in Inorganic and Metal-Organic Chemistry, Vol. 30, No. 9, 2000, pp. 1805-1816. [37] M. Salavati-Niasari, M. Shakouri-Arani and F. Davar, “Flexible Ligand Synthesis, Characterization and Cata- lytic Oxidation of Cyclohexane with Host (Nanocavity of zeoLite-Y)/Guest (Mn(II), Co(II), Ni(II) and Cu(II) Com- plexes of Tetrahydro-salophen) Nanocomposite Materi- als,” Microporous Mesoporous Mater, Vol. 116, No. 1-3, 2008, pp. 77-85. doi:10.1016/j.micromeso.2008.03.015 [38] M. Taillefer, N. Xia and A. Ouali, “Efficient Iron/Copper Co-Catalyzed Arylation of Nitrogen Nucleophiles,” Ange- wandte Chemie International Edition, Vol. 46, No. 6, 2007, pp. 934-936.doi:10.1002/anie.200603173 [39] H.-C. Ma and X.-Z. Jiang, “N-Hydroxyimides as Efficient Ligands for the Copper-Catalyzed N-Arylation of Py rrole, Imidazole, and Indole,” The Journal of Organic Chemis- try, Vol. 72, No. 23, 2007, pp. 8943-8946. doi:10.1021/jo7015983 [40] J. P. Collman, M. Zhong, L. Zeng and S. Costanzo, “The [Cu(OH)·TMEDA]2Cl2-Catalyzed Coupling of Arylboro- nic Acids with Imidazoles in Water,” The Journal of Or- ganic Chemistry, Vol. 66, No. 4, 2001, pp. 1528-1531. doi:10.1021/jo0016780
|