 World Journal of Cardiovascular Diseases, 2013, 3, 406-411 WJCD http://dx.doi.org/10.4236/wjcd.2013.36063 Published Online September 2013 (http://www.scirp.org/journal/wjcd/) Beta-adrenergic receptor polymorphisms: A basis for pharmacogenetics Efstratios K. Theofilogiannakos1*, Konstantinos Dean Boudoulas2*, Brian E. Gawronski3, Taimour Y. Langaee3, Timotheos G. Kelpis1, Antonios A. Pitsis1, Julie A. Johnson3, Harisios Boudoulas2,4# 1Agios Lukas Hospital, Thessaloniki, Greece 2The Ohio State University, Division of Cardiovascular Medicine, Columbus, Ohio, USA 3University of Florida, Department of Pharmacotherapy and Translational Research and Center for Pharmacogenomics, Gainesville, Florida, USA 4Aristotelian University of Thessaloniki, Thessaloniki, Greece Email: #boudoulas@bioacademy.gr Received 28 May 2013; revised 1 July 2013; accepted 16 July 2013 Copyright © 2013 Efstratios K. Theofilogiannakos et al. This is an open access article distributed under the Creative Commons At- tribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is prop- erly cited. ABSTRACT Aims: Polymorphisms of the β-adrenergic receptor, the frequency of which may differ in ethnic groups, alters β-receptor function. The aim of this study was to elucidate the frequency of β1 and β2-adrenergic receptor polymorphisms in healthy Greeks and to compare with those of Caucasian European (Euro) and African American (AA) origin. Methods: Ninety- nine individuals with a median age of 63 without cli- nical evidence of any disease were studied. Blood samples were obtained and common β1 and β2-adre- nergic receptor polymorphisms that change the en- coded amino acid were determined by pyrosequen- cing. Results: The most common β1-adrenergic re- ceptor polymorphism in Greeks is nucleotide substi- tut io n cy to si ne f or guanine at position 1165 (1165 C/G) resulting in amino acid substitution arginine for gly- cine at position 389 (389 Arg/Gly) with a minor allele frequency of 28% (Euro 27%, AA 42%); this poly- morphism increases the sensitivity of the β1-receptor. The most common β2-adrenergic receptor polymor- phism in Greeks is the nucleotide substitution gua- nine for adenine at position 46 (46 G/A) resulting in amino acid substitution glycine for arginine at posi- tion 16 (16 Gly/Arg) with a minor allele frequency of 38% (Euro 41%, AA 50%); this polymerphism fa- cilitates receptor down-regulation during chronic ad- renergic stimulation. Conclusion: The most common β1 and β2-adrenergic receptor polymorphisms in the Greek population are similar to those of other Euro- pean ancestry, and less common than in those of Af- rican origin indicating variability in ethnic groups. This information provides insight into common po- lymorphisms that may assist in optimizing β-anta- gonist and agoni st the r a py . Keywords: β1 and β2-Adrenergic Receptor; Polymorphism; Ethnic Variability 1. INTRODUCTION Normal function of β-adrenergic receptors plays an im- portant role for cardiovascular and neurohumoral ho- meostasis in humans. Heart rate, arterial pressure, myo- cardial contractility, glucose metabolism and other vital functions are directly related to the function of β-adren- ergic receptors, which are stimulated by secreted cate- cholamines. High adrenergic activity in certain disease states (i.e. heart failure, atrial fibrillation, neurocardio- genic syncope, other) may be deleterious. The patho- physiologic significance of β-adrenergic receptors and the effect of their blockade have been studied extensively for almost a century. Further, extrinsic stimulation or blockade of β-adrenergic receptors have been used for decades for the management of certain diseases such as heart failure, arterial hypertension, coronary artery dis- ease, atrial fibrillation and others [1]. Polymorphisms of β-adrenergic receptors (β1-receptor and β2-receptor) have been reported that alter the func- tion of the receptor [2-5]. Thus, determination of β- adrenergic receptor polymorphisms is important to better *First and second authors contributed equally to the paper and are both equally first authors. #Corresponding author. OPEN ACCESS  E. K. Theofilogiannakos et al. / World Journal of Cardiovascular Diseases 3 (2013) 406-411 407 understand their response to secreted catecholamines du- ring daily activities. In addition, identifying polymor- phisms may assist in optimizing management of patients where β-adrenergic stimulation or blockade therapy is used. Polymorphisms of β-adrenergic receptors have been studied in individuals of Caucasian European and Afri- can American origin [2]. Studies related to the frequen- cy of β2-receptor polymorphisms in the Greek populatio n with pulmonary disease have also been reported [6]; however, the frequency of β1 and β2-adrenergic receptor polymorphisms in a healthy Greek population has not been studied. The present investigation was undertaken to elucidate the frequency of β1 and β2-adrenergic recep- tor polymorphisms in healthy Greeks and to compare this frequency with those of Caucasian European and African American origin in order to determine if the variability exists in different ethnic groups. 2. METHODS 2.1. Study Population Ninety-nine individuals (70 male) with a median age of 63 years (range 31 to 80 years) without clinical evidence of disease were studied; medical history and physical examination were performed in order to exclude under- lying disease in all studied individuals. All study partici- pants were hospital employees of Agios Loukas Hospital, Thessaloniki, Greece, or volunteers that were non-blood relatives. Whole blood was obtained from each subject using standard venous puncture in which 10 mL of blood was collected into EDTA anti-coagulant tubes in order to determine β-adrenergic receptor polymorphisms. The study was approved by the Institutio nal Review Board of Agios Loukas Hospital and written informed consent was ob- tained by all participants. 2.2. Determination of β-Adrenergic Receptor Polymorphisms Genomic DNA was isolated from lymphocytes in whole blood using a commercially available kit (Qiagen DNA Blood Isolation Kit, Qiagen, Valencia, California, USA). DNA samples were genotyped for two β1-adrenergic re- ceptors (ADRB1) including: 49 Ser/Gly (amino acid sub- stitution of serine for glycine at position 49 resulting in the nucleotide substitution of adenine for guanine at po- sition 145 (145 A/G), rs1801252); and 389 Arg/Gly (amino acid substitution of arginine for glycine at posi- tion 389 resulting in the nucleotide substitution of cyto- sine for guanine at pos ition 1165 (1165 C/G), rs1801253). In addition, DNA samples were genotyped for two β2- adrenergic receptors (ADRB2) including: 16 Gly/Arg (amino acid substitution of glycine for arginine at posi- tion 16 resulting in the nu cleotide substitution of guanine for adenine at position 46 (46 G/A), rs1042713); and 27 Gln/Glu (amino acid substitution of glutamine for glu- tamic acid at position 27 resulting in the nucleotide sub- stitution of cytosine for guanine at position 79 (79 C/G), rs1042714). Single-nucleotide polymorphisms (SNPs) were determined by polymerase chain reaction (PCR) followed by pyrosequencing using a PSQ HS96A SNP reagent kit according to the manufacturer protocol (Bio- tage AB, Uppsala, Sweden) and TaqMan allelic discri- mination (Applied Biosystems, Foster City, California, USA). The PCR primers and probes for ADRB1 49 Ser/ Gly and 389 Arg/Gly (IDs C___8898508_10 and C___ 8898494_10), and ADRB2 16 Gly/Arg and 27 Gln/Glu (IDs C___2084764_20 and C___2084765_20) used in assays were purchased from Applied Biosystems (Ap- plied Biosystems, Foster City, California, USA); 5 mL reactions in a 384-well plate were prepared and the as- says were performed and analyzed according to the ma- nufacturer’s recommendations. The PCR and pyrose- quencing primers for above-mentioned SNPs have been previously reported [7]. Genotype accuracy was verified by genotyping 5% - 10% randomly selected duplicate samples for each SNP on the alternate platform. The mi- nor allele frequency was determined by allele counting, where each person has two alleles; the frequency of the least common allele is determined by: minor allele fre- quency (MAF) = (# heterozygous individuals + 2* # homozygous variant individuals)/2* total number of in- dividuals in the study. 3. RESULTS Frequencies of the β-adrenergic receptor genotypes (β1- receptor and β2-receptor) in the Greek population are shown in Table 1. Table 2 displays minor allele frequen - cies in Greeks, compared to population values for Cau- casian Europeans and African Americans. 3.1. Most Common β1-Receptor Polymorphisms The most common β1-receptor polymorphism found in the Greek population is a substitution of cytosine for guanine at position 1165 (1165 C/G) that results in the amino acid substitution of arginine for glycin e at po sition 389 (389 Arg/Gly); the minor allele frequency for this polymorphism is 28% in the Greek population. This po- lymorphism increases the sensitivity of the β1-receptor during adrenergic stimulation [1-3]. The second most common β1-receptor polymorphism found in the Greek population is a su bstitution of adenine for guanine at position 145 (145 A/G) that results in the amino acid substitution of serine for glycine at position 49 (49 Ser/Gly); the minor allele frequency for this polymorphism is 10% in the Greek population. This polymorphism does not alter the sensitivity of the β1- receptor during acute adrenergic stimulation, but may Copyright © 2013 SciRes. OPEN ACCESS 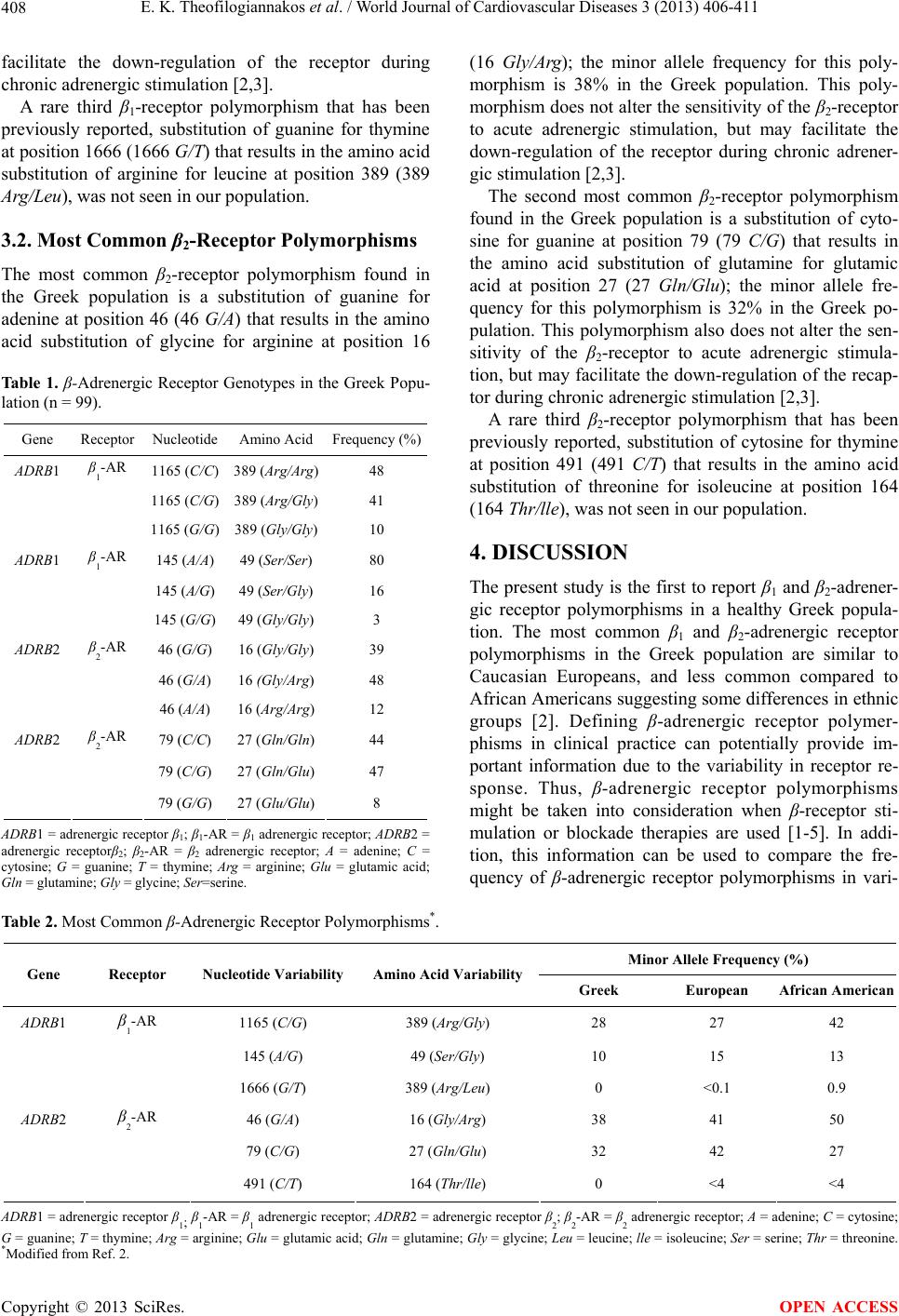 E. K. Theofilogiannakos et al. / World Journal of Cardiovascular Diseases 3 (2013) 406-411 Copyright © 2013 SciRes. 408 OPEN ACCESS facilitate the down-regulation of the receptor during chronic adrenergic stimulation [2,3]. A rare third β1-receptor polymorphism that has been previously reported, substitution of guanine for thymine at position 1666 (1 666 G/T) that results in the amino acid substitution of arginine for leucine at position 389 (389 Arg/Leu), was not seen in our population. 3.2. Most Common β2-Receptor Polymorphisms The most common β2-receptor polymorphism found in the Greek population is a substitution of guanine for adenine at position 46 (46 G/A) that results in the amino acid substitution of glycine for arginine at position 16 Tab le 1 . β-Adrenergic Receptor Genotypes in the Greek Popu- lation (n = 99). Gene Receptor Nucleotide Amino Acid Frequency (%) ADRB1 β1-AR 1165 (C/C) 389 (Arg/Arg) 48 1165 (C/G) 389 (Arg/Gly) 41 1165 (G/G) 389 (Gly/Gly) 10 ADRB1 β1-AR 145 (A/A) 49 (Ser/Ser) 80 145 (A/G) 49 (Ser/Gly) 16 145 (G/G) 49 (Gly/Gly) 3 ADRB2 β2-AR 46 (G/G) 16 (Gly/Gly) 39 46 (G/A) 16 (Gly/Arg) 48 46 (A/A) 16 (Arg/Arg) 12 ADRB2 β2-AR 79 (C/C) 27 (Gln/Gln) 44 79 (C/G) 27 (Gln/Glu) 47 79 (G/G) 27 (Glu/Glu) 8 ADRB1 = adrenergic receptor β1; β1-AR = β1 adrenergic receptor ; ADRB2 = adrenergic receptorβ2; β2-AR = β2 adrenergic receptor; A = adenine; C = cytosine; G = guanine; T = thymine; Arg = arginine; Glu = glutamic acid; Gln = glutamine; Gly = glycine ; Ser=serine. (16 Gly/Arg); the minor allele frequency for this poly- morphism is 38% in the Greek population. This poly- morphism does not alter the sensitivity of the β2-receptor to acute adrenergic stimulation, but may facilitate the down-regulation of the receptor during chronic adrener- gic stimulation [2,3]. The second most common β2-receptor polymorphism found in the Greek population is a substitution of cyto- sine for guanine at position 79 (79 C/G) that results in the amino acid substitution of glutamine for glutamic acid at position 27 (27 Gln/Glu); the minor allele fre- quency for this polymorphism is 32% in the Greek po- pulation. This polymorphism also does not alter the sen- sitivity of the β2-receptor to acute adrenergic stimula- tion, but may facilitate the down-regulation of the recap- tor during chronic adrenergic stimulation [2,3]. A rare third β2-receptor polymorphism that has been previously reported, substitution of cytosine for thymine at position 491 (491 C/T) that results in the amino acid substitution of threonine for isoleucine at position 164 (164 Thr/lle ), was not seen in our population. 4. DISCUSSION The present study is the first to report β1 and β2-adrener- gic receptor polymorphisms in a healthy Greek popula- tion. The most common β1 and β2-adrenergic receptor polymorphisms in the Greek population are similar to Caucasian Europeans, and less common compared to African Americans suggesting some differences in ethnic groups [2]. Defining β-adrenergic receptor polymer- phisms in clinical practice can potentially provide im- portant information due to the variability in receptor re- sponse. Thus, β-adrenergic receptor polymorphisms might be taken into consideration when β-receptor sti- mulation or blockade therapies are used [1-5]. In addi- tion, this information can be used to compare the fre- quency of β-adrenergic receptor polymorphisms in vari- Table 2. Most Common β-Adrenergic Receptor Polymorphisms*. Minor Allele Frequency (%) Gene Receptor Nucleotide Variability Amino Acid Variability Greek European African American ADRB1 β1-AR 1165 (C/G) 389 (Arg/Gly) 28 27 42 145 (A/G) 49 (Ser/Gly) 10 15 13 1666 (G/T) 389 (Arg/Leu) 0 <0.1 0.9 ADRB2 β2-AR 46 (G/A) 16 (Gly/Arg) 38 41 50 79 (C/G) 27 (Gln/Glu) 32 42 27 491 (C/T) 164 (Thr/lle) 0 <4 <4 ADRB1 = adrenergi c recepto r β1; β1-AR = β1 adrenergic recept or; ADRB2 = adr energi c recepto r β2; β2-AR = β2 adrenergic receptor; A = adenine; C = cytosine; G = guanine; T = thymine; Arg = arginine; Glu = glutamic acid; Gln = glutamine; Gly = glycine; Leu = leucine; lle = iso leucin e; Ser = serine; Thr = thr eonine. *Modified from Ref. 2.  E. K. Theofilogiannakos et al. / World Journal of Cardiovascular Diseases 3 (2013) 406-411 409 ous diseases (i.e. heritable diseases, arterial hypertension, coronary artery disease, other) to better tailor potential therapies. Interestingly, certain groups of patients (i.e. floppy mitral valve/mitral valve prolapse syndrome) have a greater response to isoproterenol, while elderly indivi- duals may have decreased β-receptor function [8]. It should also be kept in mind that th e response to intrin sic catecholamines during daily physical or other activities may be related to β-adrenergic receptor polymorphisms. 4.1. Pharacogenetics-Pharmacogenomics Pharmacogenetics-pharmacogenomics is the field of bio- medical sciences that studies the effect of pharmacologic agents in relatio n to genetic factors. A gene is comprised of DNA that encodes for a specific protein; a specific triplet of nucleotides in the DN A chain, c alle d a co don , is translated into a specific amino acid. A polymorphism that involves a change in on e nucleotid e is called a sing le nucleotide polymorphism (SNP) [5]. Certain polymor- phisms may be of clinical significance and may account for different responses to pharmacologic agents (phar- macogenetics). Often, however, the genetic contribution to the variability of drug response is not related to a polymorphism of one gene, but to multiple genes or to the entire genome (pharmacogenomics). Genetic factors may affect drug function by two major mechanisms: al- teration of drug metabolism (pharmacokinetics), altera- tion of the sensitivity of enzymes or receptors via which pharmacologic agents provide their effect (pharmacody- namics), or both [5,9,10]. Although pharmacogenetics-pharmacogenomics is a relatively new field in medical sciences, different re- sponses to substances possibly related to genetic factors have been reported from antiquity. Pythagoras in the 6th century B.C. observed that eating fava beans was dan- gerous to certain individuals. For this reason, he recom- mended that everyone avoid fava beans [5,11]. Today it is known that serious hemolytic anemia may occur in individuals with a genetically determined deficiency of the anti-oxidant enzyme glucose-6-phosphate dehydro- genase (G6PD) after the consumption of fava beans [1]. 4.2. Pharmacogenetics and β-Adrenergic Receptors The response to β-adrenergic receptor stimulation or blo- ckade may differ from person to person. This may be related to underlying disease, age, drug-drug interaction, and other factors; there are situations, however, where this difference cannot be explained with factors men- tioned above [2,5]. Different responses from person to person may be related to polymorphisms. Catecholamines endogenous or exogenous provide the i r effects via β-adrenergic receptors. The most common β1- receptor polymorphism found in the Greek population is 389 Arg/Gly; this polymorphism is associated with a greater response to β-adrenergic stimulation in vitro and vivo. Studies have suggested that resting heart rate and blood pressure were greater in 389 Arg/Gly individuals compared to individuals without the polymorphism, and that these individuals were more responsive to β-blockers. Further, 389 Arg/Gly polymorphism underwent a greater degree of agonist related with desensitization (down-re- gulation) compared to those without the polymorphism. Thus, 389 Arg/Gly polymorphism has a greater capacity to undergo modification under conditions of elevated catecholamines [2-4]. On the other hand, 49 Ser/Gly polymorphism does not increase the sensitivity to acute β-adrenergic stimulation, but promotes down-regulation of the receptor during chronic stimulation. Thus, β-adre- nergic receptor polymorphisms may alter the function of these receptors [2,12,13]. Medical therapy with β-blockers has been used for more than a half a century for the treatment of most car- diovascular diseases, such as acute myocardial infarction, chronic coronary artery disease, arterial hypertension, congestive heart failure, atrial fibrillation, aortic aneu- rysm-dissection, just to mention a few [1]. It has been suggested that the response to β-blockade therapy may be related to β-adrenergic receptor polymorphisms. Im- provement in left ventricular ejection fraction or in- creased survival in patients with heart failure with β- blockade therapy was greater in those with the 389 Arg/ Gly polymorphism, although these findings were not confirmed in all studies. Studies also suggest that indi- viduals with the 389 Arg/Gly polymorphism had a gre ater survival when treated with low dose β-blockade therapy compared to no ther apy [2,14-16]. Several studies in Europe and in the United States of America demonstrated that β-blockade therapy in acute myocardial infarction may decrease infarct size, inci- dence of ventricular arrhythmias and mortality [17-23]; however, a more recent study in the Chinese population indicated that metoprolol administration in acute myo- cardial infarction may not be as beneficial due to an in- crease in cardiogenic shock [24]. The discrepancy in these results may be at least partially related to the vari- ous responses to β-blockade therapy in different ethnic groups [25]. Data suggest that in arterial hypertension, β1-receptor polymorphisms influence the response and the outcome (blood pressure control and cardiovascular events) when β-blockade therapy was used; the greatest benefit was seen in patients with 389 Arg/Gly and 49 Ser/Gly poly- morphisms, however, this was not recapitulated in all studies. Some of the discrepancies may be related to dif- ferent β-blocking drugs that were used in these studies [13,26]. Similarly, in patients with atrial fibrillation the Copyright © 2013 SciRes. OPEN ACCESS  E. K. Theofilogiannakos et al. / World Journal of Cardiovascular Diseases 3 (2013) 406-411 410 effect of β-blockade therapy was associated with β- adrenergic receptor polymorphism [27]. In patients with acute coronary syndromes, the outcome related to β- blockade therapy was also dependent on the β1-receptor polymorphism; a greater benefit in survival was seen in individuals with 389 Arg/Gly and 49 Ser/Gly polymor- phisms [28,29]. Stimulation of β-adrenergic receptors may have vari- able responses due to β-adrenergic receptor polymor- phisms. Stimulation of β1-receptors is used for the treat- ment of patients with acute heart failure and/or cardio- genic shock in which the effect may be related to recep- tor polymorphism. Further, β2 agonists have been used for the treatment of chronic obstructive pulmonary dis- ease and the effect may also be related to receptor poly- morphism [1]. In conclusion, the most common β1 and β2-adrenergic receptor polymorphisms in the Greek population are si- milar to those of Caucasian European origin, and less common compared to African American origin indicating variability in ethnic groups [25]. Polymorphisms of β- adrenergic receptors may alter the response to β-adren- ergic stimulation and blockade. Further, in certain dis- eases, such as congestive heart failure, arterial hyperten- sion, atrial fibrillation, acute myocardial infarction and chronic coronary artery disease, patient outcomes when using β-blockade therapy may be related to receptor po- lymorphism. Better understanding of β-adrenergic rece- ptor function will help to optimize therapy with β-blo- cking drugs and to improve patient outcomes. The data provide important information for the clinical applica- tion of pharmacogenetics related to β-adrenergic recap- tors [30-32]. 5. AUTHOR CONTRIBUTION EKT, AAP, HB designed the research; EKT, BEG, TYL, TGK, AAP, JAJ and HB performed the research; KDB, JAJ and HB analyzed data; EKT, KDB, JAJ and HB wrote the manuscript. REFERENCES [1] Brunton, L.L. (2011) Goodman and Gilman’s the patho- physiologic basis of therapeutics. 12th Edition, The McGraw-Hill Companies, Inc., New York. [2] Johnson, J.A. and Liggett, S.B. (2 011) Cardiova scular p h a - rmacogenomics of adrenergic receptor signaling: Clinical implications and future directions. Clinical Pharmaco- logy & Therapeutics, 89, 366-378. doi:10.1038/clpt.2010.315 [3] Shin, J. and Johnson, J.A. (2007) Pharmacogenetics of beta-blockers. Pharmacotherapy, 27, 874-887. doi:10.1592/phco.27.6.874 [4] Johnson, J.A., Zineh, I., Puckett, B.J., McGorray, S.P., Yarandi, H.N. and Pauly, D.F. (2003) Beta1-adrenergic receptor polymorphisms and antihypertensive response to metoprolol. Clinical Pharmacology & Therapeutics, 74, 44-52. doi:10.1016/S0009-9236(03)00068-7 [5] Stakos, D.A. and Boudoulas, H. (2002) Pharmacogenetics and pharmacogenomics in cardiology. The Hellenic Jour- nal of Cardiology, 43, 1-15. [6] Papatheodorou, A., Makrythanasis, P., Kaliakatsos, M., Dimakou, A., Orfanidou, D., Rou ssos, C., et al. (2010) D e- velopment of novel microarray methodology for the study of mutations in the SERPINA1 and ADRB2 genes—their association with obstructive pulmonary disease and dis- seminated bronchiectasis in Greek patients. Clinical Bio- chemistry, 43, 43-50. doi:10.1016/j.clinbiochem.2009.08.026 [7] Shin, J., Lobmeyer, M.T., Gong, Y., Zineh, I., Langaee, T.Y., Yarandi, H., et al. (2007) Relation of beta(2)-adre- noceptor haplotype to risk of death and heart transplanta- tion in patients with heart failure. American Journal of Cardiology, 99, 250-255. doi:10.1016/j.amjcard.2006.08.020 [8] Boudoulas, H., Reynolds, J.C., Mazzaferri, E. and Woo- ley, C.F. (1983) Mitral valve prolapse syndrome: The ef- fect of adrenergic stimulation. Journal of the American College of Cardiology, 2, 638-644. doi:10.1016/S0735-1097(83)80303-9 [9] Voora, D. and Ginsburg, G.S. (2012) Clinical application of cardiovascular pharmacogenetics. Journal of the Ame- rican College of Cardiology, 60, 9-20. doi:10.1016/j.jacc.2012.01.067 [10] Salari, K., Watkins, H. and Ashley, E.A. (2012) Person- alized medicine: Hope or hype? The European Heart Journal, 33, 1564-1570. doi:10.1093/eurheartj/ehs112 [11] Diogenis Laertios, “Pythagoras,” Vitae Philosophoru VIII, 8.33.6- 8.34.6. [12] Humma, L.M., Puckett, B.J., Richasdson, H.E., Terra, S.G., Andrisin, T.E., Lejeune, B.L., et al. (2001) Effects of beta1-adrenergic polymorphisms on resting hemody- namics in patients undergoing diagnostic testing for is- chemia. American Journal of Cardiology, 88, 1 0 3 4-1037. doi:10.1016/S0002-9149(01)01986-5 [13] Peng, Y., Xue, H., Luo, L., Yao, W. and Li, R. (2009) Po- lymorphisms of the adrenergic receptor gene are associ- ated with essential hypertension in Chinese. Clinical Che- mistry and Laboratory Medicine, 47, 1227-1231. doi:10.1515/CCLM.2009.276 [14] Kaye, D.M., Smi rk, B., Williams, C., Je nnings, G., Esler, M. and Holst, D. (2003) Beta-adrenoreceptor genotype influences the response to carvedilol in patients with con- gestive heart failure. Pharmacogenetics, 13, 379-382. doi:10.1097/00008571-200307000-00002 [15] Borjesson, M., Magnusson, Y., Hjalmarson, A. and An- derson, B. (2000) A novel polymorphism in the gene cod- ing for the beta 1-adrenergic receptor is associated with survival in patients with heart failure. The European Heart Journal, 21, 1853-1858. doi:10.1053/euhj.1999.1994 [16] Biolo, A., Clausell, N., Santos, K.G., Salvaro, R., Ashton- Prolla, P., Borges, A., et al. (2008) Impact of beta1-adre- nergic receptor polymorphisms on susceptibility to heart failure, arrhythmogenesis, p rognosis, and response to bet a- blocker therapy. American Journal of Cardiology, 102, Copyright © 2013 SciRes. OPEN ACCESS  E. K. Theofilogiannakos et al. / World Journal of Cardiovascular Diseases 3 (2013) 406-411 Copyright © 2013 SciRes. 411 OPEN ACCESS 726-732. doi:10.1016/j.amjcard.2008.04.070 [17] Rydén, L., Ariniego, R., Arnman, K., Herlitz, J., Hjalmar- son, A., Holmberg, S., et al. (1983) A double-blind trial of metoprolol in acute myocardial infarction. Effects on ventricular tachyarrhythmias. The New England Journal of Medicine, 308, 614-618. doi:10.1056/NEJM198303173081102 [18] Roqué, F., Amuchastegui, L.M., Lopez Morillos, M.A., Mon, G.A., Girotti, A.L., Drajer, S., et al. (1987) Benefi- cial effects of timolol on infarct size and late ventricular tachycardia in patients with acute myocardial infarction. Circulation, 76, 610-617. doi:10.1161/01.CIR.76.3.610 [19] Boudoulas, H. (1990) Therapeutic interventions which may improve survival in patients with coronary artery disease. Acta Cardiologica, 45, 477-487. [20] The MIAMI Trial Research Group (1985) Metoprolol in acute myocardial infarction (MIAMI). A randomised pla- cebo-controlled international trial. The European Heart Journal, 6, 199-226. [21] Hjalmarson, A., Elmfeldt, D., Herlitz, J., Holmberg, S., Málek, I., Nyberg, G., et al. (1981) Effect on mortality of metoprolol in acute myocardial infarction. A double-blind randomised trial. Lancet, 2, 823-827. doi:10.1016/S0140-6736(81)91101-6 [22] Hjalmarson, A., Herlitz, J., Holmberg, S., Rydén, L., Swedberg, K., Vedin, A., et al. (1983) The Göteborg me- toprolol trial. Effects on mortality and morbidity in acute myocardial infarction. Circulation, 67, I26-I32. [23] Hjalmarson, A. and Olsson, G. (1991) Myocardial infarc- tion. Effects of beta-blockade. Circulation, 84, VI101- VI107. [24] Chen, Z.M., Pan, H.C., Chen, Y.P., Peto, R., Collins, R., Jiang, L.X., et al. (2005) COMMIT (ClOpidogrel and M e- toprolol in Myocardial Infarction Trial) collaborative group. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: Randomised placebo-controlled trial. Lancet, 366, 1622-1632. doi:10.1016/S0140-6736(05)67661-1 [25] Johnson, J.A. (2008) Ethnic differences in cardiovascular drug response: Potential contribution of pharmacogenet- ics. Circulation, 23, 1383-1393. doi:10.1161/CIRCULATIONAHA.107.704023 [26] Suonsyrja, T., Donner, K., Hannila-Handelberg, T., Fod- stad, H., Kontula, K. and Hiltunen, T.P. (2010) Common genetic variation of beta1- and beta2-adrenergic receptor and response to four classes of antihypertensive treatment. Pharmacogenetics and Genomics, 20, 342-345. doi:10.1097/FPC.0b013e328338e1b8 [27] Parvez, B., Chopra, N., Rowan, S., Vaglio, J.C., Muham- mad, R., Roden, D.M., et al. (2012) A common 1-adre- nergic receptor polymorphism predicts favorable respon- se to rate-control therapy in atrial fibrillation. Journal of the American College of Cardiology, 59, 49-56. doi:10.1016/j.jacc.2011.08.061 [28] Cresci, S., Dorn II, G.W., Jones, P.G., Beitelshees, A.L., Li, A.Y., Lenzini , P.A., et al. (2012) Adrenergic-pathway gene influence beta-blocker-related outcomes after acute coronary syndrome in a race-specific manner. Journal of the American College of Cardiology, 60, 898-907. doi:10.1016/j.jacc.2012.02.051 [29] Puri, R., Liew, G.Y.H., Nicholls, S.J., Nelson, A.J., Leong, D.P., Carbone, A., et al. (2012) Coronary 2-adre- noreceptors mediate endothelium-dependent vasoreactiv- ity in humans: Novel insights from an in vivo intravascu- lar ultrasound study. The European Heart Journal, 33, 495-504. doi:10.1093/eurheartj/ehr359 [30] Evans, W.E. and Relling, M.V. (1999) Pharmacogenetics: Translating functional genomics into rational therapeutics. Science, 286, 487-91. doi:10.1126/science.286.5439.487 [31] Wachter, S.B. and Gilbert, E.M. (2012) Beta-adrenergic receptors, from their discovery and characterization th rough their manipulation to beneficial clinical application. Car- diology, 122, 104-112. doi:10.1159/000339271 [32] Roden, D.M., Altman, R.B., Benowitz, N.L., et al. (2006) Pharmacogenomics: Challenges and opportunities. Annals of Internal Medicine, 145, 749-757. doi:10.7326/0003-4819-145-10-200611210-00007
|