 World Journal of Cardiovascular Diseases, 2013, 3, 40-44 WJCD http://dx.doi.org/10.4236/wjcd.2013.35A007 Published Online August 2013 (http://www.scirp.org/journal/wjcd/) Effects of omacor® on left ventricular remodelling consecutive to post myocardial infarction special issue-myocardial infarction* Bruno Le Grand Institut de Recherche Pierre Fabre, Castres, France Email: bruno.le.grand@pierre-fabre.com Received 26 June 2013; revised 27 July 2013; accepted 6 August 2013 Copyright © 2013 Bruno Le Grand. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Ventricular remodelling is the main trigger of the de- velopment of heart failure. Therefore, the reduction of structural remodelling is known to prevent the de- velopment of heart failure. The aim of the present study was to investigate the effects of OMACOR®, a well known mixture of EPA and DHA in an experi- mental model of heart failure induced by occlusion of left descending coronary artery and the reperfusion within 2 months. After a long term treatment of 2 months; OMACOR® (100 mg/kg) statistically signifi- cantly reduced the expansion of infarcted zone (35% 4%, P < 0.05, n = 9, versus 45% 3% in the vehicle group). The phosphorylation of Cx43 as biomarker of the cardiac remodelling was visualised by immunoflu- orescence in rat’s heart at the end of the study. In the vehicle-infarcted group, a significant de-phosphoryla- tion of Cx43 was observed (8.2 ± 1.0 u.a, n = 8 com- pared to 11.8 ± 1.3 u.a in the sham group, n = 9) con- firming a remodelling process in the infarcted group. In the group treated with OMACOR®, the de-phos- phorylation of Cx43 was no longer observed compar- ed to the sham group (16.4 ± 2.9 u.a, n = 9, NS). The present results demonstrate that a long term treat- ment with OMA-COR® reduced the infarcted size in experimental models of heart failure and that these anti-remodelling effects are due at least in part by re- synchronizing the gap junction activity. Keywords: Left Ventricular Remodelling; Myocardial Infarction; OMACOR® 1. INTRODUCTION Occlusion of the circumflex coronary artery results in ex- tensive myocardial necrosis and fibrosis, with a decrease in myocardial contractility and function [1-3]. Following in- farction, the myocardium undergoes a prolonged remodel- ling process that induces widespread structural changes, with the ventricle becoming considerably stiffer and less compliant [4,5]. Histologically, the most prominent chang- es are myocyte death and scar formation in the infarcted myocardium. The remodelling process is not limited to the infarct area. Changes in noninfarcted myocardium include myocyte hypertrophy, apoptosis, fiber disarray, angiogene- sis, and an increase in interstitial collagen, all of which can eventually lead to death from heart failure. OMACOR®, one of the polyunsaturated fatty acid com- pounds, has been shown to reduce cardiovascular-related morbidity and mortality in clinical trials [6,7] and has been demonstrated to impact cardiac remodelling, includ- ing hypertrophy [8,9]. However, the beneficial effects of OMACOR® on coronary artery disease are not limited to its triglyceride-lowering function but involve various pleiotropic effects on cardiac function including reduc- tion of arrhythmias, inhibition of cellular proliferation and migration, anti-inflammatory effects, and improve- ment of endothelial function [10]. Despite the wide- spread clinical use of OMACOR® for hypertriglyceride- mia and prevention of coronary artery disease, data are lacking on the effects of OMACOR® on clinical outcome in heart failure secondary to myocardial infarction (MI). Thus, the role of OMACOR® in heart failure due to MI remains controversial. Therefore, the purpose of this stu- dy was to determine whether administration of OMA- COR® during the peri-infarct period attenuates the pro- gressive LV chamber dilatation and contractile dysfunc- tion in a rat model of MI. 2. METHODS 2.1. Preparation of Animals *Competing interests statement: Bruno Le Grand is an employee of In- stitut de Recherche Pierre Fabre. The experiments were carried out according to French OPEN ACCESS  B. Le Grand / World Journal of Cardiovascular Diseases 3 (2013) 40-44 41 law and the local ethical committee guidelines for animal research. Male Sprague-dawley rats weighing 220 - 300 g at the date of the experiments were purchased from Iffa Credo (France). They were housed in the Centre de Recherche Pierre Fabre animal facilities for at least two weeks be- fore use. Throughout this period, they had free access to food and drinking water. The animal house was maintain- ed on a 12-h light/dark cycle (lights on at 7 a.m.) at an ambient temperature of 20˚C ± 2˚C. 2.2. Surgical Model of Myocardial Infarction The rats were anesthetized using Isoflurane 3% on O2. Then, the animals were intubated and ventilated at 60 re- spirations/min (2.5 ml/respiration, Ventilator model 683, Harvard Apparatus, HOLLISTON, MA, USA) while anes- thesia was maintained. Body temperature was maintained at 37˚C by a heating pad (Homeothermic blanket control unit, Harvard Apparatus, HOLLISTON, MA, USA). A left thoracotomy was performed and a silk suture (4.0) was placed around the left coronary artery ~1 mm from its origin. Both ends of the silk thread were passed through a polyethylene tube. The left coronary artery was occlu- ded by pressing the polyethylene tube against another polyethylene tube placed on the heart. After 30 min of ischemia, the polyethylene tube was removed to initiate a definitive reperfusion phase. Then, the thorax was closed and the animals regained consciousness 1 hour after the end of the ischemia. Finally, the reperfusion was perform- ed for 2 months in conscious animals. Then, OMACOR® or vehicle (olive oil) was daily administrated orally by gavage using a single administration of 100 mg/kg. 2.3. Histological Analysis and Immmunochemistry Immediately after the sacrifice, the heart was rapidly ex- cised and fixed with AFA (alcohol, formaldehyde, ace- tate) for 1 - 4 days. The ventricles were cut into five cross-sectional samples of 2 mm each. The five regions were then processed into paraffin with an automated tis- sue processor. The samples were then embedded into fresh paraffin with the apical side down. From the third block of tissue, a 3 µm section was cut and was used for Masson staining. The stained slide was then hydrated with distilled water and then incubated twice with 100% ethanol (for 1 min each). Masson stainings were used to quantitate interstitial collagen volume fractions as well as infarct sizes using a video image analysis system (LEICA QWIN, LEICA Imaging Systems Ltd., Cambridge, England). A color vi- deo camera (DXC-390P color videocamera, SONY, Paris, France) relayed the image to a computer through LEICA analysis software application. The following parameters were measured (in mm): septal wall, left ventricular free wall, endocardial and epicardial left ventricular circum- ference and endocardial and epicardial infarcts. The green color extraction was used to quantitate interstitial colla- gen. The equation used to calculate infarct size was the following: percent infarct of left ventricle = [epicardial infarct (in mm) + endocardial infarct (in mm)]/[LV epi- cardial circumference (in mm) + LV endocardial circum- ference (in mm)] × 100 (Sandmann et al., 2001). The phosphorylation of connexion 43 (Cx43) at inter- calated disks of atrial myocardium was determined by im- munofluorescence. After the sacrifice, the atria were col- lected and immediately immerged in Formalin solution (Sigma-Aldrich, HT50-1-2) for 10 - 15 minutes at room temperature. They were then washed three times for 10 minutes in tyrode buffer, mounted in embedding medium (Miles), frozen in isopentane precooled in liquid nitrogen, and stored at –80˚C. Immunostaining was performed on 7-µm-thick cross-sections. Tissue sections were perme- abilized and saturated with 0.5% Triton X-100, 1% BSA and 10% of goat, chicken and human serum in phosphate buffered saline (PBS) for 60 minutes. They were then labeled with mouse antibody to α-actinin (1:400, Zymed) or rabbit phosphospecific antibodies to P-Ser368-Cx43 (1:50, Life Technologies) in PBS containing 1% BSA, 0.5% Triton X-100 and 3% of goat, chicken and human serum for at least 2 h at room temperature. After washing in PBS, Alexa 488-conjugated chicken anti-mouse IgG and/or Alexa 594-conjugated goat anti-rabbit IgG (1:400, Life technologies) were added with 1% BSA and 3% of goat, chicken and human serum in PBS for 1 hour. After washing in PBS, coverslips were mounted with Dako fluorescent mounting medium. For the quantification of Cx43 phosphorylation, the images were captured with Olympus BX 50 microscope and DP50 camera with mag- nification 200 and a similar time exposure for all the atrial cross-sections studied. Treatment of images and quantification of phospho-Cx43 labeling at the interca- lated disks were performed with NIH Image J software program. The mean fluorescence intensity was counted on 10 intercalated structures by field and on 6 fields for each rat. Images of Figure 1(a) were captured at magnification 600 and similar time exposure with an Olympus IX 50 microscope and a Roper Scientific camera. Images were automatically collected at 0.2 μm Z-intervals with a pie- zoelectric translator (PIFOC, Karlsruhe/Palmbach, Ger- many) driven by Metamorph Software (Universal Imag- ing Corp., Downingtown, PA). Each Z-series was decon- voluted automatically using a measured Point Spread Function and an adapted constrained interactive de-con- volution algorithm. Treatment of images was performed with the NIH Image J software program. Sets of three consecutive z-images were compiled and treated with Copyright © 2013 SciRes. OPEN ACCESS 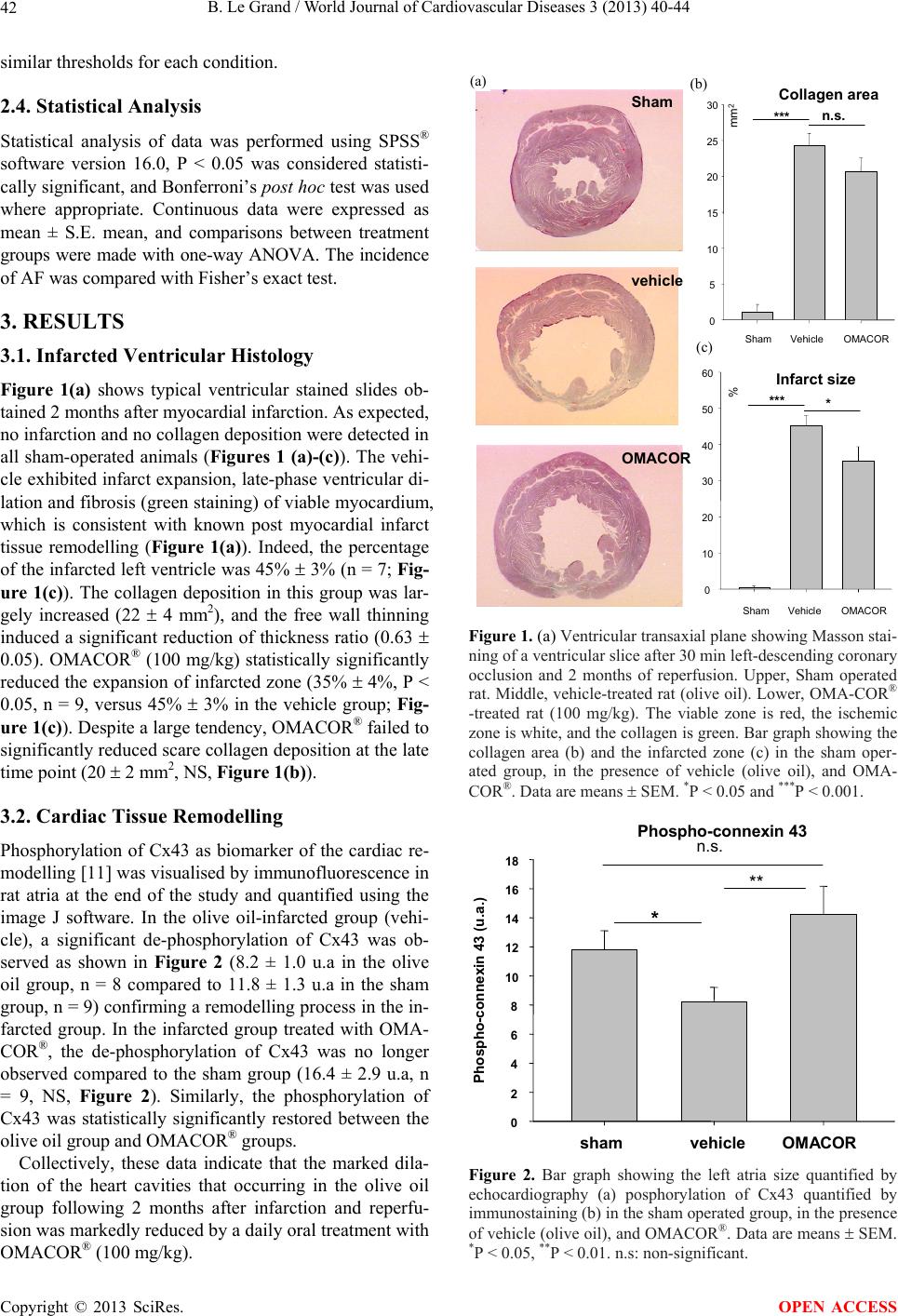 B. Le Grand / World Journal of Cardiovascular Diseases 3 (2013) 40-44 42 similar thresholds for each condition. 2.4. Statistical Analysis Statistical analysis of data was performed using SPSS® software version 16.0, P < 0.05 was considered statisti- cally significant, and Bonferroni’s post hoc test was used where appropriate. Continuous data were expressed as mean ± S.E. mean, and comparisons between treatment groups were made with one-way ANOVA. The incidence of AF was compared with Fisher’s exact test. 3. RESULTS 3.1. Infarcted Ventricular Histology Figure 1(a) shows typical ventricular stained slides ob- tained 2 months after myocardial infarction. As expected, no infarction and no collagen deposition were detected in all sham-operated animals (Figures 1 (a)-(c)). The vehi- cle exhibited infarct expansion, late-phase ventricular di- lation and fibrosis (green staining) of viable myocardium, which is consistent with known post myocardial infarct tissue remodelling (Figure 1(a)). Indeed, the percentage of the infarcted left ventricle was 45% 3% (n = 7; Fig- ure 1(c)). The collagen deposition in this group was lar- gely increased (22 4 mm2), and the free wall thinning induced a significant reduction of thickness ratio (0.63 0.05). OMACOR® (100 mg/kg) statistically significantly reduced the expansion of infarcted zone (35% 4%, P < 0.05, n = 9, versus 45% 3% in the vehicle group; Fig- ure 1(c)). Despite a large tendency, OMACOR® failed to significantly reduced scare collagen deposition at the late time point (20 2 mm2, NS, Figure 1(b)). 3.2. Cardiac Tissue Remodelling Phosphorylation of Cx43 as biomarker of the cardiac re- modelling [11] was visualised by immunofluorescence in rat atria at the end of the study and quantified using the image J software. In the olive oil-infarcted group (vehi- cle), a significant de-phosphorylation of Cx43 was ob- served as shown in Figure 2 (8.2 ± 1.0 u.a in the olive oil group, n = 8 compared to 11.8 ± 1.3 u.a in the sham group, n = 9) confirming a remodelling process in the in- farcted group. In the infarcted group treated with OMA- COR®, the de-phosphorylation of Cx43 was no longer observed compared to the sham group (16.4 ± 2.9 u.a, n = 9, NS, Figure 2). Similarly, the phosphorylation of Cx43 was statistically significantly restored between the olive oil group and OMACOR® groups. Collectively, these data indicate that the marked dila- tion of the heart cavities that occurring in the olive oil group following 2 months after infarction and reperfu- sion was markedly reduced by a daily oral treatment with OMACOR® (100 mg/kg). Sham Infarct size Collagen area C] OMACOR vehicle 0 10 20 30 40 50 60 % Sham VehicleOMACOR *** * 0 5 10 15 20 25 30 Sham VehicleOMACOR mm 2 *** n.s. (a) (b) (c) Figure 1. (a) Ventricular transaxial plane showing Masson stai- ning of a ventricular slice after 30 min left-descending coronary occlusion and 2 months of reperfusion. Upper, Sham operated rat. Middle, vehicle-treated rat (olive oil). Lower, OMA-COR® -treated rat (100 mg/kg). The viable zone is red, the ischemic zone is white, and the collagen is green. Bar graph showing the collagen area (b) and the infarcted zone (c) in the sham oper- ated group, in the presence of vehicle (olive oil), and OMA- COR®. Data are means SEM. *P < 0.05 and ***P < 0.001. shamvehicle OMACOR 0 2 4 6 8 10 12 14 16 18 Phos pho -co nn e xin43 (u.a.) * n.s. ** Phospho-connexin 43 Figure 2. Bar graph showing the left atria size quantified by echocardiography (a) posphorylation of Cx43 quantified by immunostaining (b) in the sham operated group, in the presence of vehicle (olive oil), and OMACOR®. Data are means SEM. *P < 0.05, **P < 0.01. n.s: non-significant. Copyright © 2013 SciRes. OPEN ACCESS  B. Le Grand / World Journal of Cardiovascular Diseases 3 (2013) 40-44 43 4. DISCUSSION The key finding of this study was that a 2-month treat- ment with OMACOR® led to a significant reduction in left ventricular remodelling implicated in the develop- ment of heart failure. To the best of our knowledge, no prior studies have demonstrated the long term effects of OMACOR®, alone on all of these parameters of heart founction. In the present rat model of ischemia-induced heart failure, OMACOR® (100 mg/kg) reduced the ex- pansion of the ventricular infarcted zone which was asso- ciated with a decrease of the heart remodelling and of the dephosphorylation of Cx43. The cardioprotective effects of n-3 PUFA appear to be due not through a single mode of action but to a syner- gism between multiple, intricate mechanisms that involve TG lowering, anti-inflammatory, inflammation-resolving, regulation of transcription factors and gene expression, membrane fluidity and antiarrhythmic and antithrombo- tic effects [12,13]. Both EPA and DHA components of OMACOR® have similar yet very distinctive cardiopro- tective properties. Only DHA seems to decrease blood pressure, heart rate and the number of total and small dense LDL particles. DHA also has higher potency to re- gulate the activity of several transcription factors than EPA [12,13]. The present results demonstrate that OMA- COR® (100 mg/kg) reduced the infarct size and induced a significant reduction of the ventricular dilation for 2 months after myocardial infarction. This cardioprotection mediated by OMACOR® was associated with a partial reduction of the collagen scar suggesting that the com- pound could preserve the remodelling of heart tissue con- secutively to myocardial infarction. Our findings that OMACOR® reduces left ventricular dilation after myo- cardial infarction are in agreement with previous findings demonstrating that PUFAs reduce heart failure induced by myocardial infarction in dog [14], as well as in rat with left ventricular pressure overload [13]. These protective effects of OMACOR® on post myocardial infarction in- duced ventricular dysfunction are associated with a de- crease of myocardial remodelling and alterations of Cx43 phosphorylation. Thus, two months after surgery, the in- farcted rats had a left ventricular dysfunction and an en- larged and fibrotic left ventricle. It has been previously shown that regression of the heart remodelling in treated myocardial infracted rats was associated with re-phos- phorylation and assembly of organized gap junction [11]. Thus, in the vehicle group, the large proportion of Cx43 was non-phosphorylated. Because the phosphorylation sites regulate channel properties, assembly and targeting in junctional plaques [15], the dephosphorylated Cx43 from remodelling heart is responsible for depressed cell- cell coupling [11]. Therefore, the reduction of a higher amount of non-phosphorylated Cx43 by OMACOR® and the redistribution of Cx43 suggest a re-organisation of junctional areas in heart tissue. Collectively, this cardio- protection of OMA-COR® against the dephosphorylation of Cx43 and the prevention of the atria and ventricle di- lations certainly contribute to cardioprotective properties against structural remodelling-induced heart failure. It is becoming increasingly clear from clinical and ani- mal studies that OMACOR® alters cardiac membrane pho- spholipid fatty acid composition, decreases the onset of new HF, and slows the progression of established HF [6, 7]. This effect is associated with decreased inflammation and improved resistance to arrhythmia incidence. That said, there has yet to be a definitive clinical trial with an appropriately high dose of OMACOR® (>3 g/d) or com- paring DHA to EPA in established HF. Definitive infor- mation on the optimal dose of OMACOR® is not avail- able; thus additional clinical trials are warranted. In summary, OMACOR® is a potent mixture of EPA + DHA with a high potential for the treatment of heart failure induced myocardial infarction. Indeed, the present results demonstrate that a long term treatment with OMACOR® reduces the ventricular dilation and the in- farcted size in experimental models of heart failure. The present study demonstrates that these anti-remodelling ef- fects are due at least in part by resynchronizing the gap junction activity beside the well-established cardiopro- tective mechanisms of the PUFAs. 5. ACKNOWLEDGEMENTS I am indebted to my colleagues in the Division of Cardiovascular Dis- eases II for performing the experimentations described in the present manuscript. I thank E. Dupeyron-Martel for secretarial assistance. REFERENCES [1] Bull, D.A., Bailey, S.H., Rentz, J.J., Zebrack, J.S., Lee, M., Litwin, S.E. and Kim, S.W. (2003) Effect of Terplex/ VEGF165 gene therapy on left ventricular function and structure following myocardial infarction. VEGF gene the- rapy for myocardial infarction. Journal of Control Re- lease, 93, 175-181. doi:10.1016/j.jconrel.2003.06.002 [2] Hu, N., Straub, C.M., Garzarelli, A.A., Sabey, K.H., Yock- man, J.W. and Bull, D.A. (2010) Ligation of the left cir- cumflex coronary artery with subsequent MRI and histo- pathology in rabbits. Journal of the American Association for Laboratory Animal Science, 49, 838-844. [3] Yockman, J.W., Kastenmeier, A., Erickson, H.M., Brum- bach, J.G., Whitten, M.G., Albanil, A., Li, D.Y., Kim, S.W. and Bull, D.A. (2008) Novel polymer carriers and gene constructs for treatment of myocardial ischemia and in- farction. Journal of Control Release, 132, 260-266. doi:10.1016/j.jconrel.2008.06.024 [4] Chen, J., Song, S.K., Liu, W., McLean, M., Allen, J.S., Tan, J., Wickline, S.A. and Yu, X. (2003) Remodelling of cardiac fiber structure after infarction in rats quantified with diffusion tensor MRI. American Journal of Physiol- Copyright © 2013 SciRes. OPEN ACCESS  B. Le Grand / World Journal of Cardiovascular Diseases 3 (2013) 40-44 Copyright © 2013 SciRes. 44 OPEN ACCESS ogy—Heart and Circulatory Physiology, 285, H946-H954. [5] Cleutjens, J.P., Blankesteijn, W.M., Daemen, M.J. and Smits, J.F. (1999) The infarcted myocardium: Simply dead tissue, or a lively target for therapeutic interventions? Cardiovascular Research, 44, 232-241. doi:10.1016/S0008-6363(99)00212-6 [6] Marchioli, R., Barzi, F., Bomba, E., Chieffo, C., Di Gre- gorio, D., Di Mascio, R., Franzosi, M.G., Geraci, E., Le- vantesi, G., Maggioni, A.P., Mantini, L., Marfisi, R.M., Mastrogiuseppe, G., Mininni, N., Nicolosi, G.L., Santini, M., Schweiger, C., Tavazzi, L., Tognoni, G., Tucci, C., Va- lagussa, F. and GISSI-Prevenzione Investigators (2002) Early protection against sudden death by n-3 polyunsatu- rated fatty acids after myocardial infarction: Time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Pre- venzione. Circulation, 105, 1897-903. doi:10.1161/01.CIR.0000014682.14181.F2 [7] Tavazzi, L., Maggioni, A.P., Marchioli, R., Barlera, S., Franzosi, M.G., Latini, R., Lucci, D., Nicolosi, G.L., Por- cu, M. and Tognoni, G. (2008) Effect of n-3 polyunsatu- rated fatty acids in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-con- trolled trial. Lancet, 372, 1223-1230. doi:10.1016/S0140-6736(08)61239-8 [8] Duda, M.K., O’Shea, K.M., Tintinu, A., Xu, W., Khairal- lah, R.J., Barrows, B.R., Chess, D.J., Azimzadeh, A.M., Harris, W.S., Sharov, V.G., Sabbah, H.N. and Stanley, W.C. (2009) Fish oil, but not flaxseed oil, decreases in- flammation and prevents pressure overload-induced car- diac dysfunction. Cardiovascular Research, 81, 319-327. doi:10.1093/cvr/cvn310 [9] Den Ruijter, H.M., Verkerk, A.O., Schumacher, C.A., Hou- ten, S.M., Belterman, C.N., Baartscheer, A., Brouwer, I.A., van Bilsen, M., de Roos, B. and Coronel, R. (2012) A diet rich in unsaturated fatty acids prevents progression toward heart failure in a rabbit model of pressure and vol- ume overload. Circulation: Heart Failure, 5, 376-384. doi:10.1161/CIRCHEARTFAILURE.111.963116 [10] Kromhout, D., Yasuda, S., Geleijnse, J.M. and Shimoka- wa, H. (2012) Fish oil and omega-3 fatty acids in cardio- vascular disease: Do they really work? European Heart Journal, 33, 436-443. doi:10.1093/eurheartj/ehr362 [11] Rucker-Martin, C., Milliez, P., Tan, S., Decrouy, X., Re- couvreur, M., Vranckx, R., Delcayre, C., Renaud, J.F., Du- nia, I., Segretain, D. and Hatem, S.N. (2006) Chronic he- modynamic overload of the atria is an important factor for gap junction remodelling in human and rat hearts. Cardiovascular Research, 72, 69-79. doi:10.1016/j.cardiores.2006.06.016 [12] Duda, M.K., O’Shea, K.M., Stanley, W.C. (2009) w-3 po- lyunsaturated fatty acid supplementation for the treatment of heart failure: Mechanisms and clinical potential. Car- diovascular Research, 84, 33-41. doi:10.1093/cvr/cvp169 [13] Adkins, Y. and Kelley, D.S. (2010) Mechanisms underly- ing the cardioprotective effects of omega-3 polyunsatura- ted fatty acids. The Journal of Nutritional Biochemistry, 21, 781-792. doi:10.1016/j.jnutbio.2009.12.004 [14] Billman, G.E., Nishijima, Y., Belevych, A.E., Terentyev, D., Xu, Y., Haizlip, K.M., Monasky, M.M., Hiranandani, N., Harris, W.S., Gyorke, S., Carnes, C.A. and Janssen, P.M.L. (2010) Effects of dietary omega-3 fatty acids on ventri- cular function in dogs with healed myocardial infarctions: in vivo and in vitro studies. American Journal of Physiol- ogy—Heart and Circulatory Physiology, 298, H1219- H1228. doi:10.1152/ajpheart.01065.2009 [15] Segretain, D. and Falk, M.M. (2004) Regulation of con- nexin biosynthesis, assembly, gap junction formation, and removal. Biochimica et Biophysica Acta, 1662, 3-21.
|