Y. N. FENG ET AL.
4
0.00 0.05 0.10 0.15
0.0
0.2
0.4
0.6
0.8
1.0
Residual percentage
Ca doping content
Ca doped BF O (5% Mn)
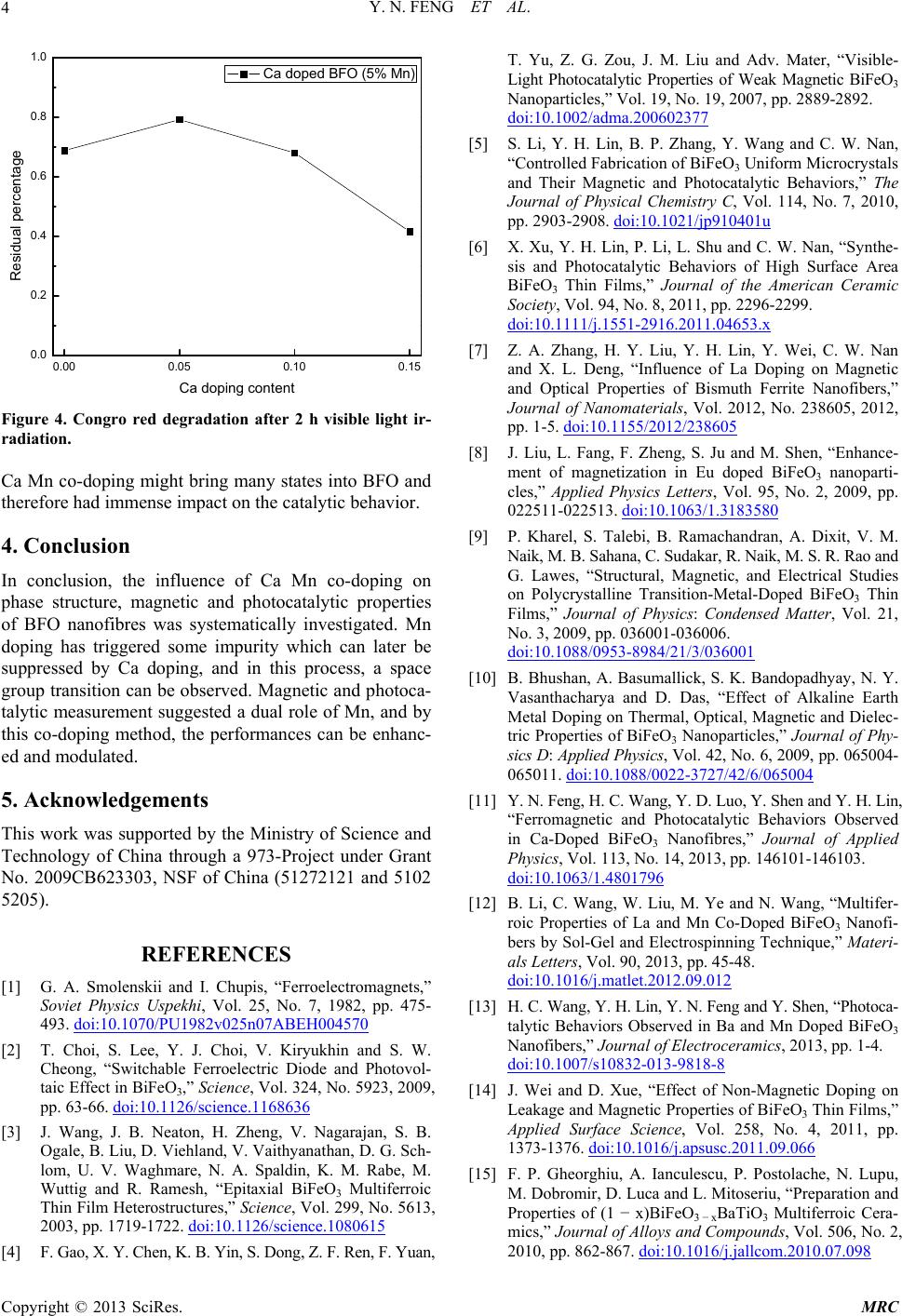
Figure 4. Congro red degradation after 2 h visible light ir-
radiation.
Ca Mn co-doping might bring many states into BFO and
therefore had immense impact on the catalytic behavior.
4. Conclusion
In conclusion, the influence of Ca Mn co-doping on
phase structure, magnetic and photocatalytic properties
of BFO nanofibres was systematically investigated. Mn
doping has triggered some impurity which can later be
suppressed by Ca doping, and in this process, a space
group transition can be observed. Magnetic and photoca-
talytic measurement suggested a dual role of Mn, and by
this co-doping method, the performances can be enhanc-
ed and modulated.
5. Acknowledgements
This work was supported by the Ministry of Science and
Technology of China through a 973-Project under Grant
No. 2009CB623303, NSF of China (51272121 and 5102
5205).
REFERENCES
[1] G. A. Smolenskii and I. Chupis, “Ferroelectromagnets,”
Soviet Physics Uspekhi, Vol. 25, No. 7, 1982, pp. 475-
493. doi:10.1070/PU1982v025n07ABEH004570
[2] T. Choi, S. Lee, Y. J. Choi, V. Kiryukhin and S. W.
Cheong, “Switchable Ferroelectric Diode and Photovol-
taic Effect in BiFeO3,” Science, Vol. 324, No. 5923, 2009,
pp. 63-66. doi:10.1126/science.1168636
[3] J. Wang, J. B. Neaton, H. Zheng, V. Nagarajan, S. B.
Ogale, B. Liu, D. Viehland, V. Vaithyanathan, D. G. Sch-
lom, U. V. Waghmare, N. A. Spaldin, K. M. Rabe, M.
Wuttig and R. Ramesh, “Epitaxial BiFeO3 Multiferroic
Thin Film Heterostructures,” Science, Vol. 299, No. 5613,
2003, pp. 1719-1722. doi:10.1126/science.1080615
[4] F. Gao, X. Y. Chen, K. B. Yin, S. Dong, Z. F. Ren, F. Yuan,
T. Yu, Z. G. Zou, J. M. Liu and Adv. Mater, “Visible-
Light Photocatalytic Properties of Weak Magnetic BiFeO3
Nanoparticles,” Vol. 19, No. 19, 2007, pp. 2889-2892.
doi:10.1002/adma.200602377
[5] S. Li, Y. H. Lin, B. P. Zhang, Y. Wang and C. W. Nan,
“Controlled Fabrication of BiFeO3 Uniform Microcrystals
and Their Magnetic and Photocatalytic Behaviors,” The
Journal of Physical Chemistry C, Vol. 114, No. 7, 2010,
pp. 2903-2908. doi:10.1021/jp910401u
[6] X. Xu, Y. H. Lin, P. Li, L. Shu and C. W. Nan, “Synthe-
sis and Photocatalytic Behaviors of High Surface Area
BiFeO3 Thin Films,” Journal of the American Ceramic
Society, Vol. 94, No. 8, 2011, pp. 2296-2299.
doi:10.1111/j.1551-2916.2011.04653.x
[7] Z. A. Zhang, H. Y. Liu, Y. H. Lin, Y. Wei, C. W. Nan
and X. L. Deng, “Influence of La Doping on Magnetic
and Optical Properties of Bismuth Ferrite Nanofibers,”
Journal of Nanomaterials, Vol. 2012, No. 238605, 2012,
pp. 1-5. doi:10.1155/2012/238605
[8] J. Liu, L. Fang, F. Zheng, S. Ju and M. Shen, “Enhance-
ment of magnetization in Eu doped BiFeO3 nanoparti-
cles,” Applied Physics Letters, Vol. 95, No. 2, 2009, pp.
022511-022513. doi:10.1063/1.3183580
[9] P. Kharel, S. Talebi, B. Ramachandran, A. Dixit, V. M.
Naik, M. B. Sahana, C. Sudakar, R. Naik, M. S. R. Rao and
G. Lawes, “Structural, Magnetic, and Electrical Studies
on Polycrystalline Transition-Metal-Doped BiFeO3 Thin
Films,” Journal of Physics: Condensed Matter, Vol. 21,
No. 3, 2009, pp. 036001-036006.
doi:10.1088/0953-8984/21/3/036001
[10] B. Bhushan, A. Basumallick, S. K. Bandopadhyay, N. Y.
Vasanthacharya and D. Das, “Effect of Alkaline Earth
Metal Doping on Thermal, Optical, Magnetic and Dielec-
tric Properties of BiFeO3 Nanoparticles,” Journal of Phy-
sics D: Applied Physics, Vol. 42, No. 6, 2009, pp. 065004-
065011. doi:10.1088/0022-3727/42/6/065004
[11] Y. N. Feng, H. C. Wang, Y. D. Luo, Y. Shen and Y. H. Lin,
“Ferromagnetic and Photocatalytic Behaviors Observed
in Ca-Doped BiFeO3 Nanofibres,” Journal of Applied
Physics, Vol. 113, No. 14, 2013, pp. 146101-146103.
doi:10.1063/1.4801796
[12] B. Li, C. Wang, W. Liu, M. Ye and N. Wang, “Multifer-
roic Properties of La and Mn Co-Doped BiFeO3 Nanofi-
bers by Sol-Gel and Electrospinning Technique,” Materi-
als Letters, Vol. 90, 2013, pp. 45-48.
doi:10.1016/j.matlet.2012.09.012
[13] H. C. Wang, Y. H. Lin, Y. N. Feng and Y. Shen, “Photoca-
talytic Behaviors Observed in Ba and Mn Doped BiFeO3
Nanofibers,” Journal of Electroceramics, 2013, pp. 1-4.
doi:10.1007/s10832-013-9818-8
[14] J. Wei and D. Xue, “Effect of Non-Magnetic Doping on
Leakage and Magnetic Properties of BiFeO3 Thin Films,”
Applied Surface Science, Vol. 258, No. 4, 2011, pp.
1373-1376. doi:10.1016/j.apsusc.2011.09.066
[15] F. P. Gheorghiu, A. Ianculescu, P. Postolache, N. Lupu,
M. Dobromir, D. Luca and L. Mitoseriu, “Preparation and
Properties of (1 − x)BiFeO3 − xBaTiO3 Multiferroic Cera-
mics,” Journal of Alloys and Compounds, Vol. 506, No. 2,
2010, pp. 862-867. doi:10.1016/j.jallcom.2010.07.098
Copyright © 2013 SciRes. MRC