 Pharmacology & Pharmacy, 2013, 4, 13-18 doi:10.4236/pp.2013.45A003 Published Online August 2013 (http://www.scirp.org/journal/pp) 13 Analysis of the 3' Variable Region of Cytotoxin-Associated Gene A (cagA) in Helicobacter pylori Isolates in China Boqing Li*#, Donglong Du*, Wanju Sun, Qizhi Cao, Zhen Zhang, Zhenzhen Du Department of Pathogen Biology, Binzhou Medical College, Binzhou, China. Email: #sdliboqing@163.com Received May 14th, 2013; revised June 25th, 2013; accepted July 4th, 2013 Copyright © 2013 Boqing Li et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Infection by the bacterium Helicobacter pylori is a putative cause of various gastric disorders, including gastric adeno- carcinoma. Incident rates are associated with variants of the H. pylori virulence factor cytotoxin-associated gene A pro- tein (CagA), encoded by the gene cagA. However, these variants have not been characterized in China, where gastric cancer is common. We investigated the diversity of CagA variants in H. pylori strains isolated from a Chinese popula- tion. The 3' variable region of cagA genes from 66 clinical isolates in China were amplified by polymerase chain reac- tion, sequenced, aligned, and analyzed. All 66 H. pylori strains were CagA-positive, of which 93.9% were East Asian type and the tyrosine phosphorylation motifs (TPMs) were EPIYA-ABD. The remainder was Western type, in which TPMs were EPIYA-ABC. Interestingly, two of sixty-two strains (3.2%) of the East Asian type were mutated into ESIYA-B, whereas all four Western type (100%) strains were mutated into EPIYT-B. Both of the two strains with Western-type CagA obtained from gastric cancer patients contained a distinguished mutation on the first residue fol- lowing the EPIYA site in the EPIYA-A motif. The predominant CagA type in these H. pylori strains isolated from Chi- nese patients in China was East Asian, with TPMs EPIYA-ABD, and there existed mutations in both the East Asian and Western type CagA. Keywords: Helicobacter pylori; CagA; 3' Variable Region; China; Gastritis; Gastric Cancer 1. Introduction Helicobacter pylori, a spiral microaerophilic gram-nega- tive bacterium, colonizes the stomachs of 50% or more people worldwide [1]. It is the causative agent of many peptic disorders, including gastritis, gastric and duodenal ulcers, and gastric mucosa-associated lymphoid tissue lymphoma. H. pylori infection is also a recognized risk factor for gastric adenocarcinoma [2,3]. However, not all people with H. pylori infections will suffer these dis- eases—about 30% of the population of Western countries is infected but the rate of gastric cancer is only 0.1% - 1%. In East Asian countries such as China, Japan, and Korea, the prevalence of H. pylori infection is 60% - 88%, and H. pylori-associated gastric cancers occur more frequently than in Western countries [4-6]. Many epide- miological studies have suggested that progression from H. pylori infection to adverse effects is controlled by combinations of genetic variants in the host, environ- mental factors, and H. pyl o ri gene polymorphisms [7-9]. An H. pylori virulence marker found in most isolates in high-risk areas of the world is a 40-kb cytotoxin-as- sociated gene (cag) pathogenicity island, which forms a type IV secretion system for delivering bacterial gene products into host gastric epithelial cells. One of these products is the oncoprotein CagA (encoded by the gene cagA), putatively responsible for most malignancies as- sociated with the bacterium [10]. CagA has a variable C- terminus containing structural tyrosine phosphorylation motifs (TPMs) within repetitive amino acid sequences of Glu-Pro-Ile-Tyr-Ala (EPIYA) [11]. There are four known EPIYA motifs (EPIYA-A, EPIYA-B, EPIYA-C and EPI YA-D), differentiated by specific flanking amino acids [12,13]. Two types of CagA, Western and East Asian, have been described. Western CagA has EPIYA-A and EPI YA-B segments followed by (1 to 3) EPIYA-C segments. In the East Asian form, a EPIYA-D segment substitutes for the EPIYA-C segments of the Western CagA. When a CagA molecule is injected into the host cell, its tyrosi- nes are phosphorylated and it binds to the N- and C-SH2 *Contributed equally. #Corres ondin autho . Copyright © 2013 SciRes. PP  Analysis of the 3' Variable Region of Cytotoxin-Associated Gene A (cagA) in Helicobacter pylori Isolates in China 14 domains of a SHP-2 tyrosine phosphatase of the host, initiating a cascade of abnormal enzymatic responses and changes in cellular phenotype. The greater binding affin- ity of East Asian CagA to SHP-2 makes East Asian CagA more virulent than the Western type; the degree of pathogenicity of Western CagA is associated with the number of EPIYA-C motifs [12-15]. Variations in the TPMs of the 3’ region of CagA have been reported in Western countries, and these variations are associated with different peptic disorders. Yet to the best of our knowledge, there have been no systematic studies of CagA variants in China or their association with diseases, although China is representative of the East Asian area and gastric cancer is common [16], with incidence rates in Shanghai (the latest statistics available) reported at 34.2 and 17.3 per 100,000 men and women, respectively [17]. It is not known if the EPIYA motifs of CagA in Chinese H. pylori strains are East Asian, or whether TPM types in China are associated with gastric cancer. In this study, we investigated the diversity of CagA found in H. pylori strains isolated from a Chinese popu- lation. We amplified the 3' region of cagA genes from 66 H. pylori clinical isolates, and then sequenced, aligned and compared the amino acid sequences of correspond- ing regions. 2. Materials and Methods 2.1. Patients Gastric tissue biopsy samples from the antrum were ob- tained via endoscopy of 66 patients (46 men and 20 women; mean age 49.8 y, range 20 - 82 y) at three hos- pitals in Shandong, China. These patients were diagnosed with gastritis (n = 31), gastric ulcer (n = 11), duodenal ulcer (n = 6), or gastric cancer (n = 18), respectively. 2.2. Strain Culture and DNA Extraction To examine the gastric biopsy specimens for the pres- ence of H. pylori, the tissues were ground and then cul- tured on Campylobacter agar base plates containing 10% sheep blood and vancomycin (6 μg/mL), trimethoprim (5 μg/mL), polymyxin B (4 μg/mL), amphotericin B (2.5 μg/mL) under microaerophilic conditions (5% O2, 10% CO2, 85% N2) at 37˚C for 72 h. The bacteria were har- vested and genomic DNA was extracted using a QIAamp DNA mini kit (Qiagen, Hilden, Germany) in accordance with the instructions of the manufacturer. 2.3. Detection of CagA and EPIYA Motifs by PCR The 3' variable region and TPMs of cagA were amplified by PCR using the primers shown in Table 1. All the primers were designed using “Primer Premier 5.0” soft- Table 1. PCR primers and the expected size of PCR prod- ucts. Primer Sequence (5’-3’) Size (bp) cagF CCTAGTCGGTAATGGGTTAT cagRa TTAATGCGTGTGTGGCTG 659 cagAa CTTGTCCTGTTTTCTTTTTATT 172 cagBa GCATTTACCTTTTTAGCAACT 224 cagCa CCGAGATCATCAATCGTAG 380 cagDa TGCTTGATTTGCCTCATC 396 aWith the forward primer cagF. ware based on the homologous nucleotide sequence of cagA gene among strains ATCC26695 and F32, repre- sentative strains of Western and East Asian types, pub- lished on the PubMed. Primers cagF and cagR were used to amplify the full-length of the 3' variable region; prim- ers cagA, cagB, cagC and cagD together with cagF were used to detect the EPIYA-A, -B, -C and -D motifs, re- spectively. A reaction mixture contained 2.5 μL of each primer, 5 μL of genomic DNA, 25 μL 2 × Taq PCR MasterMix, and ddH2O to a total volume of 50 μL. PCR conditions consisted of pre-denaturation for 5 min at 95˚C; 30 cy- cles of 30 s at 94˚C, 30 s at 52˚C, and 1 min at 72˚C; and a final extension for 5 min at 72˚C. Five μL of each PCR product was then resolved via electrophoresis on a 1% agarose gel and observed under UV light after staining with ethidium bromide. 2.4. Alignment and Analysis of the Amino Acid Sequences The PCR products of the cagF and cagR primers were sequenced by Shanghai Majorbio. Alignment and analy- sis of the sequences in the 3' variable region were carried out using DNAStar 5.0 software. 3. Results The gene amplification and sequencing protocols were successful, and all the H. pylori strains detected in this study were cagA-positive (cagA+). Representative PCR products are shown in Figures 1(a) and (b). The prevalence of East Asian CagA (62/66, 93.9%,) was significantly higher than that of Western CagA (4/66, 6.1%; Table 2). Among the strains isolated from gastric cancer patients specifically, 88.9% (16/18) were East Asian CagA, and 11.1% (2/18) were Western CagA, while the strains from the remaining patients were 95.8% (46/48) East Asian and 4.2% (2/48) Western. All the TPMs in East Asian-type strains were EPIYA-ABD, while all the Western-type were EPIYA-ABC. No EPIYA-ABCC or -ABCCC TPMs were found. Interest- ingly, 3.2% of the East Asian-type strains (2/62, gastritis Copyright © 2013 SciRes. PP 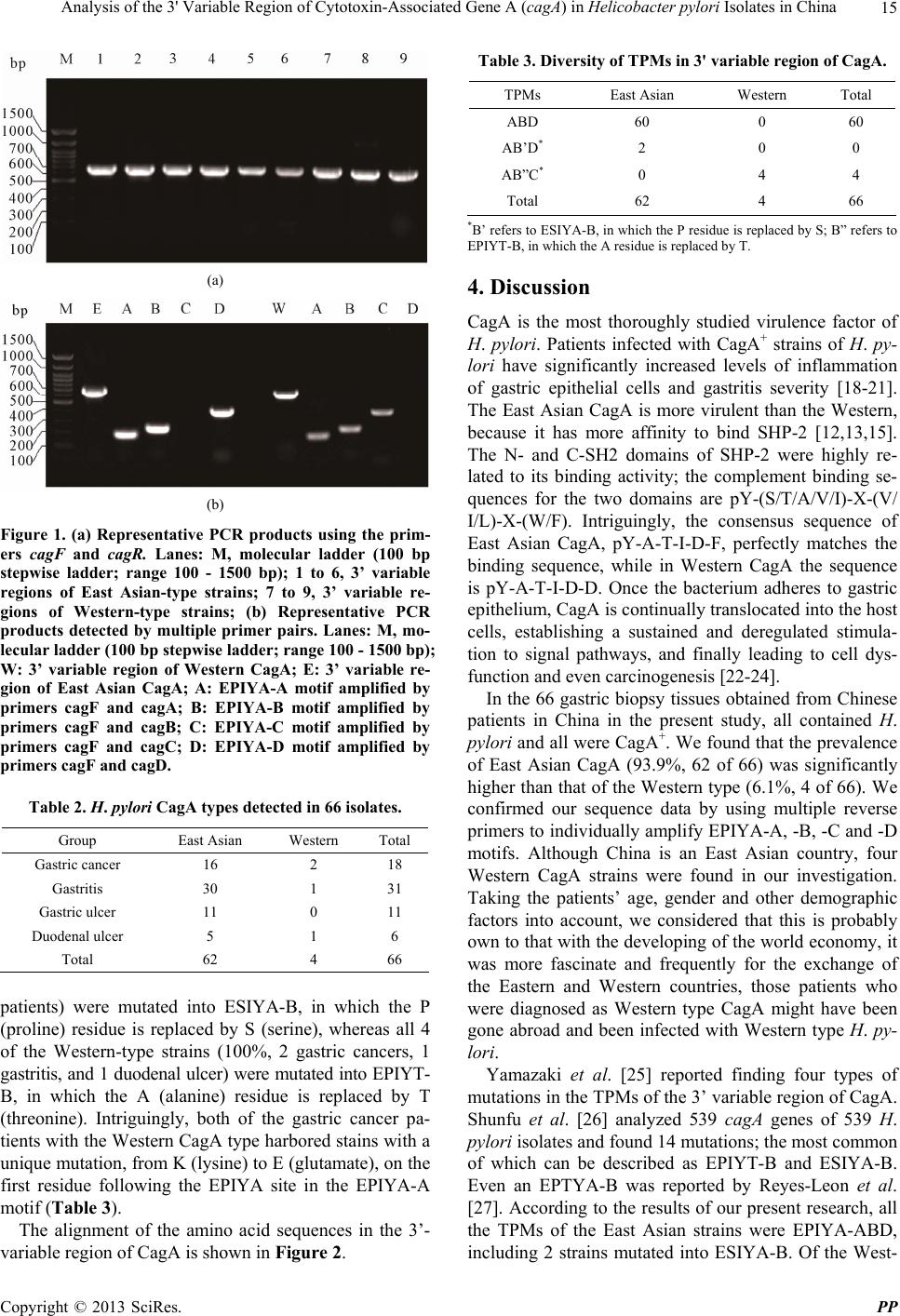 Analysis of the 3' Variable Region of Cytotoxin-Associated Gene A (cagA) in Helicobacter pylori Isolates in China 15 (a) (b) Figure 1. (a) Representative PCR products using the prim- ers cagF and cagR. Lanes: M, molecular ladder (100 bp stepwise ladder; range 100 - 1500 bp); 1 to 6, 3’ variable regions of East Asian-type strains; 7 to 9, 3’ variable re- gions of Western-type strains; (b) Representative PCR products detected by multiple primer pairs. Lanes: M, mo- lecular ladder (100 bp stepwise ladder; range 100 - 1500 bp); W: 3’ variable region of Western CagA; E: 3’ variable re- gion of East Asian CagA; A: EPIYA-A motif amplified by primers cagF and cagA; B: EPIYA-B motif amplified by primers cagF and cagB; C: EPIYA-C motif amplified by primers cagF and cagC; D: EPIYA-D motif amplified by primers cagF and cagD. Table 2. H. pylori CagA types detected in 66 isolates. Group East Asian Western Total Gastric cancer 16 2 18 Gastritis 30 1 31 Gastric ulcer 11 0 11 Duodenal ulcer 5 1 6 Total 62 4 66 patients) were mutated into ESIYA-B, in which the P (proline) residue is replaced by S (serine), whereas all 4 of the Western-type strains (100%, 2 gastric cancers, 1 gastritis, and 1 duodenal ulcer) were mutated into EPIYT- B, in which the A (alanine) residue is replaced by T (threonine). Intriguingly, both of the gastric cancer pa- tients with the Western CagA type harbored stains with a unique mutation, from K (lysine) to E (glutamate), on the first residue following the EPIYA site in the EPIYA-A motif (Table 3). The alignment of the amino acid sequences in the 3’- variable region of CagA is shown in Figure 2. Table 3. Diversity of TPMs in 3' variable region of CagA. TPMs East Asian Western Total ABD 60 0 60 AB’D* 2 0 0 AB”C* 0 4 4 Total 62 4 66 *B’ refers to ESIYA-B, in which the P residue is replaced by S; B” refers to EPIYT-B, in which the A residue is replaced by T. 4. Discussion CagA is the most thoroughly studied virulence factor of H. pylori. Patients infected with CagA+ strains of H. py- lori have significantly increased levels of inflammation of gastric epithelial cells and gastritis severity [18-21]. The East Asian CagA is more virulent than the Western, because it has more affinity to bind SHP-2 [12,13,15]. The N- and C-SH2 domains of SHP-2 were highly re- lated to its binding activity; the complement binding se- quences for the two domains are pY-(S/T/A/V/I)-X-(V/ I/L)-X-(W/F). Intriguingly, the consensus sequence of East Asian CagA, pY-A-T-I-D-F, perfectly matches the binding sequence, while in Western CagA the sequence is pY-A-T-I-D-D. Once the bacterium adheres to gastric epithelium, CagA is continually translocated into the host cells, establishing a sustained and deregulated stimula- tion to signal pathways, and finally leading to cell dys- function and even carcinogenesis [22-24]. In the 66 gastric biopsy tissues obtained from Chinese patients in China in the present study, all contained H. pylori and all were CagA+. We found that the prevalence of East Asian CagA (93.9%, 62 of 66) was significantly higher than that of the Western type (6.1%, 4 of 66). We confirmed our sequence data by using multiple reverse primers to individually amplify EPIYA-A, -B, -C and -D motifs. Although China is an East Asian country, four Western CagA strains were found in our investigation. Taking the patients’ age, gender and other demographic factors into account, we considered that this is probably own to that with the developing of the world economy, it was more fascinate and frequently for the exchange of the Eastern and Western countries, those patients who were diagnosed as Western type CagA might have been gone abroad and been infected with Western type H. py- lori. Yamazaki et al. [25] reported finding four types of mutations in the TPMs of the 3’ variable region of CagA. Shunfu et al. [26] analyzed 539 cagA genes of 539 H. pylori isolates and found 14 mutations; the most common of which can be described as EPIYT-B and ESIYA-B. Even an EPTYA-B was reported by Reyes-Leon et al. [27]. According to the results of our present research, all the TPMs of the East Asian strains were EPIYA-ABD, including 2 strains mutated into ESIYA-B. Of the West- Copyright © 2013 SciRes. PP 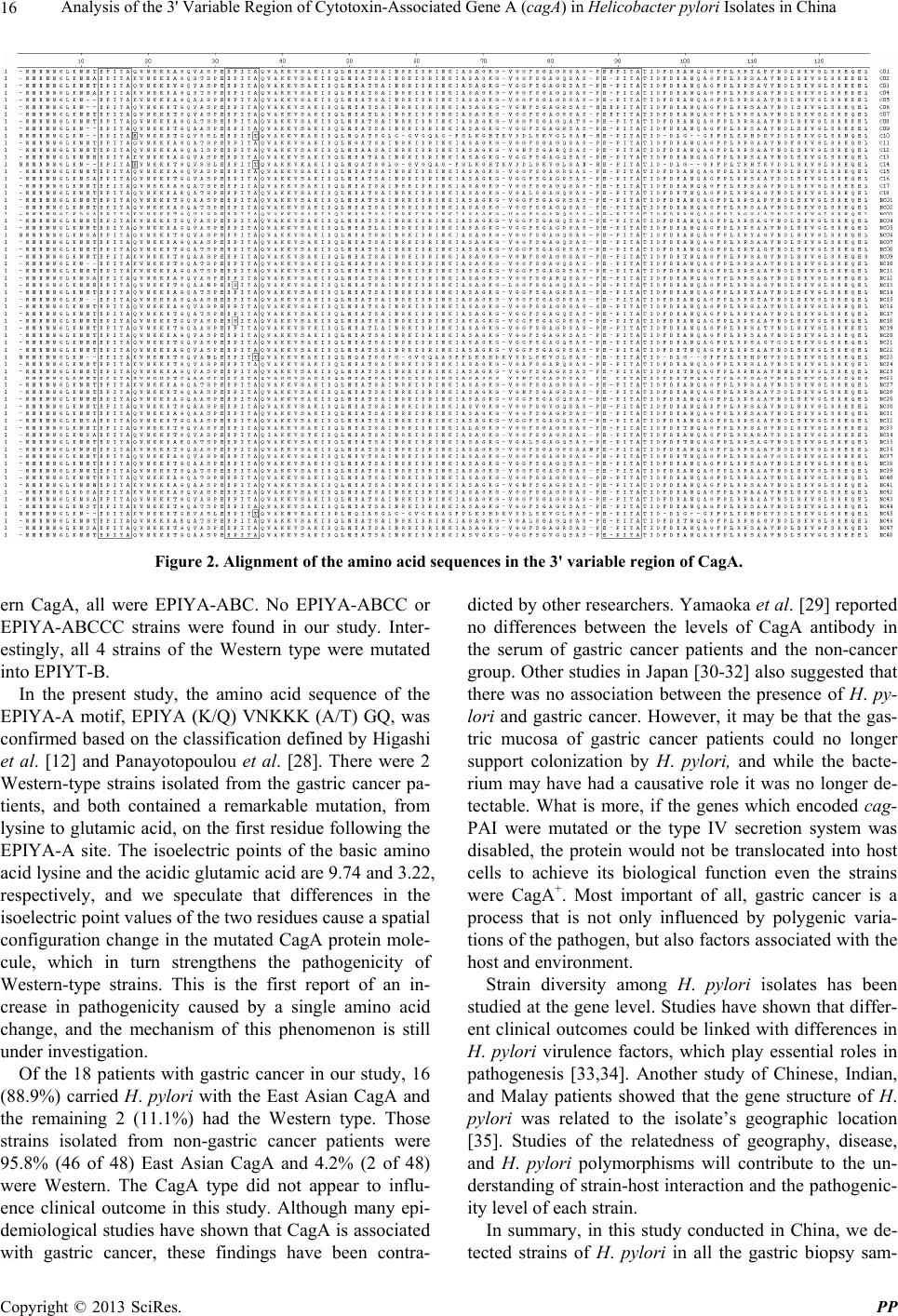 Analysis of the 3' Variable Region of Cytotoxin-Associated Gene A (cagA) in Helicobacter pylori Isolates in China Copyright © 2013 SciRes. PP 16 Figure 2. Alignment of the amino acid sequences in the 3' variable region of CagA. ern CagA, all were EPIYA-ABC. No EPIYA-ABCC or EPIYA-ABCCC strains were found in our study. Inter- estingly, all 4 strains of the Western type were mutated into EPIYT-B. In the present study, the amino acid sequence of the EPIYA-A motif, EPIYA (K/Q) VNKKK (A/T) GQ, was confirmed based on the classification defined by Higashi et al. [12] and Panayotopoulou et al. [28]. There were 2 Western-type strains isolated from the gastric cancer pa- tients, and both contained a remarkable mutation, from lysine to glutamic acid, on the first residue following the EPIYA-A site. The isoelectric points of the basic amino acid lysine and the acidic glutamic acid are 9.74 and 3.22, respectively, and we speculate that differences in the isoelectric point values of the two residues cause a spatial configuration change in the mutated CagA protein mole- cule, which in turn strengthens the pathogenicity of Western-type strains. This is the first report of an in- crease in pathogenicity caused by a single amino acid change, and the mechanism of this phenomenon is still under investigation. Of the 18 patients with gastric cancer in our study, 16 (88.9%) carried H. pylori with the East Asian CagA and the remaining 2 (11.1%) had the Western type. Those strains isolated from non-gastric cancer patients were 95.8% (46 of 48) East Asian CagA and 4.2% (2 of 48) were Western. The CagA type did not appear to influ- ence clinical outcome in this study. Although many epi- demiological studies have shown that CagA is associated with gastric cancer, these findings have been contra- dicted by other researchers. Yamaoka et al. [29] reported no differences between the levels of CagA antibody in the serum of gastric cancer patients and the non-cancer group. Other studies in Japan [30-32] also suggested that there was no association between the presence of H. py- lori and gastric cancer. However, it may be that the gas- tric mucosa of gastric cancer patients could no longer support colonization by H. pylori, and while the bacte- rium may have had a causative role it was no longer de- tectable. What is more, if the genes which encoded cag- PAI were mutated or the type IV secretion system was disabled, the protein would not be translocated into host cells to achieve its biological function even the strains were CagA+. Most important of all, gastric cancer is a process that is not only influenced by polygenic varia- tions of the pathogen, but also factors associated with the host and environment. Strain diversity among H. pylori isolates has been studied at the gene level. Studies have shown that differ- ent clinical outcomes could be linked with differences in H. pylori virulence factors, which play essential roles in pathogenesis [33,34]. Another study of Chinese, Indian, and Malay patients showed that the gene structure of H. pylori was related to the isolate’s geographic location [35]. Studies of the relatedness of geography, disease, and H. pylori polymorphisms will contribute to the un- derstanding of strain-host interaction and the pathogenic- ity level of each strain. In summary, in this study conducted in China, we de- tected strains of H. pylori in all the gastric biopsy sam-  Analysis of the 3' Variable Region of Cytotoxin-Associated Gene A (cagA) in Helicobacter pylori Isolates in China 17 ples, all were CagA positive, and the prevalence of the East Asian CagA was considerably higher than that of the Western CagA. There was not sufficient evidence to indicate that the type of TPM was related to different clinical outcomes. EPIYA-ABD and EPIYA-ABC were the most common types found, and mutations existed in both the East Asian- and Western-type CagA. 5. Acknowledgements This report is based on work supported in part by grants from the NSFC (81072429), Program for New Century Excellent Talents in University (NCET-11-1026), and grants-in-aid for Shandong Province Outstanding Young Scientist Research Award Fund (2010BSB140), Colleges and universities in Shandong province science and tech- nology projects (J10LF21) and Yantai Municipal Science and Technology Development Program (2010172). REFERENCES [1] A. Covacci, J. L. Telford, G. Del Giudice, J. Parsonnet and R. Rappuoli, “Helicobacter pylori Virulence and Ge- netic Geography,” Science, Vol. 284, No. 5418, 1999, pp. 1328-1333.doi:10.1126/science.284.5418.1328 [2] J. C. Atherton, “The Pathogenesis of Helicobacter pylori- Induced Gastro-Duodenal Diseases,” Annual Review of Pathology, Vol. 1, 2006, pp. 63-96. doi:10.1146/annurev.pathol.1.110304.100125 [3] M. J. Blaser, “Helicobacter pylori and Gastric Diseases,” British Medical Journal, Vol. 316, No. 7143, 1998, pp. 1507-1510. doi:10.1136/bmj.316.7143.1507 [4] E. Ogorodnik and R. D. Raffaniello, “Analysis of the 3′-Variable Region of the cagA Gene from Helicobacter pylori Strains Infecting Patients at New York City Hospi- tals,” Microbial Pathogenesis, Vol. 56, 2012, pp. 29-34. doi:10.1016/j.micpath.2012.10.003 [5] C. Chomvarin, K. Phusri, K. Sawadpanich, P. Mairiang, W. Namwat, C. Wongkham and C. Hahnvajanawong, “Prevalence of CagA EPIYA Motifs in Helicobacter py- lori among Dyspeptic Patients in Northeast Thailand,” The Southeast Asian Journal of Tropical Medicine and Public Health, Vol. 43, No. 1, 2012, pp. 105-115. [6] L. Shokrzadeh, K. Baghaei, Y. Yamaoka, H. Dabiri, F. Jafari, N. Sahebekhtiari, A. Tahami, M. Sugimoto, H. Zojaji and M. R. Zali, “Analysis of 3′-End Variable Re- gion of the CagA Gene in Helicobacter pylori Isolated from Iranian Population,” Journal of Gastroenterology and Hepatology, Vol. 25, No. 1, 2010, pp. 172-177. doi:10.1111/j.1440-1746.2009.05979.x [7] S. Jang, K. R. Jones, C. H. Olsen, Y. M. Joo, Y. J. Yoo, I. S. Chung, J. H. Cha and D. S. Merrell, “Epidemiological Link between Gastric Disease and Polymorphisms in Vac A and CagA,” Journal of Clinical Microbiology, Vol. 48, No. 2, 2010, pp. 559-567. doi:10.1128/JCM.01501-09 [8] S. Kumar, A. Kumar and V. K. Dixit, “Diversity in the Cag Pathogenicity Island of Helicobacter pylori Isolates in populations from North and South India,” Journal of Medical Microbiology, Vol. 59, No. 1, 2010, pp. 32-40. doi:10.1099/jmm.0.013763-0 [9] S. Satomi, A. Yamakawa, S. Matsunaga, R. Masaki, T. Inagaki, T. Okuda, H. Suto, Y. Ito, Y. Yamazaki, M. Ku- riyama, Y. Keida, H. Kutsumi and T. Azuma, “Relation- ship between the Diversity of the cagA Gene of Helico- bacter pylori and Gastric Cancer in Okinawa, Japan,” Journal of Gastroenterology, Vol. 41, No. 7, 2006, pp. 668-673. doi:10.1007/s00535-006-1838-6 [10] S. Odenbreit, J. Püls, B. Sedlmaier, E. Gerland, W. Fis- cher and R. Haas, “Translocation of Helicobacter pylori CagA into Gastric Epithelial Cells by Type IV Secre- tion,” Science, Vol. 287, No. 5457, 2000, pp. 1497-1500. doi:10.1126/science.287.5457.1497 [11] R. H. Argent, Y. Zhang and J. C.Atherton, “Simple Me- thod for Determination of the Number of Helicobacter pylori CagA Variable-Region EPIYA Tyrosine Phospho- rylation Motifs by PCR,” Journal of Clinical Microbiol- ogy, Vol. 43, No. 2, 2005, pp. 791-795. doi:10.1128/JCM.43.2.791-795.2005 [12] H. Higashi, R. Tsutsumi, A. Fujita, S. Yamazaki, M. Asaka, T. Azuma and M. Hatakeyama, “Biological Activ- ity of the Helicobacter pylori Virulence Factor CagA Is Determined by Variation in the Tyrosine Phosphorylation Sites,” Proceedings of the National Academy of Sciences of the USA, Vol. 99, No. 22, 2002, pp. 14428-14433. doi:10.1073/pnas.222375399 [13] M. Hatakeyama, “Oncogenic Mechanisms of the Helico- bacter pylori CagA Protein,” Nature Reviews Cancer, Vol. 4, No. 9, 2004, pp. 688-694. doi:10.1038/nrc1433 [14] R. M. Ferreira, J. C. Machado, M. Leite, F. Carneiro and C. Figueiredo, “The Number of Helicobacter pylori CagA EPIYA C Tyrosine Phosphorylation Motifs Influences the Pattern of Gastritis and the Development of Gastric Carci- noma,” Histopathology, Vol. 60, No. 6, 2012, pp. 992- 998. doi:10.1111/j.1365-2559.2012.04190.x [15] D. De Souza, L. J. Fabri, A. Nash, D. J. Hilton, N. A. Nicola and M. Baca, “SH2 Domains from Suppressor of Cytokine Signaling-3 and Protein Tyrosine Phosphatase SHP-2 Have Similar Binding Specificities,” Biochemistry, Vol. 41, No. 29, 2002, pp. 9229-9236. doi:10.1021/bi0259507 [16] Z. J. Pan, R. W. van der Hulst, M. Feller, S. D. Xiao, G. N. Tytgat, J. Dankert and A. van der Ende, “Equally High Prevalences of Infection with CagA-Positive Helicobac- ter pylori in Chinese Patients with Peptic Ulcer Disease and Those with Chronic Gastritis-Associated Dyspepsia,” Journal of Clinical Microbiology, Vol. 35, No. 6, 1997, pp. 1344-1347. [17] P. Bertuccio, L. Chatenoud, F. Levi, D. Praud, J. Ferlay, E. Negri, M. Malvezzi and C. La Vecchia, “Recent Pat- terns in Gastric Cancer: A Global Overview,” Interna- tional Journal of Cancer, Vol. 125, No. 3, 2009, pp. 666- 673. doi:10.1002/ijc.24290 [18] R. M. Peek Jr., S. F. Moss, K. T. Tham, G. I. Pérez-Pérez, S. Wang, G. G. Miller, J. C. Atherton, P. R. Holt and M. J. Blaser, “Helicobacter pylori CagA+ Strains and Dissocia- Copyright © 2013 SciRes. PP  Analysis of the 3' Variable Region of Cytotoxin-Associated Gene A (cagA) in Helicobacter pylori Isolates in China Copyright © 2013 SciRes. PP 18 tion of Gastric Epithelial Cell Proliferation from Apopto- sis,” Journal of the National Cancer Institute, Vol. 89, No. 12, 1997, pp. 863-868. doi:10.1093/jnci/89.12.863 [19] H. Higashi, K. Yokoyama, Y. Fujii, S. Ren, H. Yuasa, I. Saadat, N. Murata-Kamiya, T. Azuma and M. Hatake- yama, “EPIYA Motif Is a Membrane-Targeting Signal of Helicobacter pylori Virulence Factor CagA in Mammal- ian Cells,” Journal of Biological Chemistry, Vol. 280, No. 24, 2005, pp. 23130-23137. doi:10.1074/jbc.M503583200 [20] M. Naito, T. Yamazaki, R. Tsutsumi, H. Higashi, K. Onoe, S. Yamazaki, T. Azuma and M. Hatakeyama, “In- fluence of EPIYA-Repeat Polymorphism on the Phos- phorylation-Dependent Biological Activity of Helicobac- ter pylori CagA,” Gastroenterology, Vol. 130, No. 4, 2006, pp. 1181-1190. doi:10.1053/j.gastro.2005.12.038 [21] M. Suzuki, H. Mimuro, K. Kiga, M. Fukumatsu, N. Ishi- jima, H. Morikawa, S. Nagai, S. Koyasu, R. H. Gilman, D. Kersulyte, D. E. Berg and C. Sasakawa, “Helicobacter pylori CagA Phosphorylation-Independent Function in Epithelial Proliferation and Inflammation,” Cell Host & Microbe, Vol. 5, No. 1, 2009, pp. 23-34. doi:10.1016/j.chom.2008.11.010 [22] M. Asahi, T. Azuma, S. Ito, Y. Ito, H. Suto, Y. Nagai, M. Tsubokawa, Y. Tohyama, S. Maeda, M. Omata, T. Su- zuki and C. Sasakawa, “Helicobacter pylori CagA Protein Can Be Tyrosine Phosphorylated in Gastric Epithelial Cells,” The Journal of Experimental Medicine, Vol. 191, No. 4, 2000, pp. 593-602. doi:10.1084/jem.191.4.593 [23] T. Azuma, S. Yamazaki, A. Yamakawa, M. Ohtani, A. Muramatsu, H. Suto, Y. Ito, M. Dojo, Y. Yamazaki, M. Kuriyama, Y. Keida, H. Higashi and M. Hatakeyama, “Association between Diversity in the Src Homology 2 Domain-Containing Tyrosine Phosphatase Binding Site of Helicobacter pylori CagA Protein and Gastric Atroph and Cancer,” The Journal of Infectious Diseases, Vol. 189, No. 5, 2004, pp. 820-827. doi:10.1086/381782 [24] H. Higashi, R. Tsutsumi, S. Muto, T. Sugiyama, T. Azu- ma, M. Asaka and M. Hatakeyama, “SHP-2 Tyrosine Phosphatase as an Intracellular Target of Helicobacter py- lori CagA Protein,” Science, Vol. 295, No. 5555, 2002, pp. 683-686. doi:10.1126/science.1067147 [25] S. Yamazaki, A. Yamakawa, T. Okuda, M. Ohtani, H. Suto, Y. Ito, Y. Yamazaki, Y. Keida, H. Higashi, M. Ha- takeyama and T. Azuma, “Distinct Diversity of vacA, cagA, and cagE Genes of Helicobacter pylori Associated with Peptic Ulcer in Japan,” Journal of Clinical Microbi- ology, Vol. 43, No. 8, 2005, pp. 3906-3916. doi:10.1128/JCM.43.8.3906-3916.2005 [26] X. Shunfu, Z. Guoxin, S. Ruihua, H. Bo and M. Yi, “Po- lymorphism of Variable Regions of CagA Protein,” Chi- nese Journal of Gastroenterology, Vol. 12, No. 6, 2007, pp. 357-361. [27] A. Reyes-Leon, J. C. Atherton, R. H. Argent, J. L. Puente and J. Torres, “Heterogeneity in the Activity of Mexican Helicobacter pylori Strains in Gastric Epithelial Cells and Its Association with Diversity in the cagA Gene,” Infec- tion and Immunity, Vol. 75, No. 7, 2007, pp. 3445-3454. doi:10.1128/IAI.01951-06 [28] E. G. Panayotopoulou, D. N. Sgouras, K. Papadakos, A. Kalliaropoulos, G. Papatheodoridis, A. F. Mentis and A. J. Archimandritis, “Strategy to Characterize the Number and Type of Repeating EPIYA Phosphorylation Motifs in the Carboxyl Terminus of CagA Protein in Helicobacter pylori Clinical,” Journal of Clinical Microbiology, Vol. 45, No. 2, 2007, pp. 488-495. doi:10.1128/JCM.01616-06 [29] Y. Yamaoka, T. Kodama, K. Kashima and D. Y. Graham, “Antibody against Helicobacter pylori CagA and VacA and the Risk for Gastric Cancer,” Journal of Clinical Pa- thology, Vol. 52, No. 3, 1999, pp. 215-218. doi:10.1136/jcp.52.3.215 [30] S. Backert, T. Schwarz, S. Miehlke, C. Kirsch, C. Som- mer, T. Kwok, M. Gerhard, U. B. Goebel, N. Lehn, W. Koenig and T. F. Meyer, “Functional Analysis of the Cag Pathogenicity Island in Helicobacter pylori Isolates from Patients with Gastritis, Peptic Ulcer, and Gastric Cancer,” Infection and Immunity, Vol. 72, No. 2, 2004, pp. 1043- 1056. doi:10.1128/IAI.72.2.1043-1056.2004 [31] Y. Hirata, A. Yanai, W. Shibata, Y. Mitsuno, S. Maeda, K. Ogura, H. Yoshida, T. Kawabe, M. Omata, “Func- tional Variability of CagA Gene in Japanese Isolates of Helicobacter pylori,” Gene, Vol. 343, No. 1, 2004, pp. 165-172. doi:10.1016/j.gene.2004.08.026 [32] D. Nishiya, T. Shimoyama, T. Yoshimura, M. Tanaka, S. Fukuda and A. Munakata, “Genes Inside the cagPAI of Helicobacter pylori Are Not Associated with Gastric Can- cer in Japan.” Hepatogastroenterology, Vol. 51, No. 57, 2004, pp. 891-894. [33] S. Sahara, M. Sugimoto, R. K. Vilaichone, V. Mahachai, H. Miyajima, T. Furuta and Y. Yamaoka, “Role of Heli- cobacter pylori cagA EPIYA Motif and vacA Geno- Types for the Development of Gastrointestinal Diseases in Southeast Asian Countries: A Meta-Analysis,” BMC Infectious Diseases, Vol. 12, 2012, p. 223. doi:10.1186/1471-2334-12-223 [34] A. Karlsson, A. Ryberg, M. Nosouhi Dehnoei, K. Borch and H. J. Monstein, “Association between cagA and vacA Genotypes and Pathogenesis in a Helicobacter pylori In- fected Population from South-Eastern Sweden,” BMC Microbiology, Vol. 12, 2012, p. 129. doi:10.1186/1471-2180/12-129 [35] H. M. Schmidt, K. L. Goh, K. M. Fock, I. Hilmi, S. Dhamodaran, D. Forman and H. Mitchell, “Distinct cagA EPIYA Motifs Are Associated with Ethnic Diversity in Malaysia and Singapore,” Helicobacter, Vol. 14, No. 4, 2009, pp. 256-263. doi:10.1111/j.1523-5378.2009.00684.x
|