 Journal of Cosmetics, Dermatological Sciences and Applications, 2013, 3, 26-34 http://dx.doi.org/10.4236/jcdsa.2013.33A1004 Published Online September 2013 (http://www.scirp.org/journal/jcdsa) Mutations with Hair Shape Phenotypes Abnormalities— The Morphogenetic Waves and Related Diseases Junmin Wang1, Guannan Wang2, Jintao Zhang1* 1Laboratory Animal Center, Zhengzhou University, Zhengzhou, China; 2College of Life Sciences, Henan Agricultural University, Zhengzhou, China. Email: *zhangjt66@sina.com Received June 10th, 2013; revised July 8th, 2013; accepted July 16th, 2013 Copyright © 2013 Junmin Wang et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Hair morphology is one of the most conspicuous features of human variation. The hair follicle has attracted significant attention as a model for the investigation of diverse biological problems. Whereas, very little is known about the genes influencing the morphology and structure of the hair shaft. Curly hair is very common character of hair phenotypes of human, while most congenital curl occurs owing to genetic factors and some are closely related with genetic diseases. This review highlights current related genes reported affecting hair curliness and human diseases which are due to gene mutations. Keywords: Waved Hair; Curly Hair; Gene Mutations; Diseases 1. Introduction A mammal’s pelage is generally one of its first notice- able attributes and is aesthetically pleasing. Moreover, the skin is an essential organ which protects the organism from invasion of pathogens and chemicals and prevents the escape of liquids and nutrients [1]. Its ectodermal appendages, such as hair, feather and tooth, are attractive models for understanding the mechanisms underlying epithelial mesenchymal interactions [2]. Hair is composed of terminally differentiated, dead keratinocytes (trichocytes), which are compacted into a fibre of amazing tensile strength, the hair shaft. Hair morphogenesis and epidermal development are orches- trated by an array of cytokines and growth factors [3]. The presence of hair is characteristic for mammals, in which it exerts a wide range of tasks, including physical protection, thermal insulation, camouflage, dispersion of sweat and sebum, sensory and tactile functions, and so- cial interactions [4]. In human society, hair is of enor- mous, psychosocial importance, and many human dis- eases are associated with abnormalities in hair follicle morphogenesis, cycling, and structure. A hair grows from the papilla and with the exception of that point of generation is made up of dead, cornified cells. It consists of a shaft that projects above the skin, and a root that is imbedded in the skin. Its basic compo- nents are keratin (a protein), melanin (a pigment), and trace quantities of metallic elements [5]. These elements are deposited in the hair during its growth and/or ab- sorbed by the hair from an external environment. After a period of growth, the hair remains in the follicle in a resting stage to eventually be sloughed from the body. As the place of origin of the hair, the structural change of hair follicle could directly cause the change of hair phe- notype [6,7]. The hair follicle represents an attractive experimental system because of its accessibility, dispensability, and self-renewal capacity. Owing to its complex but highly organised architecture, this mini-organ can serve as an excellent model for investigating aspects of stem cell biology, cell lineage specification, cell differentiation, patterning processes, and cell-cell interactions [8,9]. In addition, hair follicles and shafts are of significant cos- metic relevance. The follicle provides the source for hair production and, thus, eventually determining appearance by affecting the hair shaft’s structure and shape. Hair features are not only affected by the follicle’s capacity to give rise to a normal shaft but also by the so-called hair growth cycle which controls the periodic growth and shedding of hair. Human hair is one of most keratinous fibres. There are various fibre shapes in human hair and it is commonly *Corresponding author. Copyright © 2013 SciRes. JCDSA  Mutations with Hair Shape Phenotypes Abnormalities—The Morphogenetic Waves and Related Diseases 27 accepted that the curliness of hair fibres is roughly clas- sified by their ethnic origin in the three major ethnic groups: African hair which has a strong curl shape, Cau- casian hair which has a moderately waved shape, and Asian hair which is apt to have a comparatively straight shape [10]. The corresponding difference in the internal nanostructure, however, still remains unrevealed. The structured pattern of hair is determined by their length, width, and shape. Whereas the prototype hair is straight, hair can adopt different shapes owing to bend- ing. In principle, bending could be achieved by diverse means. Differences in cell proliferation on opposite sides of the hair follicle would inevitably give rise to hair cur- vature [11]. Understanding the factors that contribute to the curly morphology of human hair is important for an- thropological and physiological studies. According to a recent report, hair curling in man is a consequence of different proliferation rates within the hair follicle that appear to be reflected by the shape of the follicle [12,13]. This correlation is reminiscent of the potential link be- tween follicle and hair size. Curliness has commonly been assessed using words such as straight, wavy, curly and frizzy, a variety of at- tributes of subjective nature with no clear definition and limits [14]. Curly hair is very common character of hair phenotypes of human, which is caused by many reasons. Most congenital curl occurs as the result of genetic fac- tors and some are closely related with genetic diseases. Genetic analyses of common diseases in humans have re- vealed that gene mutations are involved in diseases. Ge- nome sequencing projects of various mammalian species followed by comparative genome analyses have revealed that a large number of genes are shared among species. Thus, it is thought that mutations found in model animals and animals carrying such mutations are of large signifi- cance in studying hair growth regulation and the rela- tionship with some hereditary diseases. 2. The Curly Hair-Specific Genes Several genetic alterations with different follicular local- izations of the primary aberration give rise to curly or wavy hair and curly pelage is an easily recognized trans- species coat anomaly, moreover, several detailed studies in various mammalian species. In mice, Caracul (Ca) mice, a dominant mutation mapped to mouse chromo- some 15 and missense point to a single amino acid ex- change at the beginning of the a-helical rod domain of Krt71, a few amino acids apart from four identified Ca alleles possess curly hair and vibrissae after birth [15]. two novel krt 71rco12 and rco13 mutant mice, displaying a wavy pelage and curly vibrissae, have been identified as missense point mutations in the first exon of the krt 71gene [16]. In rat, the autosomal dominant Rex (Re) mutation in the Krt71 gene, on chromosome 7, causes wavy body hair in Re/+ and body hair loss in Re/Re rats after the first molt. The homozygote exhibits more waved pelage and smaller body size and histological analysis of 1-month-old mice revealed bent hair follicles and fragile hair shafts, vibrissae of the homozygote are more strongly curled than those in the heterozygote [17,18]. Recently, genome-wide single-nucleotide polymor- phism (SNP) association studies led to candidate gene screening for the curly/wavy coat of the portuguese water dog. A SNP in keratin-71 (KRT71) was shown to cause a nonsynonymous mutation in exon 2, having been re- cently identified in curly hair in dogs [19]. In cat, a com- plex sequence alteration of the KRT 71 gene, also caus- ing a splice variation, was identified in the Devon Rex breed with curly coats [20]. In cattle, an autosomal re- cessive form has been described in Hereford cattle, an 8-bp deletion mutation occurring in exon 1 causes an early truncated KRT71 protein resulting in a curly-hair coat [21]. More gene mutations affecting the morphoge- netic waves are showed in the Table 1. 3. Hair Curliness-Related Inherited Diseases 3.1. Pseudofolliculitis Barbae (PFB) Pseudofolliculitis barbae, a common human hair disorder, showing a chronic, irritating, and potentially disfiguring condition that develops as a result of attempts to elimi- nate hair from the beard area, usually by shaving [56]. The disease is, however, not gender-specific, nor re- stricted to the face, but can occur in any hairy skin region upon regular shaving or other means of hair removal [57]. Compared to Caucasian males, black males are distinctly more susceptible to developing PFB due to their genetic predisposition for strongly curved hairs and the study showed that incidence rate of the disorder can affect up to 1 out of every 5 Caucasian individuals while it occurs much more commonly in black persons [58]. The muta- tion analysis of K75 and the IRS keratins in a three-gen- eration Caucasian family whose male members suffered from relatively severe PFB symptoms revealed that af- fected males exhibited a heterozygous point mutation in the KRT75 gene. The mutation was also present in a fe- male member of the family, however, this individual did not shave nor remove hairs by other means, and she was free of symptoms. Clinical features include the appear- ance of inflammatory papules and pustules. Molecular analysis in a family study and a large-scale investigation of randomly sampled PFB-affected and -unaffected indi- viduals showed that an unusual single-nucleotide poly- morphism, which gives rise to a disruptive Ala12Thr substitution in the 1A a-helical segment of the compan- ion layer-specific keratin K6hf of the hair follicle, is par- tially responsible for the phenotypic expression and represents an additional genetic risk factor for PFB [59]. Copyright © 2013 SciRes. JCDSA 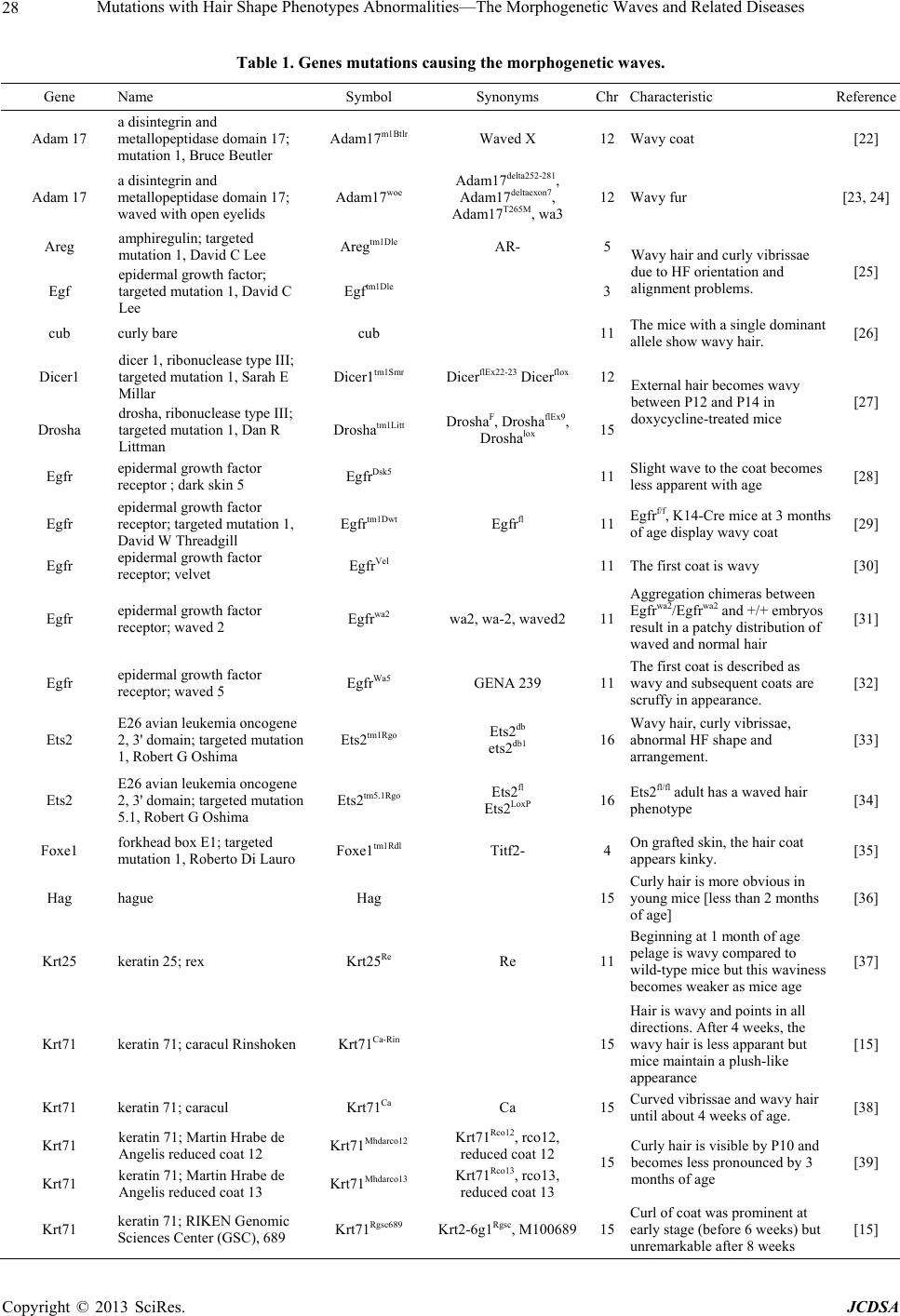 Mutations with Hair Shape Phenotypes Abnormalities—The Morphogenetic Waves and Related Diseases Copyright © 2013 SciRes. JCDSA 28 Table 1. Genes mutations causing the morphogenetic waves. Gene Name Symbol Synonyms ChrCharacteristic Reference Adam 17 a disintegrin and metallopeptidase domain 17; mutation 1, Bruce Beutler Adam17m1Btlr Waved X 12 Wavy coat [22] Adam 17 a disintegrin and metallopeptidase domain 17; waved with open eyelids Adam17woe Adam17delta252-281, Adam17deltaexon7, Adam17T265M, wa3 12 Wavy fur [23, 24] Areg amphiregulin; targeted mutation 1, David C Lee Aregtm1Dle AR- 5 Egf epidermal growth factor; targeted mutation 1, David C Lee Egftm1Dle 3 Wavy hair and curly vibrissae due to HF orientation and alignment problems. [25] cub curly bare cub 11 The mice with a single dominant allele show wavy hair. [26] Dicer1 dicer 1, ribonuclease type III; targeted mutation 1, Sarah E Millar Dicer1tm1Smr DicerflEx22-23 Dicerflox 12 Drosha drosha, ribonuclease type III; targeted mutation 1, Dan R Littman Droshatm1Litt DroshaF, DroshaflEx9, Droshalox 15 External hair becomes wavy between P12 and P14 in doxycycline-treated mice [27] Egfr epidermal growth factor receptor ; dark skin 5 EgfrDsk5 11 Slight wave to the coat becomes less apparent with age [28] Egfr epidermal growth factor receptor; targeted mutation 1, David W Threadgill Egfrtm1Dwt Egfrfl 11 Egfrf/f, K14-Cre mice at 3 months of age display wavy coat [29] Egfr epidermal growth factor receptor; velvet EgfrVel 11 The first coat is wavy [30] Egfr epidermal growth factor receptor; waved 2 Egfrwa2 wa2, wa-2, waved2 11 Aggregation chimeras between Egfrwa2/Egfrwa2 and +/+ embryos result in a patchy distribution of waved and normal hair [31] Egfr epidermal growth factor receptor; waved 5 EgfrWa5 GENA 239 11 The first coat is described as wavy and subsequent coats are scruffy in appearance. [32] Ets2 E26 avian leukemia oncogene 2, 3' domain; targeted mutation 1, Robert G Oshima Ets2tm1Rgo Ets2db ets2db1 16 Wavy hair, curly vibrissae, abnormal HF shape and arrangement. [33] Ets2 E26 avian leukemia oncogene 2, 3' domain; targeted mutation 5.1, Robert G Oshima Ets2tm5.1Rgo Ets2fl Ets2LoxP 16 Ets2fl/fl adult has a waved hair phenotype [34] Foxe1 forkhead box E1; targeted mutation 1, Roberto Di Lauro Foxe1tm1Rdl Titf2- 4 On grafted skin, the hair coat appears kinky. [35] Hag hague Hag 15 Curly hair is more obvious in young mice [less than 2 months of age] [36] Krt25 keratin 25; rex Krt25Re Re 11 Beginning at 1 month of age pelage is wavy compared to wild-type mice but this waviness becomes weaker as mice age [37] Krt71 keratin 71; caracul Rinshoken Krt71Ca-Rin 15 Hair is wavy and points in all directions. After 4 weeks, the wavy hair is less apparant but mice maintain a plush-like appearance [15] Krt71 keratin 71; caracul Krt71Ca Ca 15 Curved vibrissae and wavy hair until about 4 weeks of age. [38] Krt71 keratin 71; Martin Hrabe de Angelis reduced coat 12 Krt71Mhdarco12 Krt71Rco12, rco12, reduced coat 12 Krt71 keratin 71; Martin Hrabe de Angelis reduced coat 13 Krt71Mhdarco13 Krt71Rco13, rco13, reduced coat 13 15 Curly hair is visible by P10 and becomes less pronounced by 3 months of age [39] Krt71 keratin 71; RIKEN Genomic Sciences Center (GSC), 689 Krt71Rgsc689 Krt2-6g1Rgsc, M10068915 Curl of coat was prominent at early stage (before 6 weeks) but unremarkable after 8 weeks [15] 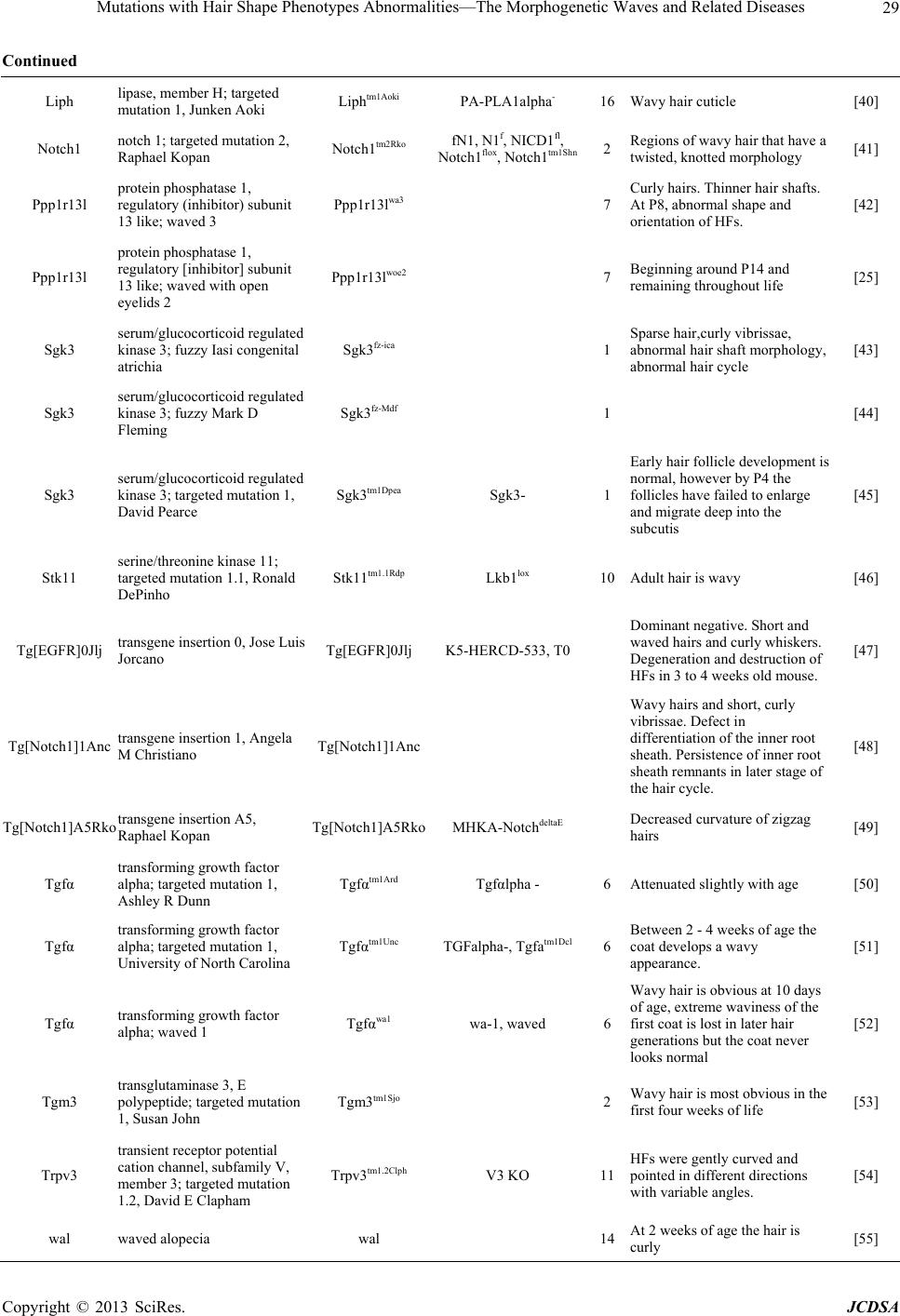 Mutations with Hair Shape Phenotypes Abnormalities—The Morphogenetic Waves and Related Diseases 29 Continued Liph lipase, member H; targeted mutation 1, Junken Aoki Liphtm1Aoki PA-PLA1alpha- 16 Wavy hair cuticle [40] Notch1 notch 1; targeted mutation 2, Raphael Kopan Notch1tm2Rko fN1, N1f, NICD1fl, Notch1flox, Notch1tm1Shn 2 Regions of wavy hair that have a twisted, knotted morphology [41] Ppp1r13l protein phosphatase 1, regulatory (inhibitor) subunit 13 like; waved 3 Ppp1r13lwa3 7 Curly hairs. Thinner hair shafts. At P8, abnormal shape and orientation of HFs. [42] Ppp1r13l protein phosphatase 1, regulatory [inhibitor] subunit 13 like; waved with open eyelids 2 Ppp1r13lwoe2 7 Beginning around P14 and remaining throughout life [25] Sgk3 serum/glucocorticoid regulated kinase 3; fuzzy Iasi congenital atrichia Sgk3fz-ica 1 Sparse hair,curly vibrissae, abnormal hair shaft morphology, abnormal hair cycle [43] Sgk3 serum/glucocorticoid regulated kinase 3; fuzzy Mark D Fleming Sgk3fz-Mdf 1 [44] Sgk3 serum/glucocorticoid regulated kinase 3; targeted mutation 1, David Pearce Sgk3tm1Dpea Sgk3- 1 Early hair follicle development is normal, however by P4 the follicles have failed to enlarge and migrate deep into the subcutis [45] Stk11 serine/threonine kinase 11; targeted mutation 1.1, Ronald DePinho Stk11tm1.1Rdp Lkb1lox 10 Adult hair is wavy [46] Tg[EGFR]0Jlj transgene insertion 0, Jose Luis Jorcano Tg[EGFR]0Jlj K5-HERCD-533, T0 Dominant negative. Short and waved hairs and curly whiskers. Degeneration and destruction of HFs in 3 to 4 weeks old mouse. [47] Tg[Notch1]1Anc transgene insertion 1, Angela M Christiano Tg[Notch1]1Anc Wavy hairs and short, curly vibrissae. Defect in differentiation of the inner root sheath. Persistence of inner root sheath remnants in later stage of the hair cycle. [48] Tg[Notch1]A5Rko transgene insertion A5, Raphael Kopan Tg[Notch1]A5Rko MHKA-NotchdeltaE Decreased curvature of zigzag hairs [49] Tgfα transforming growth factor alpha; targeted mutation 1, Ashley R Dunn Tgfαtm1Ard Tgfαlpha - 6 Attenuated slightly with age [50] Tgfα transforming growth factor alpha; targeted mutation 1, University of North Carolina Tgfαtm1Unc TGFalpha-, Tgfatm1Dcl 6 Between 2 - 4 weeks of age the coat develops a wavy appearance. [51] Tgfα transforming growth factor alpha; waved 1 Tgfαwa1 wa-1, waved 6 Wavy hair is obvious at 10 days of age, extreme waviness of the first coat is lost in later hair generations but the coat never looks normal [52] Tgm3 transglutaminase 3, E polypeptide; targeted mutation 1, Susan John Tgm3tm1Sjo 2 Wavy hair is most obvious in the first four weeks of life [53] Trpv3 transient receptor potential cation channel, subfamily V, member 3; targeted mutation 1.2, David E Clapham Trpv3tm1.2Clph V3 KO 11 HFs were gently curved and pointed in different directions with variable angles. [54] wal waved alopecia wal 14 At 2 weeks of age the hair is curly [55] Copyright © 2013 SciRes. JCDSA  Mutations with Hair Shape Phenotypes Abnormalities—The Morphogenetic Waves and Related Diseases Copyright © 2013 SciRes. JCDSA 30 3.2. Tricho-Dento-Osseous (TDO) Tricho-dento-osseous syndrome is a rare human genetic disorder first distinguished by Lichtenstein et al., in 1972 [60]. It is a highly penetrant autosomal dominant trait characterized by curly kinky hair in infancy, enamel hy- poplasia, taurodontism, thickening of cortical bones and variable expression of craniofacial morphology [61]. Diagnostic criteria are based on the generalized enamel defects, severe taurodontism especially of the mandibular first permanent molars, an autosomal dominant mode of inheritance, and at least one of the other features (i.e., nail defects, bone sclerosis, and curly, kinky or wavy hair present at a young age that may straighten out later). Kinky or tightly curled hair at birth may be a characteris- tic and distinguishing feature in many families and aid in diagnosing TDO from hypomaturation-type amelogene- sis imperfecta [62,63]. TDO syndrome is considered the ectodermal dysplasia with a high penetrance even if the individual signs and symptoms can be present in variable intensities. Genetic studies have shown a mutation in the DLX3 gene on chromosome 17q21 and a 4 bp deletion in the DLX3 gene associated with TDO, which has also been identified [64]. Some families have been reported to have wavy hair or curly hair at birth that straightened out a few years later [65-67]. Seow [68] reported that the hair defects may vary among affected members of the same family. Mayer et al. [62] reported that an 8-year-old girl with TDO syndrome had uncombable hair, enamel hy- poplasia and enlarged pulp chambers of the molar teeth. Electron microscopic examination of the curly hair showed a flattened hair shaft with longitudinal grooves. 3.3. Oculo-Dento-Digital Dysplasia (ODDD) Oculo-dento-digital dysplasia is a rare autosomal domi- nant congenital disorder caused by mutations in con- served domains of the gap junction alpha 1 gene (GJA1 or Connexin 43 (CX43)) located on chromosome 6q21- q23.2 with two exons separated by an 11-kb intron [69]. Abnormalities observed in ODDD affect the eye, denti- tion, and digits of the hands and feet [70]. Patients pre- sent with a characteristic facial appearance, narrow nose, and hypoplastic alae nasi. Neurological problems are known to occur as well as conductive hearing loss, car- diac defects, and anomalies of the skin, hair, and nails. Curly/kinky hair with features of early trichorrhexis nodosa was identified in a 13 years old girl with ODDD [71], this in accord with the observation by Kjaer et al., who found curly hair in seven out of nine affected sub- jects harboring a mutation in the Cx43 gene in a Danish family over five generations [72]. 3.4. Woolly Hair (WH) Woolly hair is a group of hair shaft disorders, which re- fers to an abnormal variant of fine, tightly curled hair that often exhibits decreased pigmentation. Hutchinson et al. [73] classified woolly hair into 3 variants: woolly hair nevus, autosomal dominant hereditary woolly hair, and autosomal recessive familial woolly hair. Since then, WH has also been observed in association with several ge- netic conditions, such as Naxos disease and Carvajal syndrome, both of which are characterized by cardio- myopathy, palmoplantar keratoderma, and WH, and are caused by mutations in the plakoglobin [74] and desmo- plakin [75] genes, respectively. Furthermore, most nota- bly is Noonan syndrome and cardiofaciocutaneous (CFC) syndrome. Andy J. Chien described a family with woolly hair and ulerythema ophryogenes spanning four genera- tions, which have been associated with Noonan syn- drome and CFC, and he found that this family did not exhibit any of the other findings characteristic of either Noonan syndrome or CFC, similar to a previously de- scribed pedigree with hereditary woolly hair [76]. In ad- dition to these syndromes, WH without associated find- ings (non-syndromic WH) has also been described [77]. 4. Acknowledgements This publication was supported by grants from China Natural Science Foundation (#31071923). REFERENCES [1] A. Abbasi, “Molecular Evolution of HR, A Gene That Regulates the Postnatal Cycle of the Hair Follicle,” Scien- tific Reports, Vol. 1, No. 32, 2011, pp.1-7. doi:10.1038/srep00032 [2] J. Hwang, T. Mehrani, S. E. Millar and M. I. Morasso, “Dlx3 is a Crucial Regulator of Hair Follicle Differen- tiation and Cycling,” Development, Vol. 135, No. 18, 2008, pp. 3149-3159. doi:10.1242/dev.022202 [3] E. Fuchs and S. Raghavan, “Getting under the Skin of Epidermal Morphogenesis,” Nature Reviews Genetics, Vol. 3, No. 3, 2002, pp. 199-209. doi:10.1038/nrg758 [4] M. R. Schneider, R. Schmidt-Ullrich and R. Paus, “The Hair Follicle as A Dynamic Miniorgan,”Current Biology, Vol. 19, No. 3, 2009, pp. 132-142 doi:10.1016/j.cub.2008.12.005 [5] M. A. Rogers, L. Langbein, S. Praetzel-Wunder, H. Win- ter and J. Schweizer, “Human Hair Keratin-Associated Proteins (KAPs),” International Review of Cytology, Vol. 251, 2006, pp. 209-263. doi:10.1016/S0074-7696(06)51006-X [6] L. Langbein and J. Schweizer, “Keratins of the Human Hair Follicle,” International Review of Cytology, Vol. 243, 2005, pp.1-78. doi:10.1016/S0074-7696(05)43001-6 [7] Y. Shimomura and M. Ito, “Human Hair Keratin-Associ- ated Proteins,” Journal of Investigative Dermatology Symposium Proceedings, Vol. 10, No. 3, 2005, pp. 230- 233. doi:10.1111/j.1087-0024.2005.10112.x [8] E. Fuchs, “Skin Stem Cells, Rising to the Surface,” The  Mutations with Hair Shape Phenotypes Abnormalities—The Morphogenetic Waves and Related Diseases 31 Journal of Cell Biology, Vol. 180, No. 2, 2008, pp. 273- 284. doi:10.1083/jcb.200708185 [9] E. Fuchs and V. Horsley, “More Than One Way to Skin,” Genes Development, Vol. 22, No. 8, 2008, pp. 976-985. doi:10.1101/gad.1645908 [10] Y. Kajiura, S. Watanabe, T. Itou, K. Nakamura, A. Iida, K. Inoue, N. Yagi, Y. Shinohara and Y. Amemiya, “Struc- tural Analysis of Human Hair Single Fibres by Scanning Microbeam SAXS,” Journal of Structural Biology, Vol. 155, No. 3, 2006, pp. 438-444. doi:10.1016/j.jsb.2006.04.008 [11] T. Schlake, “Determination of Hair Structure and Shape,” Seminars in Cell& Developmental Biology, Vol. 18, No. 2, 2007, pp. 267-273. doi:10.1016/j.semcdb.2007.01.005 [12] S. Thibaut, O. Gaillard, P. Bouhanna, D. W. Cannell and B. A. Bernard, “Human Hair Shape Is Programmed From the Bulb,” British Journal of Dermatology, Vol. 152, No.4, 2005, pp. 632- 638. doi:10.1111/j.1365-2133.2005.06521.x [13] Lindelof, B. Forslind, M. A. Hedblad and U. Kaveus, “Human Hair Form Morphology Revealed by Light and Scanning Electron Microscopy and Computer Aided Three-Dimensional Reconstruction,” Archives of Derma- tology, Vol. 124, No. 9, 1988, pp. 1359-1363. doi:10.1001/archderm.1988.01670090015003 [14] G. Loussouarn, A.L. Garcel, I. Lozano, C. Collaudin, C. Porter, S. Panhard, D. Saint-Léger and R. de La Mettrie, “Worldwide Diversity of Hair Curliness: A New Method of Assessment,” International Journal of Dermatology, Vol. 46, Suppl. 1, 2007, pp. 2-6. doi:10.1111/j.1365-4632.2007.03453.x [15] Y. Kikkawa, A. Oyama, R. Ishii, I. Miura, T. Amano, Y. Ishii, Y. Yoshikawa, H. Masuya, S. Wakana, T. Shiroishi, C. Taya and H. Yonekawa, “A Small Deletion Hotspot in the Type II Keratin Gene Mk6irs1/Krt2-6g on Mouse Chromosome 15, A Candidate for Causing the Wavy Hair of the Caracul (Ca) Mutation,” Genetics, Vol. 165, No. 2, 2003, pp. 721-733. [16] F. Runkel, M. Klaften, K. Koch, V. Böhnert, H. Büssow, H. Fuchs, T. Franz and M. Hrabé de Angelis, “Morphol- ogic and Molecular Characterization of Two Novel Krt71 (Krt2-6g) Mutations: Krt71rco12 and Krt71rco13,” Mam- malian Genome, Vol. 17, No. 12, 2006, pp. 1172-1182. doi:10.1007/s00335-006-0084-9 [17] T. Kuramoto, R. Hirano, M. Kuwamura and T. Serikawa, “Identification of the Rat Rex Mutation as A 7-Bp Dele- tion at Splicing Acceptor Site of the Krt71 Gene,” The Journal of Veterinary Medical Science, Vol. 72, No. 7, 2010, pp. 909-912. doi:10.1292/jvms.09-0554 [18] T. Kuramoto, M. Yokoe, K. Yagasaki, T. Kawaguchi, K. Kumafuji and T. Serikawa, “Genetic Analyses of Fancy Rat-Derived Mutations,” Experimental Animals, Vol. 59, No. 2, 2010, pp. 147-155. doi:10.1538/expanim.59.147 [19] E. Cadieu, M. W. Neff, P. Quignon, K. Walsh, K. Chase, H. G. Parker, B. M. VonHoldt, A.Rhue, A. Boyko, A. Byers, A. Wong, D. S. Mosher, A. G. Elkahloun, T. C. Spady, C. André, K. Gordon Lark, M. Cargill, C. D. Bustamante, R. K. Wayne and E. A. Ostrander, “Coat Variation in the Domestic Dog is Governed by Variants in Three Genes,” Science, Vol. 326, No. 5949, 2009, pp. 150-153. doi:10.1126/science.1177808 [20] B. Gandolfi, C. A. Outerbridge, L. G. Beresford, J. A. Myers, M. Pimentel, H. Alhaddad, J. C. Grahn, R. A. Grahn and L. A. Lyons, “The Naked Truth: Sphynx and Devon Rex Cat Breed Mutations in KRT71,” Mammalian Genome, Vol. 21, No. 9-10, 2010, pp.509-515. doi:10.1007/s00335-010-9290-6 [21] A. D. Markey, J. F. Taylor, R. D. Schnabel, S. D. McKay, M. C. McClure and J. E. Beever, “A Deletion Mutation in Krt71 is Associated with Congenital Hypotrichosis in Hereford Cattle,” Plant & Animal Genomes XVIII Con- ference, San Diego, 9-13 January 2010, p. 552. [22] K. Brandla, L.Sun, C. Neppl, O. M. Siggsa, S. M. Le Gallc, W. Tomisatoa, X.H. Li, X. Du, D. N. Maennel, C. P. Blobel and B. Beutler, “MyD88 Signaling in Nonhe- matopoietic Cells Protects Mice Against Induced Colitis by Regulating Specific EGF Receptor Ligands,” Pans, Vol. 107, 2010, pp. 19967-19972. doi:10.1073/pnas.1014669107 [23] B. Chang, N. L. Hawes, R. E. Hurd, J. Wang, D. Howell, M. T. Davisson, T. H. Roderick, S. Nusinowitz and J. R. Heckenlively, “Mouse Models of Ocular Diseases,” Vis- ual Neuroscience, Vol. 22, No. 5, 2005, pp. 587-593. [24] L. Hassemer, S. M. Le Gall, R. Liegel, M. McNally, B. Chang, C. J. Zeiss, R. D. Dubielzig, K. Horiuchi, T. Ki- mura, Y. Okada, C. P. Blobel and D. J. Sidjanin, “The Waved with Open Eyelids (Woe) Locus is A Hypomor- phic Mouse Mutation in Adam17,” Genetics, Vol. 185, No. 1, 2010, pp. 245-255. doi:10.1534/genetics.109.113167 [25] N. C. Luetteke, T. H. Qiu, S. E. Fenton, K. L. Troyer, R. F. Riedel, A. Chang and D. C. Lee, “Targeted Inactiva- tion of the EGF and Amphiregulin Genes Reveals Dis- tinct Roles for EGF Receptor Ligands in Mouse Mam- mary Gland Development,” Development, Vol. 126, No. 12, 1999, pp. 2739-2750. [26] K. R. Johnson, P. W. Lane, S. A. Cook, B. S. Harris, P. F. Ward-Bailey, R. T. Bronson, B. L. Lyons, L. D. Shultz and M. T. Davisson, “Curly Bare (Cub), A New Mouse Mutation on Chromosome 11 Causing Skin and Hair Abnormalities, and A Modifier Gene (Mcub) on Chro- mosome 5,” Genomics, Vol. 81, No. 1, 2003, pp. 6-14. doi:10.1242/dev.070920 [27] M. Teta, Y. S. Choi, T. Okegbe, O. H. Tam, M. M. Chong, J. T. Seykora, A. Nagy, D. R. Littman, T. Andl and S. E. Millar, “Inducible Deletion of Epidermal Dicer and Drosha Reveals Multiple Functions for Mirnas in Postnatal Skin,” Development, Vol. 139, No. 8, 2012, pp. 1405-1406. [28] K. R. Fitch, K. A. McGowan, C. D. van Raamsdonk, H. Fuchs, D. Lee, A. Puech, Y. Hérault, D. W. Threadgill, M. Hrabé de Angelis and G. S. Barsh, “Genetics of Dark Skin in Mice,” Genes & Development, Vol. 17, No. 2, 2003, pp. 214-228. doi:10.1101/gad.1023703 [29] T. C. Lee and D. W. Threadgill, “Generation and Valida- tion of Mice Carrying a Conditional Allele of The Epi- dermal Growth Factor Receptor,” Genesis, Vol. 47, No. 2, 2009, pp. 85-92. doi:10.1002/dvg.20464 Copyright © 2013 SciRes. JCDSA  Mutations with Hair Shape Phenotypes Abnormalities—The Morphogenetic Waves and Related Diseases 32 [30] X. Du, K. Tabeta, K. Hoebe, H. Liu, N. Mann, S. Mudd, K. Crozat, S. Sovath, X. Gong and B. Beutler, “Velvet, A Dominant Egfr Mutation That Causes Wavy Hair and Defective Eyelid Development in Mice,” Genetics, Vol. 166, No. 1, 2004, pp. 331-340. doi:10.1534/genetics.166.1.331 [31] A. Mclaren, “The Microscopic Appearance of Waved-2 Mouse Hairs,” Genetics Research, Vol. 17, No. 3, 1971, pp. 257-260. doi:10.1017/S0016672300012271 [32] C. Thaung, K. West, B. J. Clark, L. McKie, J. E. Morgan, K. Arnold, P. M. Nolan, J. Peters, A. J. Hunter, S. D. Brown, I. J. Jackson and S. H. Cross, “Novel ENU-In- duced Eye Mutations in The Mouse: Models for Human Eye Disease,” Human Molecular Genetics, Vol. 11, No. 7, 2002, pp.755-767. doi:10.1093/hmg/11.7.755 [33] H. Yamamoto, M. L. Flannery, S. Kupriyanov, J. Pearce, S. R. McKercher, G. W. Henkel, R. A. Maki, Z. Werb and R. G. Oshima, “Defective Trophoblast Function in Mice with A Targeted Mutation of Ets2,” Genes & De- velopment, Vol. 12, No. 9, 1998, pp. 1315-1326. doi:10.1101/gad.12.9.1315 [34] G. Wei, R. Srinivasan, C. Z. Cantemir-Stone, S. M. Sharma, R. Santhanam, M. Weinstein, N. Muthusamy, A. K. Man, R. G. Oshima, G. Leone and M. C. Ostrowski, “Ets1 and Ets2 are Required for Endothelial Cell Survival During Embryonic Angiogenesis,” Blood, Vol. 114, No. 5, 2009, pp. 1123-1130. doi:10.1182/blood-2009-03-211391 [35] A. Brancaccio, A. Minichiello, M. Grachtchouk, D. An- tonini, H. Sheng, R. Parlato, N. Dathan, A. A. Dlugosz and C. Missero, “Requirement of the Forkhead Gene Foxe1, a Target of Sonic Hedgehog Signaling, in Hair Follicle Morphogenesis,” Human Molecular Genetics, Vol. 13, No. 21, 2004, pp. 2595-2606. doi:10.1093/hmg/ddh292 [36] C. Poirier, A. Yoshiki, K. Fujiwara, J. L. Guénet and M. Kusakabe, “Hague (Hag): A New Mouse Hair Mutation with an Unstable Semidominant Allele,” Genetics, Vol. 162, No. 2, 2002, pp. 831-840. [37] S. Tanaka, I. Miura, A. Yoshiki, Y. Kato, H. Yokoyama, A. Shinogi, H. Masuya, S. Wakana, M. Tamura and T. Shiroishi, “Mutations in the Helix Termination Motif of Mouse Type I IRS Keratin Genes Impair the Assembly of Keratin Intermediate Filament,” Genomics, Vol. 90, No. 6, 2007, pp. 703-711. doi:10.1016/j.ygeno.2007.07.013 [38] L. C. Dunn, “Caracul a Dominant Mutation,” The Journal of Heredity, Vol. 28, No. 10, 1937, pp. 334. [39] F. Runkel, M. Klaften, K. Koch, V. Böhnert, H. Büssow, H. Fuchs, T. Franz and M. H. de Angelis, “Morphologic and Molecular Characterization of Two Novel Krt71 (Krt2-6g) Mutations: Krt71rco12 and Krt71rco13,” Mam- malian Genome, Vol. 17, No. 12, 2006, pp. 1172-1182. doi:10.1007/s00335-006-0084-9 [40] A. Inoue, N. Arima, J. Ishiguro, G. D. Prestwich, H. Arai and J. Aoki, “LPA-Producing Enzyme PA-PLA1 α Regu- lates Hair Follicle Development by Modulating EGFR Signalling,” The EMBO Journal, Vol. 30, No. 20, 2011, pp. 4248-4260. [41] Y. H. Pan, M. H. Lin, X. L.Tian, H. T. Cheng, T. Gridley, J. Shen and R. Kopan, “Γ-Secretase Functions Through Notch Signaling to Maintain Skin Appendages But Is Not Required for Their Patterning or Initial Morphogenesis,” Developmental Cell, Vol. 7, No. 5, 2004, pp. 731-743. doi:10.1016/j.devcel.2004.09.014 [42] J. Herron, C. Rao, S. Liu, L.Laprade, J. A. Richardson, E. Olivieri, C. Semsarian, S. E. Millar, L. Stubbs and D. R. Beier, “A Mutation in NFkB Interacting Protein 1 Results in Cardiomyopathy and Abnormal Skin Development in Wa3 Mice,” Human Molecular Genetics, Vol. 14, No. 5, 2005, pp. 667-677. doi:10.1093/hmg/ddi063 [43] L. Mecklenburg, D. J. Tobin, M. V. Cirlan, C. Craciun and R. Paus, “Premature Termination of Hair Follicle Morphogenesis and Accelerated Hair Follicle Cycling in Iasi Congenital Atrichia (Fzica) Mice Points to Fuzzy as a Key Element of Hair Cycle Control,” Experimental Der- matology, Vol. 14, No. 8, 2005, pp. 561-570. doi:10.1111/j.0906-6705.2005.00343.x [44] D. R. Campagna, A. O. Custodio, B. B. Antiochos, M. V. Cirlan and M. D. Fleming, “Mutations in the Serum/ Glu- cocorticoid Regulated Kinase 3 (Sgk3) Are Responsible for the Mouse Fuzzy (Fz) Hair Phenotype,” Journal of Investive Dermatology, Vol. 128, No. 3, 2008, pp. 730- 732. doi:10.1038/sj.jid.5701089 [45] J. A. McCormick, Y. X. Feng, K. Dawson, M. J. Behne, B. Yu, J. Wang, A. W. Wyatt, G. Henke, F. Grahammer, T. M. Mauro, F. Lang and D. Pearce, “Targeted Disrup- tion of the Protein Kinase SGK3/CISK Impairs Postnatal Hair Follicle Development,” Molecular Biology of the Cell, Vol. 15, No. 9, 2004, pp. 4278-4288. doi:10.1091/mbc.E04-01-0027 [46] S. Gurumurthy, A. F. Hezel, E. Sahin, J. H. Berger, M. W. Bosenberg and N. Bardeesy, “LKB1 Deficiency Sensi- tizes Mice to Carcinogen-Induced Tumorigenesis,” Can- cer Research, Vol. 68, No. 1, 2008, pp. 55-63. doi:10.1158/0008-5472.CAN-07-3225 [47] R. Murillas, F. Larcher, C. J. Conti, M. Santos, A. Ullrich and J. L. Jorcano, “Expression of a Dominant Negative Mutant of Epidermal Growth Factor Receptor in the Epi- dermis of Transgenic Mice Elicits Striking Alterations in Hair Follicle Development and Skin Structure,” The EMBO Journal, Vol. 14, No. 21, 1995, pp. 5216-5223. [48] H. Uyttendaele, A. A. Panteleyev, D. de Berker, D. T. Tobin and A. M. Christiano, “Activation of Notch1 in the Hair Follicle Leads to Cell-Fate Switch and Mohawk Alopecia,” Differentiation, Vol. 72, No. 8, 2004, pp. 396- 409. doi:10.1111/j.1432-0436.2004.07208006.x [49] M. H. Lin, C. Leimeister, M. Gessler and R. Kopan, “Activation of the Notch Pathway in the Hair Cortex Leads to Aberrant Differentiation of the Adjacent Hair- Shaft Layers,” Development, Vol. 127, No. 11, 2000, pp. 2421-2432. [50] B. Mann, K. J. Fowler, A. Gabriel, E. C. Nice, R. L. Williams and A. R. Dunn, “Mice with a Null Mutation of the TGF Alpha Gene Have Abnormal Skin Architecture, Wavy Hair, and Curly Whiskers and Often Develop Cor- neal Inflammation, ” Cell, Vol. 73, No. 2, 1993, pp. 249- 261. doi:10.1016/0092-8674(93)90227-H [51] N. C. Luetteke, T. H. Qiu, R. L. Peiffer, P. Oliver, O. Smithies and D. C. Lee, “TGF Alpha Deficiency Results Copyright © 2013 SciRes. JCDSA  Mutations with Hair Shape Phenotypes Abnormalities—The Morphogenetic Waves and Related Diseases 33 in Hair Follicle and Eye Abnormalities in Targeted and Waved-1 Mice,” Cell, Vol. 73, No. 2, 1993, pp. 263-278. doi:10.1016/0092-8674(93)90228-I [52] M. J. Trigg, “Hair Growth in Mouse Mutants Affecting Coat Texture,” Journal of Zoology, Vol. 168, No. 2, 1972, pp. 165-198. [53] S. John, L. Thiebach, C. Frie, S. Mokkapati, M. Bechtel, R. Nischt, S. Rosser-Davies, M. Paulsson and N. Smyth, “Epidermal Transglutaminase (Tgase 3) Is Required for Proper Hair Development, but Not the Formation of the Epidermal Barrier,” PLoS ONE, Vol. 7, No. 4, 2012, Ar- ticle ID: e34252. doi:10.1371/journal.pone.0034252 [54] X. Cheng, J. Jin, L. Hu, D. Shen, X. P. Dong, M. A. Samie, J. Knoff, B. Eisinger, M. L. Liu, S. M. Huang, M. J. Caterina, P. Dempsey, L. E. Michael, A. A. Dlugosz, N. C. Andrews, D. E. Clapham and H. Xu, “TRP Channel Regulates EGFR Signaling in Hair Morphogenesis and Skin Barrier Formation,” Cell, Vol. 141, No. 2, 2010, pp. 331-343. doi:10.1016/j.cell.2010.03.013 [55] P. Baden, M. F.Champliaud, J. P. Sundberg and A. Viel, “Targeted Deletion of the Sciellin Gene Resulted in Nor- mal Development and Maturation,” Genesis, Vol. 42, No. 4, 2005, pp. 219-228. doi:10.1002/gene.20133 [56] H. Winter, D. Schissel, D. A. Parry, T. A. Smith, M. Lio- vic, E. Birgitte Lane, L. Edler, L. Langbein, L. F. Jave- Suarez, M. A. Rogers, J. Wilde, G. Peters and J. Schwei- zer, “An Unusual Ala12Thr Polymorphism in the 1A α- Helical Segment of the Companion Layer-Specific Kera- tin K6hf: Evidence for a Risk Factor in the Etiology of the Common Hair Disorder Pseudofolliculitis Barbae,” Jour- nal of Investigative Dermatology, Vol. 122, No. 3, 2004, pp. 652-657. doi:10.1111/j.0022-202X.2004.22309.x [57] J. Schweizer, L. Langbein, M. A. Rogers and H. Winter, “Hair Follicle-Specific Keratins and Their Diseases,” Ex- perimental Cell Research, Vol. 313, No. 10, 2007, pp. 2010-2020. doi:10.1016/j.yexcr.2007.02.032 [58] M. Ribera, N. Fernández-Chico and M. Casals, “Pseudo- folliculitis Barbae,” Actas Dermosifiliog á ficas, Vol. 101, No. 9, 2010, pp. 749-757. doi:10.1016/j.ad.2010.03.011 [59] C. Chamcheu, I. A. Siddiqui, D. N. Syed, V. M. Adhami, M. Liovic and H. Mukhtar, “Keratin Gene Mutations in Disorders of Human Skin and Its Appendages,” Archives of Biochemistry and Biophysics, Vol. 508, No. 2, 2011, pp. 123-137. doi:10.1016/j.abb.2010.12.019 [60] J. Lichtenstein, R. Warson, R. Jorgenson, J. P. Dorst and V. A. McKusick, “The Tricho-Dento-Osseous (TDO) Syn- drome,” The American Journal of Human Genetics, Vol. 24, No. 5, 1972, pp. 569-582. [61] T. Nguyen, C. Phillips, S. Frazier-Bower and T. Wright, “Craniofacial Variations in the Tricho-Dento-Osseous Syn- drome,” Clinical Genetics, Vol. 83, No. 4, 2013, pp. 375- 379. doi:10.1111/j.1399-0004.2012.01907.x [62] E. Mayer, C. Baal, M. Litschauer-Poursadrollah, W. Hem- mer and R. Jarisch, “Uncombable Hair and Atopic Der- matitis in a Case of Trichodento-Osseous Syndrome,” Journal der Deutschen Dermatologischen Gesellschaft, Vol. 8, No. 2, 2010, pp. 102-104. doi:10.1111/j.1610-0387.2009.07159_supp.x [63] R. P. Elzay and D. H. Chamberlain, “Differential Diagno- sis of Enlarged Dental Pulp Chambers: A Case Report of Amelogenesis Imperfecta with Taurodontism,” ASDC Jour- nal of Dentistry Children, Vol. 53, No. 5, 1986, pp. 388- 390. [64] B. Al-Batayneh, “Tricho-Dento-Osseous Syndrome: Diag- nosis and Dental Management,” International Journal of Dentistry, Vol. 2012, No. 2012, 2012, Article ID: 514692. doi:10.1155/2012/514692 [65] S. D. Shapiro, F. L. Quattromani, R. J. Jorgenson and R. S. Young, “Tricho-Dento-Osseous Syndrome: Heteroge- neity or Clinical Variability,” American Journal of Medi- cal Genetics, Vol. 16, No. 2, 1983, pp. 225-236. doi:10.1002/ajmg.1320160212 [66] Duverger, D. Lee, M. Q. Hassan, S. X. Chen, F. Jaisser, J. B. Lian and M. I. Morasso, “Molecular Consequences of A Frameshifted DLX3 Mutant Leading to Tricho-Dento- Osseous Syndrome,” The Journal of Biological Chemistry, Vol. 283, No. 29, 2008, pp. 20198-20208. doi:10.1074/jbc.M709562200 [67] M. Melnick, E. D. Shields and A. H. El-Kafrawy, “Tri- chodento-Osseous Syndrome: A Scanning Electron Micro- scopic Analysis,” Clinical Genetics, Vol. 12, No. 1, 1977, pp. 17-27. doi:10.1111/j.1399-0004.1977.tb00896.x [68] W. K. Seow, “Trichodentoosseous (TDO) Syndrome: Case Report and Literature Review,” Pediatric Dentistry, Vol. 15, No. 5, 1993, pp. 355-361. [69] W. A. Paznekas, B. Karczeski, S. Vermeer, R. B. Lowry, M. Delatycki, F. Laurence, P. A. Koivisto, L. van Mal- dergem, S. A. Boyadjiev, J. N. Bodurtha and E. W. Jabs, “GJA1 Mutations, Variants, and Connexin 43 Dysfunc- tion as It Relates to the Oculodentodigital Dysplasia Phe- notype,” Human Mutation, Vol. 30, No. 5, 2009, pp. 724- 733. doi:10.1002/humu.20958 [70] W. A. Paznekas, S. A. Boyadjiev, R. E. Shapiro, O. Daniels, B. Wollnik, C. E. Keegan, J. W. Innis, M. B. Dinulos, C. Christian, M. C. Hannibal and E. W. Jabs, “Connexin 43 (GJA1) Mutations Cause the Pleiotropic Phenotype of Oculodentodigital Dysplasia,” The Ameri- can Journal of Human Genetics, Vol. 72, No. 2, 2003, pp. 408-418. doi:10.1086/346090 [71] K. W. Kjaer, L. Hansen, H. Eiberg, P. Leicht, J. M. Opitz and N. Tommerup, “Novel Connexin 43 (GJA1) Mutation Causes Oculo-Dento-Digital Dysplasia with Curly Hair,” American Journal of Medical Genetics, Vol. 127A, No. 2, 2004, pp. 152-157. doi:10.1002/ajmg.a.20614 [72] S. C. Kelly, P. Ratajczak, M. Keller, S. M. Purcell, T. Griffin and G. Richard, “A Novel GJA 1 Mutation in Oculodento-Digital Dysplasia with Curly Hair and Hy- perkeratosis,” European Journal of Dermatology, Vol. 16, No. 3, 2006, pp. 241-245. [73] P. E. Hutchinson, R. J. Cairns and R. S. Wells, “Woolly Hair: Clinical and General Aspects,” Transactions of the St. Johns Hospital Dermatological Society, Vol. 60, No. 2, 1974, pp. 160-177. [74] G. McKoy, N. Protonotarios, A. Crosby, A. Tsatsopoulou, A. Anastasakis, A. Coonar, M. Norman, C. Baboonian, S. Jeffery and W. J. McKenna, “Identification of a Deletion in Plakoglobin in Arrhythmogenic Right Ventricular Car- diomyopathy with Palmoplantar Keratoderma and Woolly Copyright © 2013 SciRes. JCDSA  Mutations with Hair Shape Phenotypes Abnormalities—The Morphogenetic Waves and Related Diseases Copyright © 2013 SciRes. JCDSA 34 Hair (Naxos Disease),” The Lancet, Vol. 355, No. 9221, 2000, pp. 2119-2124. doi:10.1016/S0140-6736(00)02379-5 [75] E. E. Norgett, S. J. Hatsell, L. Carvajal-Huerta, J. C. Ca- bezas, J. Common, P. E. Purkis, N. Whittock, I. M. Leigh, H. P. Stevens and D. P. Kelsell, “Recessive Mutation in Desmoplakin Disrupts Desmoplakin—Intermediate Fila- ment Interactions and Causes Dilated Cardiomyopathy, Woolly Hair and Keratoderma,” Human Molecular Gene- tics, Vol. 9, No. 18, 2000, pp. 2761-2766. doi:10.1093/hmg/9.18.2761 [76] A. J. Chien, M. C. Valentine and V. P. Sybert, “Heredi- tary Woolly Hair and Keratosis Pilaris,” Journal of the American Academy of Dermatology, Vol. 54, No. 2, 2006, pp. S35-S39. doi:10.1016/j.jaad.2005.01.092 [77] Y. Shimomura, M. Wajid, L. Petukhova, L. Shapiro and A. M. Christiano, “Mutations in the Lipase H Gene Un- derlie Autosomal Recessive Woolly Hair/Hypotrichosis,” Journal of Investigative Dermatology, Vol. 129, No. 3, 2009, pp. 622-628. doi:10.1038/jid.2008.290
|