 Journal of Cosmetics, Dermatological Sciences and Applications, 2013, 3, 175-189 http://dx.doi.org/10.4236/jcdsa.2013.33028 Published Online September 2013 (http://www.scirp.org/journal/jcdsa) 175 Combined Effects of Capsaicin and HA14-1 in Inducing Apoptosis in Melanoma Cells Claudia M. G. Marques1, Catherine Dibden2, Sarah Danson3, John W. Haycock1, Sheila MacNeil1 1University of Sheffield, North Campus, Kroto Research Institute, Sheffield, UK; 2Department of Clinical Biochemistry Northern General Hospital, Sheffield, UK; 3Academic Unit of Clinical Oncology, Weston Park Hospital, Sheffield, UK. Email: s.macneil@sheffield.ac.uk Received March 12th, 2013; revised April 15th, 2013; accepted April 24th, 2013 Copyright © 2013 Claudia M. G. Marques et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Abnormal regulation of apoptosis is an important aspect of tumour development. Capsaicin, an extract of red chilli pe- ppers, has been shown to inhibit growth of melanoma and other malignant cell lines and HA14-1 is an organic com- pound that directly induces apoptosis by binding to Bcl-2 protein. The aim of this work was to investigate whether com- bination therapy with capsaicin and HA14-1 might hold any promise for the treatment of melanoma. Three melanoma cell lines of a range of aggressive potential, melanocytes and fibroblasts were examined, looking at the effects of both drugs singly and in co mbination on cell viability and ind uction of apoptosis. This comparative stud y showed that mela- noma cells and melanocytes have a similar sensitiv ity to capsaicin while fibrob lasts are more resistant to it. HA14-1, as expected, induced apoptosis in all cells at relatively low concentrations. A combination of the two agen ts produced the expected results of an add itive effect for 2 (HBL and A375SM) out of 3 melanoma cell lines in inducing apoptosis, but encouragingly for the most metastatically aggressive cancer cell line (C8161), a combination of the two showed a syn- ergistic induction of apoptosis. Keywords: Capsaicin; HA14-1; Bcl-2 Inhibitors; Melano ma; Apoptosis 1. Introduction Many studies have suggested a strong association be- tween inflammation and cancer [1-6]. A recent review shows a significant decline in cancer risk with increasing intake of non-steroidal anti-inflammatory drugs (NSAIDs) (primarily aspirin and ibuprofen) for at least four major types of cancer: colon, breast, lung and prostate [7]. Pre- clinical investigations also provide consistent evidence that both selective and non-selective NSAIDs effectively inhibit chemically-induced carcinogenesis of epithelial tumours [8]. In 1998, capsaicin, a common component which pro- duces the burning hot sensation of chilli-laced spicy foods (8-methyl N-vanillyl 6-nonamide), was used as an anti-inflammatory/anti-cancer medication [9] as a treat- ment for human bladder cancer. In this study, 20 patients had repeated instillations of intravesical capsaicin (at 1 - 2 mmol/l) over 5 years. Throughou t th e fo llowing 5 years with capsaicin treatment, there was no further progres- sion of the cancer in these patients. With respect to melanoma Morré et al. [10] reported that NADH oxidase activity was inhibited preferentially in A-375 melanoma cells but not in primary melanocytes, by capsaicin. They speculated that the inhibition of cell surface NADH oxidase activity may be correlated to the effects of capsaicin in inhibiting growth and inducing apoptosis. Brar et al. [11] reported that catalase, N-acetylcysteine, ebselen, dicumarol and capsaicin also inhibited growth of melanoma and other malignant cell lines. These results raise the possibility that ROS (Reactive Oxygen Species) produced endogenously by mechanisms involving the NAD (P) H: quinone oxidoredutase (NQO) can constitu- tively activate NF-kappaB in an autocrine fashion. These authors suggested that there was potential for new anti- oxidant strategies for interruptio n of oxidant signallin g of melanoma cell growth. Treatments for melanoma remain challenging. While the outcome for superficial melano mas remains good, the treatment for tumours which have invaded and spread to distant lymph nodes or other sites in the body surgery is not a very useful option. Ongoing clinical trials continue to evaluate chemotherapy approaches to the treatment of Copyright © 2013 SciRes. JCDSA  Combined Effects of Capsaicin and HA14-1 in Inducing Apoptosis in Melanoma Cells 176 metastatic melanoma. Chemotherapy, with Dacarbazine (DTIC) and temozolomide (Temodar) is currently used, however, there has been no improvement over 5-year sur- vival for patients with advanced melanoma [12]. Recent- ly Oblimersen, an antisense oligo nuc leotide that stops th e translation of Bcl-2 mRNA to protein, was found to sig- nificantly improve progression-free survival when admi- nistered in combination with dacarbazine in patients with normal lactate dehydrogenase [13,14]. Additionally more recently there are now 2 new treatments for melanoma- ipilimumab, a CTLA-4 targeting agent [15] and vemu- rafenib, a BRAF inhibitor, [16] both of which have im- proved on dacarbazine and temozolomide. It has been suggested by some authors that another promising can- didate might be HA14-1 as an inducer of apoptosis in drug resistant cancer either as a monotherapy or in com- bination with current cancer therapies [17]. HA14-1 bi- nds to Bcl-2 protein and induces apoptosis of tumour cells [18]. The regulation of apoptosis may be a central part of tumour development. Bcl-2 over-expression has been linked to tumour development and is associated with inhibition of apoptosis and also with chemotherapy resistance [19]. Accordingly the aim of this study was to investigate the effects of capsaicin and HA14-1 used individually and combined, in reducing melanoma cell viability and in inducing apop tosis in three melanoma cell lines to assess whether any useful synergy could be obtained by a com- bination of these agents. 2. Methods 2.1. Culture of Melanoma Cells The human C8161 melanoma line was established from an abdominal wall metastasis of a menopausal woman with recurrent melanoma (kindly donated by Pr ofessor F. Meyskens, University of California, Irvine, USA, via Professor M. Edwards, University of Glasgow, UK). Cells were cultured in Eagle’s modified essential medium (EMEM) supplemented with 10% (v/v) FCS, 2 mM L- Glutamine, 100 units/ml penicillin, and 100 g/ml strep- tomycin sulphate, 1.2 mg/ml amphotericin B, 1.5% (v/v) (of 100 × stock solution) vitamin concentrate, 1 mM so- dium pyruvate, 1% (v/v) non essential amino acids (NEA) and 0.187% (w/v) sodium hydrogen carbonate (Sigma, Poole, Dorset, UK). Cells were incubated at 37˚C in a humidified 5% carbon dioxide/95% air environment un- der standard conditions. The melanoma cell line A375-SM was a generous gift from Professor I. J. Fidler (USA) via Professor M. J. Humphries (University of Manchester, UK). This cell line was established in culture fro m a lymph node metas- tasis. These cells are heterogeneous in nature and a highly metastatic variant (A375-SM) was established in culture from lung metastasis produced by parental A375 cells growing subcutaneously in nude mice [20]. These cells were cultured in Eagle’s modified essential medium as described for C8161 cells. The human cutaneous cell line HBL used was originally established and described by Professor Ghanem Ghanem (Laboratory of Oncology and Experimental Surgery, Free University of Brussels, Belgium) from a lymph node metastasis of a modular malignant melanoma [21]. Cells were cultured in Ham’s F10 medium (Gibco; Paisley, Scotland) supplemented with 5% (v/v) foetal calf serum (FCS), 5% (v/v) newborn calf serum (NBCS; Advanced Protein Products, UK), 2 mM L-Glutamine, 100 units/ml penicillin, 100 g/ml St reptomyci n, 25 0 g/ml amphotericin. Each cell line was subcultured when 80% - 90% con- fluent using 0.02% (w/v) EDTA for five minutes. Cells were used between passages 20 and 50. Some melanoma cells were incubated prior to use with TNF- (300 units/ml to 1000 units/ml) Sigma; Poole, Dorset, UK. 2.2. Culture of Melanocytes Fibroblasts and melanocytes were established from skin donations from patients undergoing elective abdomino- plasties and breast reductions who gave written informed consent for excised skin to be used for research purposes on an anonymous basis. The Kroto Research Laboratory holds a Human Tissue Authority Research Tissue Bank License for anonymous tissue donations for research purposes. Melanocytes were isolated from harvested split-thick- ness skin grafts (STSGs) obtained from routine plastic surgery of breast reduction and abdominoplasties as pre- viously described [22]. The STSGs were harvested and placed in sterile saline and stored at 4˚C until processing (within 24 h). Samples of this skin were cut into 0.5 cm2 pieces using a scalpel blade and were incubated overnight (12 - 24 h) at 4˚C in 0.1% w/v trypsin. FCS was added to neutralize the trypsin and the epidermal and dermal layers were carefully separated using a pair of forceps with fine poi- nts. A scalpel blade was used for gently scraping basal keratinocytes and melanocytes from the under surface of the epidermis and the papillary surface of the dermis. The cells were collected into a mixture of FCS and PBS in sterile 25 ml universal containers. The cell sus- pension was then centrifuged at 200 g (1000 rpm) for 5 min. Once isolated, cell suspensions were seeded at 4 × 106 cells/T25 flask in a low calcium MCDB 153 basal media supplemented with 2% chelated FCS, 25 µg/ml bovine pituitary extract (BPE), 0.6 ng/ml basic fibro- blasts growth factor (bFGF), 10 µg/ml insulin, 10 µg/ml transferrin, 2.8 µg/ml hydrocortisone, 2 mM/l L-gluta- mine, 100 IU/ml; 100 µg/ml penicillin/streptomycin, 10 U/ml nystatin, 1 µg/ml α-tocopherol, 100 ng/ml cholera Copyright © 2013 SciRes. JCDSA  Combined Effects of Capsaicin and HA14-1 in Inducing Apoptosis in Melanoma Cells 177 toxin and 10 nM/l phorbol 12-myristate 13-acetate (PMA). Geneticin (100 µg/ml) was added to the media over the first few days to prevent fibroblast contamina- tion. Melanocyte cultures were fed twice weekly with this Melanocyte Growth Medium. For co-culture, mela- nocytes were trypsinised using 2 ml of a 1:1 mixture of 0.1% w/v trypsin and 0.02% w/v EDTA. Once detached 1:2 trypsin inhibitor ( TI) was used to block the effects of trypsin and melanocyte growth medium was added to the cells prior centrifuging. Cells were re-suspended in this medium and seeded at the desired density. Melanocytes were not used for experiments after passage 4. 2.3. Culture of Fibroblasts STSGs were trypsinized as described previously for the isolation of keratinocytes. The epidermal and dermal lay- ers were separated and the dermal parts of the specimens were collected. These dermal samples were washed sev- eral times in sterile PBS and then finely minced with a scalpel blade. The dermal mince was incubated at 37˚C overnight in 10 ml of a 0.5% collagenase A solution. The following day, the collagenase digest was spun down in a centrifuge at 2000 g for 10 min, the supernatan t was dis- carded and the pellet of cells was re-suspended in Fibro- blasts Culture Medium. Cells were passaged when fibro- blasts reached 80% - 90% confluence using 2 ml of a 1:1 mixture of 0.1% w/v trypsin and 0.02 5 w/v EDTA per flask. Fibroblasts were used between passages 4 and 9. 2.4. Preparation of Capsaicin (8-Methyl-N-Vanillyl-Trans-6-Nonenamide) and HA14-1 (2-Amino-6-Bromo-α-Cyano-3- (Ethoxycarbonyl)-4H-1-Benzopyran-4- Acetic Acid Ethyl Ester) A stock solution of 0.33 M capsaicin (Sigma-AldrichTM) was made by dissolving 50 mg in 0.5 ml of ethanol. Cap- saicin stock was then dissolved in cell culture medium. A stock solution of 0.02 M HA14-1 (TocrisTM) was made by dissolving 10 mg in 1 ml of ethanol. This HA14-1 stock was then dissolved in cell culture medium. Camp- tothecin (Sigma-AldrichTM) was used as a positive con- trol to induce apoptosis. 2.5. MTT (3-(4,5-Dimethyl Thiazol-2-yl)-2,5-Diphenyl Tetrazolium Bromide) Assay MTT (Sigma-AldrichTM) acts as an artificial hydrogen acceptor substrate producing a coloured formazan prod- uct which is then eluted from the cells using acidified isopropanol. This cytobiochemical assay provides a di- rect indication of the viability of cells and can be used to provide an indirect reflection of cell number. MTT assays were carried out to assess the impact of drugs on the vi- ability of melanoma cells and fibroblasts. Cells were seeded in a 24 well plate at concentrations of 5 × 104 cells/ml per well and incubated for 72 h. Media was then removed and either ibuprofen, capsaicin, HA14-1 or a combination of capsaicin and HA14-2 were added to the cells and incubated for a further 24 h period prior to as- sessment of cell viability. Cells were then washed in PBS. MTT solution 1 ml of 0.5 mg/ml in PBS was added to each well and incubated at 37˚C for 40 minutes. This was then removed and 300 l acidified isopropanol was ad- ded to each well to elute the stain. The optical density was measured using a plate reader set at 540 nm wave- length with a reference wavelengt h of 63 0 n m. 2.6. Assessment of Apoptosis by Annexin-V The Guava NexinTM assay exploits the use of Annexin V which has a strong affinity and specificity in the presence of calcium for phosphatidylserine (PS). The externalisa- tion of PS to the cell surface is one of the early apoptotic events. The kit includes Annexin V conjugated phyco- erythrin (PE; λex = 480 nm; λem = 578 nm) and 7-ami- noactinomycin D (7-AAD; λex = 555 nm; λem = 655 nm), a viability stain. 7-AAD is excluded from live cells and binds to GC rich regions of DNA within the cell al- lowing identification and distinguishing between apop- totic cells (Annexin V positive) in early (7-AAD negative) and late (7-AAD positive) stages. Annexin V is able to access internal PS as a result of increased permeability so simultaneous staining with 7-AAD is essential. Melanoma cells were plated at a density of 5 × 104 cells/ml per well in 24 well plates (Costar) containing EMEM or HAMS plus 10% (v/v) FCS and incubated for 72 hours. After this period media was removed and 1 ml of capsaicin or HA14-1 or both at desired concentrations were added to the cells and incub ated for 24 hours. Con- trol samples were incubated with medium alone. Camp- tothecin (20 M) was used as a positive control. Cells were prepared for flow cytometry by removing the culture medium, washing with PBS (×2) and deta- ched using 0.02% (w/v) EDTA (Sigma). Aspirated cul- ture medium and PBS washes (potentially containing late apoptotic and dead cells) were combined together with detached cells and centrifuged at 1000 rpm for 5 minutes. Cell pellets were re-suspended in 150 µl cold PBS buffer and divided into three parallel samples. Samples were then used to measur e cellular viability o r investigate ph o- sphatidylserine (PS) externalisation. Cells (~1 × 105) suspended in 100 µl cold PBS buffer were washed once with 1 ml ice cold 1 × Nexin® buffer (Guava Technologies) and centrifuged at 1000 rpm for 5 minutes. Cells were re-suspended in 40 µl ice-cold 1 × Nexin® buffer and incubated on ice in the dark with 5 µl annexin-V PE and 5 µl 7-AAD for 20 minutes. 450 µl 1 × Nexin® buffer was added to each tube, gently agitated Copyright © 2013 SciRes. JCDSA 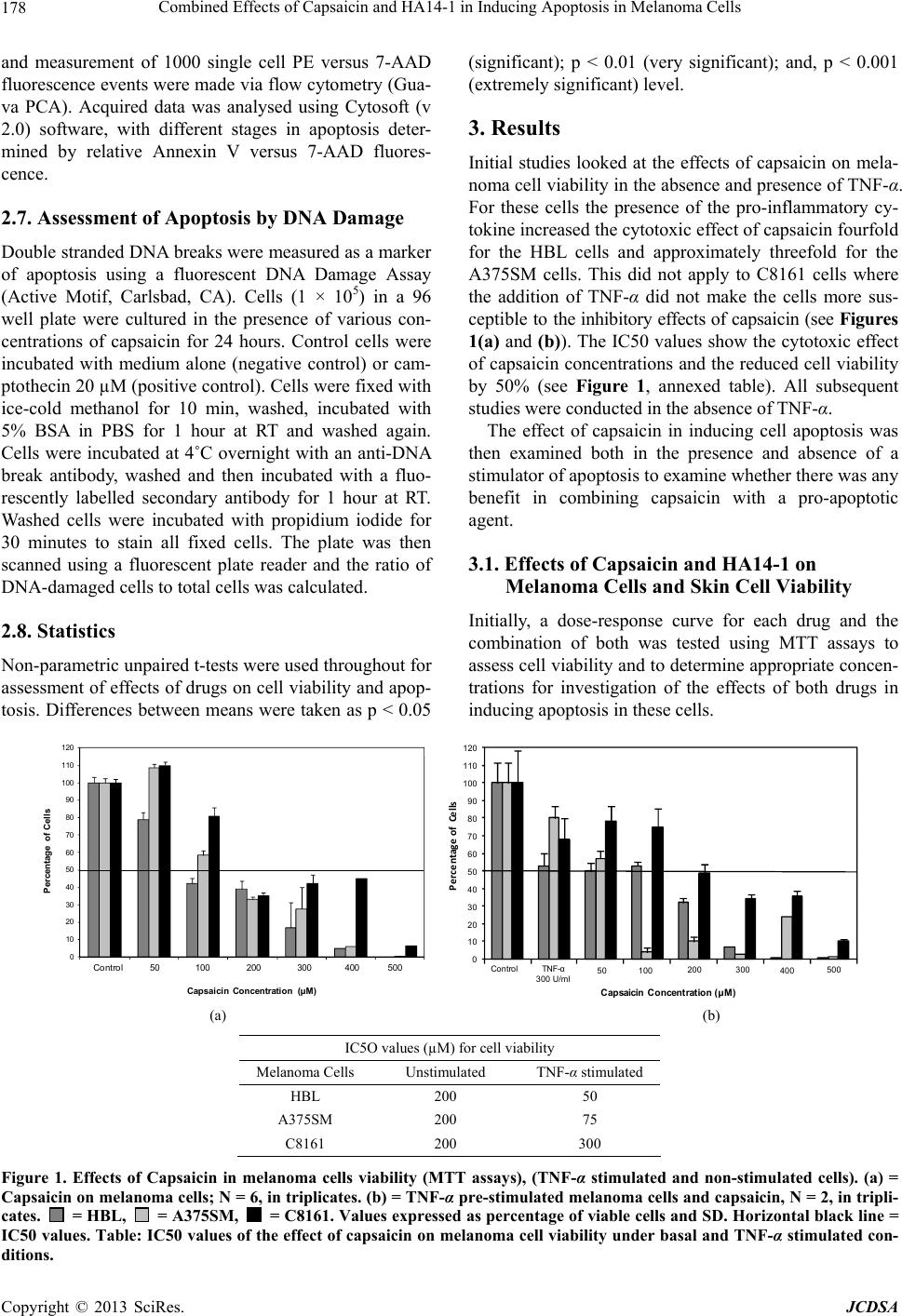 Combined Effects of Capsaicin and HA14-1 in Inducing Apoptosis in Melanoma Cells Copyright © 2013 SciRes. JCDSA 178 and measurement of 1000 single cell PE versus 7-AAD fluorescence events were made via flow cytometry (Gua- va PCA). Acquired data was analysed using Cytosoft (v 2.0) software, with different stages in apoptosis deter- mined by relative Annexin V versus 7-AAD fluores- cence. (significant); p < 0.01 (very significant); and, p < 0.001 (extremely significant) level. 3. Results Initial studies looked at the effects of capsaicin on mela- noma cell viability in the ab sence and presence of TNF-α. For these cells the presence of the pro-inflammatory cy- tokine increased th e cyto toxic effect of capsaicin fourfold for the HBL cells and approximately threefold for the A375SM cells. This did not apply to C8161 cells where the addition of TNF-α did not make the cells more sus- cep tib le to the inhibitory effects of capsaicin (see Figures 1(a) and (b)). The IC50 values show the cytotoxic effect of capsaicin con centrations and the reduced cell viability by 50% (see Figure 1, annexed table). All subsequent studies were conducted in the absence of TNF-α. 2.7. Assessment of Apoptosis by DNA Damage Double stranded DNA breaks were measured as a marker of apoptosis using a fluorescent DNA Damage Assay (Active Motif, Carlsbad, CA). Cells (1 × 105) in a 96 well plate were cultured in the presence of various con- centrations of capsaicin for 24 hours. Control cells were incubated with medium alone (negative control) or cam- ptothecin 20 µM (positive contro l). Cells were fixed with ice-cold methanol for 10 min, washed, incubated with 5% BSA in PBS for 1 hour at RT and washed again. Cells were incubated at 4˚C overnight with an anti-DNA break antibody, washed and then incubated with a fluo- rescently labelled secondary antibody for 1 hour at RT. Washed cells were incubated with propidium iodide for 30 minutes to stain all fixed cells. The plate was then scanned using a fluorescent plate reader and the ratio of DNA-damaged cells to total cells was calculated. The effect of capsaicin in inducing cell apoptosis was then examined both in the presence and absence of a stimulator of apoptosis to examine whether there was any benefit in combining capsaicin with a pro-apoptotic agent. 3.1. Effects of Capsaicin and HA14-1 on Melanoma Cells and Skin Cell Viability Initially, a dose-response curve for each drug and the combination of both was tested using MTT assays to assess cell viability and to determine ap propriate concen- trations for investigation of the effects of both drugs in inducing apop tosis in these cells. 2.8. Statistics Non-parametric unpaired t-tests were used throughout for assessment of effects of drugs on cell viability and apop- tosis. Differences between means were taken as p < 0.05 0 10 20 30 40 50 60 70 80 90 100 110 120 PercentageofCells Control TNF-α 300 U/ml50 100 200 300 400 500 C ap saicin C on cen t rat i on (µ M ) 0 10 20 30 40 50 60 70 80 90 100 110 120 Percentage of Cells Capsai cin Concentrati on (µM) Control 50100200300400500 (a) (b) IC5O values (µM) for cell viability Melanoma Cells Unstimulated TNF-α stimulated HBL 200 50 A375SM 200 75 C8161 200 300 Figure 1. Effects of Capsaicin in melanoma cells viability (MTT assays), (TNF-α stimulated and non-stimulated cells). (a) = Capsaicin on melanoma cells; N = 6, in triplicates. (b) = TNF-α pre-stimulated me lanoma cells and capsaicin, N = 2, in tripli- cates. = HBL, = A375SM, = C8161. Values expressed as percentage of viable cells and SD. Horizontal black line = IC50 values. Table: IC50 values of the effect of capsaicin on melanoma cell viability under basal and TNF-α stimulated con- ditions. 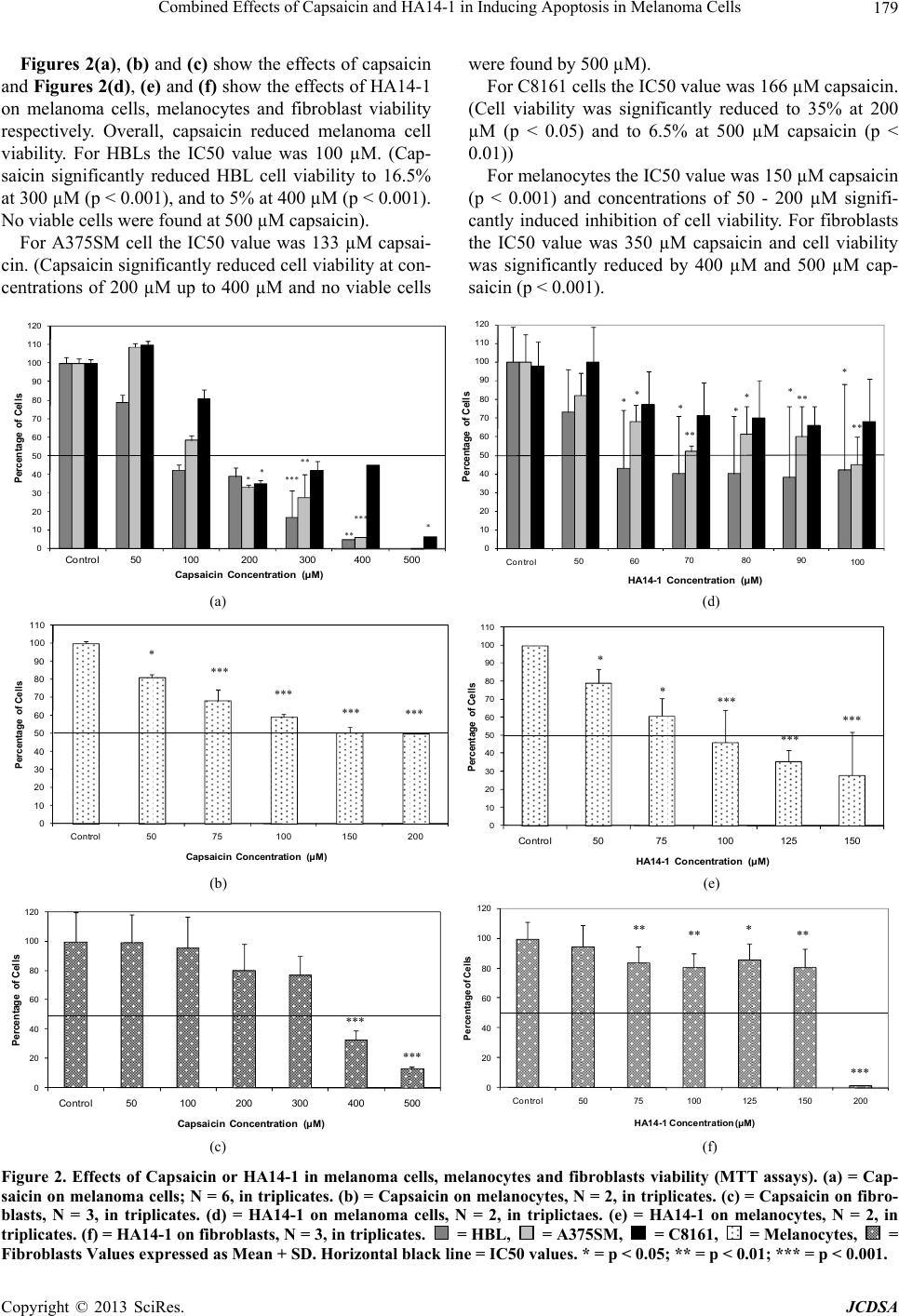 Combined Effects of Capsaicin and HA14-1 in Inducing Apoptosis in Melanoma Cells 179 Figures 2(a), (b) and (c) show the effects of capsaicin and Figures 2(d), (e) and (f) show the effects of HA14-1 on melanoma cells, melanocytes and fibroblast viability respectively. Overall, capsaicin reduced melanoma cell viability. For HBLs the IC50 value was 100 µM. (Cap- saicin significantly reduced HBL cell viability to 16.5% at 300 µM (p < 0.001), and to 5% at 400 µM (p < 0.001). No viable cells were found at 500 µM capsaicin). For A375SM cell the IC50 value was 133 µM capsai- cin. (Capsaicin significantly redu ced cell viability at con- centrations of 200 µM up to 400 µM and no viable cells were foun d by 500 µM). For C8161 cells the IC50 value was 166 µM capsaicin. (Cell viability was significantly reduced to 35% at 200 µM (p < 0.05) and to 6.5% at 500 µM capsaicin (p < 0.01)) For melanocytes the IC50 value was 150 µM capsaicin (p < 0.001) and concentrations of 50 - 200 µM signifi- cantly induced inhibition of cell viability. For fibroblasts the IC50 value was 350 µM capsaicin and cell viability was significantly reduced by 400 µM and 500 µM cap- saicin (p < 0.001). 0 10 20 30 40 50 60 70 80 90 100 110 120 Percentage of Cells Capsaicin Concentration (µM) Control 50100200300400500 ** *** *** ** * 0 10 20 30 40 50 60 70 80 90 100 110 120 Percentage of Cells HA14-1 Concentration (µM) Control 5060 708090100 ** ** * ** * ** *** (a) (d) 0 10 20 30 40 50 60 70 80 90 100 110 Control5075100 150 200 Percentage of Cells Capsaicin Concentration (µM) * *** *** *** *** 0 10 20 30 40 50 60 70 80 90 100 110 Control 5075100125150 Percentage of Cells HA14-1 Concentration (µM) * **** *** *** (b) (e) 0 20 40 60 80 100 120 Control50100 200 300 400 500 Percentage of Cells Capsaicin Concentration (µM ) *** *** 0 20 40 60 80 100 120 Control5075100 125 150 200 Percentage of Cells HA14-1 Concentration (µM) ** ** *** *** (c) (f) Figure 2. Effects of Capsaicin or HA14-1 in melanoma cells, melanocytes and fibroblasts viability (MTT assays). (a) = Cap- saicin on melanoma cells; N = 6, in triplicates. (b) = Capsaicin on melanocytes, N = 2, in triplicates. (c) = Capsaicin on fibro- blasts, N = 3, in triplicates. (d) = HA14-1 on melanoma cells, N = 2, in triplictaes. (e) = HA14-1 on melanocytes, N = 2, in triplicates. (f) = HA14-1 on fibroblasts, N = 3, in triplicates. = HBL, = A375SM, = C8161, = Melanocytes, = Fibroblasts Values expre sse d as M ean + SD. Horizontal black line = IC50 values. * = p < 0.05; ** = p < 0.01; *** = p < 0.001. Copyright © 2013 SciRes. JCDSA  Combined Effects of Capsaicin and HA14-1 in Inducing Apoptosis in Melanoma Cells 180 In summary, the three melanoma cells and melano- cytes showed a similar sensitivity to capsaicin with cap- saicin concentrations above 200 µM becoming cytotoxic. However, fibroblasts tolerated capsaicin up to 300 µM without any effect with an IC50 of around 350 µM, showing these cells were more resistant to the metabolic inhibitory effects of capsaicin. HA14-1 also reduced melanoma cell viability. The IC50 value for HA14-1 in HBL cells was around 60 µM (p < 0.05). For A 375SM cells the IC50 value was around 100 µM HA14-1. For C8161 cells the IC50 value was greater than 100 µM HA14-1. For melanocytes the IC50 value was around 100 µM HA14-1 (see Figure 2(e)). Fibroblasts coped relatively well with HA14-1 up to concentrations of 150 µM but 200 µM reduced viability to only 1.3% (see Figure 2(f)). In summary, the IC50 values for melanoma cells and normal skin cells were: HBL (55 µM) followed by mela- nocytes (80 µM) , A375SM (95 µM), C8161 (higher than 100 µM) and fibroblasts (180 µM). In conclusion, these results suggest that melanoma cells and melanocytes had similar sensitiv ities to HA14-1 whilst fibroblasts were more resistant to HA14-1 (by a factor of two-fold). 3.2. Effects of Combined HA14-1 and Capsaicin on Melanoma and Skin Cell Viability Based on their IC50 values a combination of both drugs was then used to investigate their combined effect on me- lanoma cells, melanocytes and fibroblasts viability. The predicted values from an additive effect of using both agents were also calculated. Figure 3 illustrates the effects of HA14-1 and capsai- cin alone and in combination on melanoma cells (Fig- ures 3(a), (b) and (c)), melanocytes (Figure 3(d)) and fibroblasts (Figure 3(e)). For HBL cells both agents significantly reduced cell viability to 50% (p < 0.05). This was not significantly different to the predicted additive effects of these two agents which predicted a reduction in HBL viability to 30%. For A375SM cell the combined drugs reduced viability to 57% (p < 0.001). This was not significantly different to the predicted additive value of reduction in A375SM cell viability to 47%. For C8161 cells the two drugs combined reduced vi- ability to 56% (p < 0.001) which was exactly the pre- dicted value for an ad ditive effect (57%) of these agents. For melanocytes the combined agents reduced viabil- ity to 19% (p < 0.05) which was exactly as predicted for an additive effect of these agents. For fibroblasts the combination of the two agents re- duced viab ility to only 0.4% (p < 0.001 ) which showed a strong synergistic effect as the predicted effect was 35% (p < 0.001). However, it should be noted that higher con- centrations of HA14-1 and capsaicin were deliberately used for these cells as they had been shown to be more resistant to both of these agents compared to the other melanoma cells and melanocytes. 3.3. Effects of Capsaicin and HA14-1 Alone and in Combination in Inducing Apoptosis in Melanoma Cells and Skin Cells Figure 4 shows the effects of increasing capsaicin in in- ducing apoptosis in melanoma cells and in fibroblasts using the Annexin-V assay for apoptosis. For HBL cells (Figure 4(a)), a significant increase in late apoptosis with reduced cell viability started at cap- saicin concentration of 300 µM. For A375SM cells (Fig- ure 4(b)) capsaicin significantly increased late apoptotic cells and reduced cell viability at 400 µM. For C8161 cells (Figure 4(c)) capsaicin increased nuclear debris and reduced cell viability at around 400 µM capsaicin. Over- all, fibroblasts coped well with capsaicin at the highest concentration tested, 500 µM, see Figure 4(d). The concentration of capsaicin which reduced cell vi- ability to 50% was calculated to be 220 µM for HBL cells, 350 µM for A375SM cells and 320 µM for C8161 cells. Capsaicin had no effect on fibroblasts at concen- trations up to 500 µM. 20 µM camptothecin was used as a positive control and significantly induced apoptosis in 2 out of 3 mela- noma cell lines and fibroblasts. This was not significant for HBL cells due to sample variation. 3.4. Effects of Capsaicin in Inducing Apoptosis in Melanoma Cells Assessed by DNA Damage Assessment The effects of capsaicin in inducing apoptosis in mela- noma cells were also investigated using a DNA Damage assay. For HBL cells (see Figure 5) capsaicin concentrations of 300 µM, 400 µM, and 500 µM significantly reduced HBL cells viability to 57.8%, 57.1% and 48% (p < 0.001 in all cases). The IC50 value was aro und 480 µM capsai- cin. A375SM cell viability was significantly reduced to 68.7% at 300 µM capsaicin concentrations (p < 0.01) and the IC50 value was 440 µM. The IC50 value for C8161 cells was 500 µM capsaicin (p < 0.05). Fibroblasts were not affected by the effects of cap- saicin up to 500 µM (p > 0.05). 20 µM camptothecin, which was used as a positive control, significantly reduced viability of HBL and A375- SM cells (p < 0.001) whilst not significantly reducing C8161 cell viability (p > 0.05). 20 µM camptothecin h ad Copyright © 2013 SciRes. JCDSA 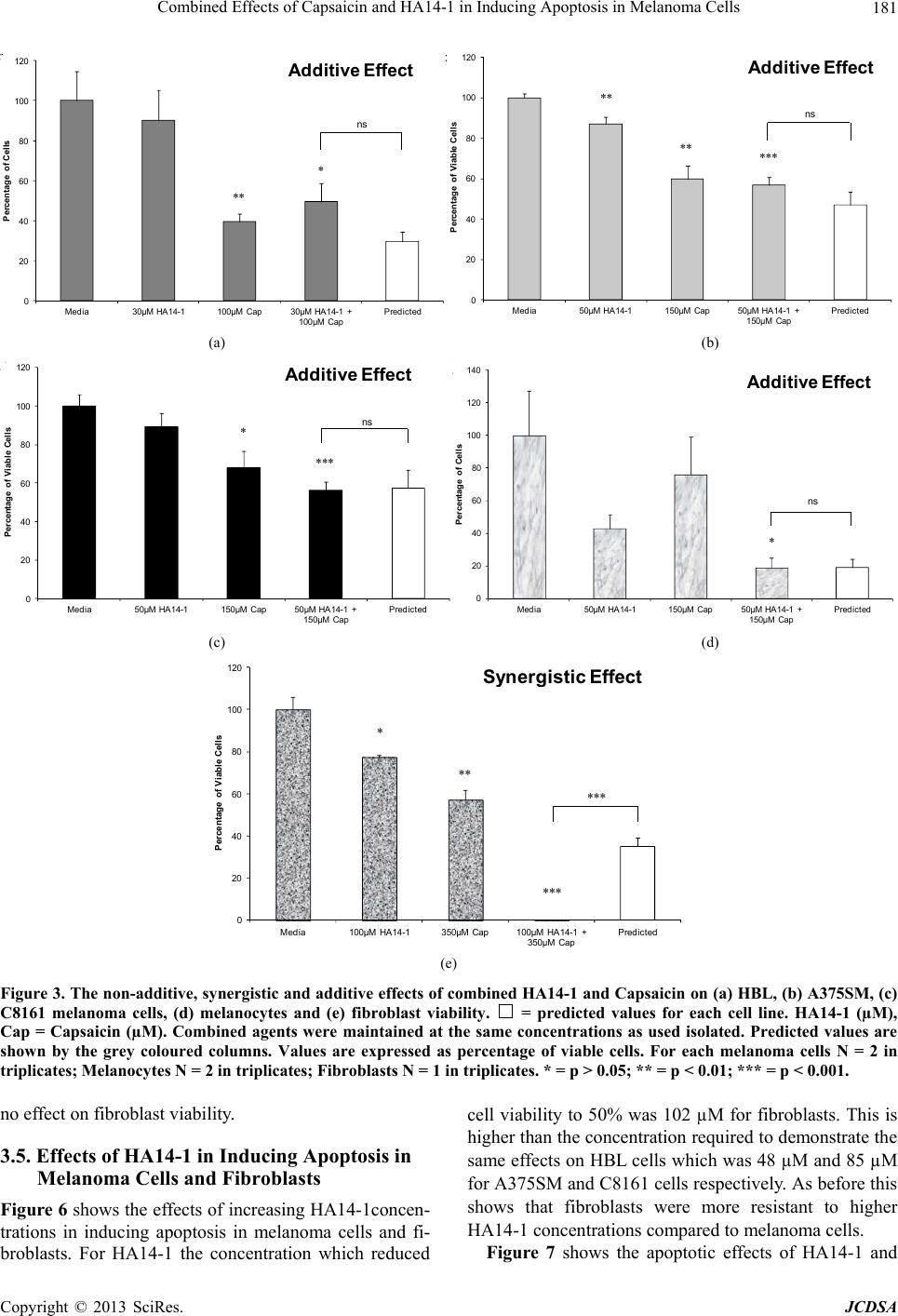 Combined Effects of Capsaicin and HA14-1 in Inducing Apoptosis in Melanoma Cells 181 0 20 40 60 80 100 120 Media30µM HA14-1100µM Cap30µM HA14-1 + 100µM Cap Predicted Percentage of Cells Additive Effect ** * ns 0 20 40 60 80 100 120 Media50µM HA14-1150µM Cap50µM HA14-1 + 150µM Cap Predicted Percentage of Viable Cells Additive Effect ** ** *** ns (a) (b) 0 20 40 60 80 100 120 Media50µM HA14-1150µM Cap50µM HA14-1 + 150µM Cap Predicted Per centage of V i ab l e Cel ls Additive Effect * *** ns 0 20 40 60 80 100 120 140 Media50µM HA14-1150µM Cap50µM HA14-1 + 150µM Cap Predicted Percentage of Cells Additive Effect * ns (c) (d) 0 20 40 60 80 100 120 Media100µM HA14-1350µM Cap100µM HA14-1 + 350µM Cap Predicted Per centag e of V i ab l e Cells S ner istic Effect * ** *** *** (e) Figure 3. The non-additive, synergistic and additive effects of combined HA14-1 and Capsaicin on (a) HBL, (b) A375SM, (c) C8161 melanoma cells, (d) melanocytes and (e) fibroblast viability. = predicted values for each cell line. HA14-1 (µM), Cap = Capsaicin (µM). Combined agents were maintained at the same concentrations as used isolated. Predicted values are shown by the grey coloured columns. Values are expressed as percentage of viable cells. For each melanoma cells N = 2 in triplicates; Melanocytes N = 2 in triplicates; Fibroblasts N = 1 in triplicates. * = p > 0.05; ** = p < 0.01; *** = p < 0.001. no effect on fibroblast viability. 3.5. Effects of HA14-1 in Inducing Apoptosis in Melanoma Cells and Fibroblasts Figure 6 shows the effects of increasing HA14-1concen - trations in inducing apoptosis in melanoma cells and fi- broblasts. For HA14-1 the concentration which reduced cell viability to 50% was 102 µM for fibroblasts. This is higher than the co ncentration required to demonstrate the same effects on HBL cells which was 48 µM and 85 µM for A375SM and C816 1 cells respectively. As before this shows that fibroblasts were more resistant to higher HA14-1 concentration s compared to melanoma cells. Figure 7 shows the apoptotic effects of HA14-1 and Copyright © 2013 SciRes. JCDSA 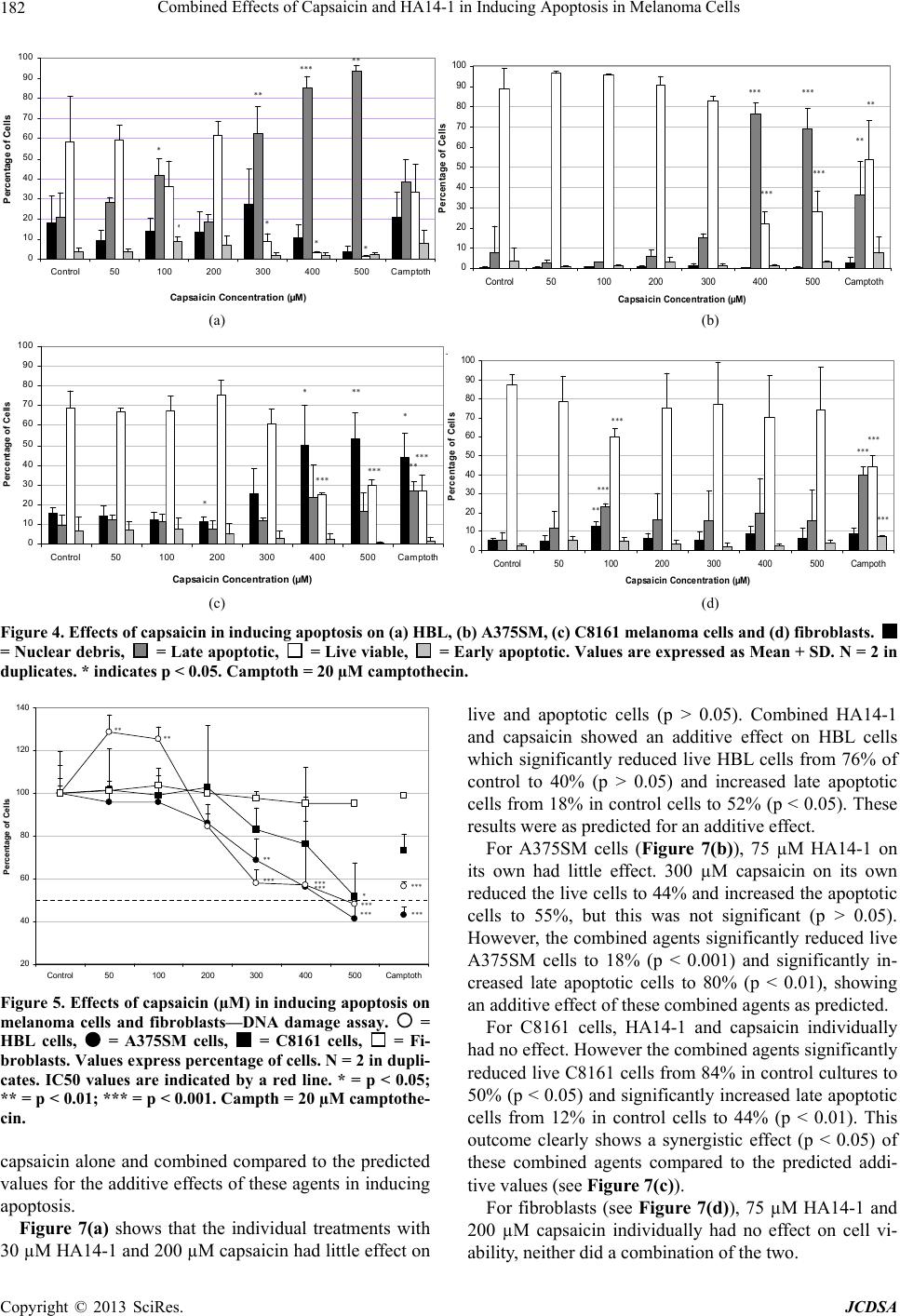 Combined Effects of Capsaicin and HA14-1 in Inducing Apoptosis in Melanoma Cells 182 0 10 20 30 40 50 60 70 80 90 100 Control50100 200 300400500Camptoth Capsaicin Concentration (µM) Percentage of Cells * *** ** ** * * * 0 10 20 30 40 50 60 70 80 90 100 Control50100200300400500 Camptoth Capsaicin Concentration (µM) Percentage of Cells *** *** ** ** *** *** (a) (b) 0 10 20 30 40 50 60 70 80 90 100 Control50100 200300 400500Camptoth Capsai cin Concentration (µM) Percentage of Cells *** ** * * *** *** *** 0 10 20 30 40 50 60 70 80 90 100 Control50100 200300 400500Campoth Capsaicin Concentration (µM) Percentage of Cells *** ** *** *** *** *** (c) (d) Figure 4. Effects of capsaicin in inducing apoptosis on (a) HBL, (b) A375SM, (c) C8161 melanoma cells and (d) fibroblasts. = Nuclear debris, = Late apoptotic, = Live viable, = Early apoptotic. Values are expressed as Mean + SD. N = 2 in duplicates. * indicates p < 0.05. Camptoth = 20 µM camptothecin. 20 40 60 80 100 120 140 Control50100200300400500Camptoth Percentage of Cells ** *** ** *** *** ** *** *** *** * *** Figure 5. Effects of capsaici n (µM) in inducing apoptosis on melanoma cells and fibroblasts—DNA damage assay. = HBL cells, = A375SM cells, = C8161 cells, = Fi- broblasts. Values expre ss percentage of cells. N = 2 in dupli- cates. IC50 values are indicated by a red line. * = p < 0.05; ** = p < 0.01; *** = p < 0.001. Campth = 20 µM camptothe- cin. capsaicin alone and combined compared to the predicted values for the add itive effects of these agents in inducing apoptosis. Figure 7(a) shows that the individual treatments with 30 µM HA14-1 and 200 µM capsaicin had little effect on live and apoptotic cells (p > 0.05). Combined HA14-1 and capsaicin showed an additive effect on HBL cells which significantly reduced live HBL cells from 76% of control to 40% (p > 0.05) and increased late apoptotic cells from 18% in control cells to 52% (p < 0.05). These results were as predicted for an additive effect. For A375SM cells (Figure 7(b)), 75 µM HA14-1 on its own had little effect. 300 µM capsaicin on its own reduced the live cells to 44% and increased the apoptotic cells to 55%, but this was not significant (p > 0.05). However, the combined agents significantly reduced live A375SM cells to 18% (p < 0.001) and significantly in- creased late apoptotic cells to 80% (p < 0.01), showing an additive effect of these combined agents as predicted. For C8161 cells, HA14-1 and capsaicin individually had no effect. However the combined agents significantly reduced live C8161 cells from 84% in control cultures to 50% (p < 0.05) and signif icantly increased late apoptotic cells from 12% in control cells to 44% (p < 0.01). This outcome clearly shows a synergistic effect (p < 0.05) of these combined agents compared to the predicted addi- tive values (see Figure 7(c)). For fibroblasts (see Figure 7(d)), 75 µM HA14-1 and 200 µM capsaicin individually had no effect on cell vi- ability, neither did a combination of the two. Copyright © 2013 SciRes. JCDSA  Combined Effects of Capsaicin and HA14-1 in Inducing Apoptosis in Melanoma Cells 183 0 10 20 30 40 50 60 70 80 90 100 Control2030405060 HA14-1 Conce ntration (µM) Percentage of Cells ** ** ** ** *** * *** ** * 0 10 20 30 40 50 60 70 80 90 100 Control 5075100125150 HA14-1 Concentration (µM) Percentage of Cells *** *** ** *** ** * (a) (b) 0 10 20 30 40 50 60 70 80 90 100 Control 5075100125150 HA14-1 Concentrati on (µM) Percentage of Cells *** *** ** * *** 0 10 20 30 40 50 60 70 80 90 100 Control 5075100125150 HA14-1 Concentration (µM) Percentage of Cells (c) (d) Figure 6. Effects of HA14-1 on (a) HBL, (b) A375SM, (c) C8161 melanoma cells and (d) fibroblasts. Values are expressed as Mean + SD. = Nuclear debris, = Late apoptotic, = Live viable, = Early apoptotic. Values are expressed as Mean + SD. N = 2 in duplicates. Fibr oblasts N = 1 in duplicates. * = p < 0.05; ** = p < 0.01; *** = p < 0.001. 0 10 20 30 40 50 60 70 80 90 100 Control30µM HA14-1200µM Cap30µM HA14-1 + 200µM Cap Predicted Percentage of Cells Additive Effect * * 0 10 20 30 40 50 60 70 80 90 100 Control75µM HA14-1300µM Cap75µM HA14-1 + 300µM Cap Predict ed Percentage of Cells *** ** ** Additive Effect (a) (b) 0 10 20 30 40 50 60 70 80 90 100 Control75µM HA14-1300µM Cap75µM HA14-1 + 300µM Cap Predicted Percentage of Cells ** * * * Synergistic Effect 0 10 20 30 40 50 60 70 80 90 100 Control 75µM HA14-1 200µM Cap75µM HA14-1 + 250µM Cap Predict ed Percentage of Cells (c) (d) Figure 7. Effects of HA14-1 and Capsaicin on (a) HBL, (b) A375SM, (c) C8161 melanoma cells and (d) fibroblasts. = Nu- clear debris, = Late apoptotic, = Live viable, = Early apoptotic. Values are expressed as Mean + SD. N = 2 in du- plicates. For fibroblasts N = 1 in duplicates. * = p < 0.05; ** = p < 0.01; *** = p < 0.001. Ta bl e 1 summarises the effects of capsaicin and HA- 14-1 on melanoma cells, melanocytes and fibroblasts in reducing cell viability and inducing apoptosis comparing the three different methods used. Copyright © 2013 SciRes. JCDSA 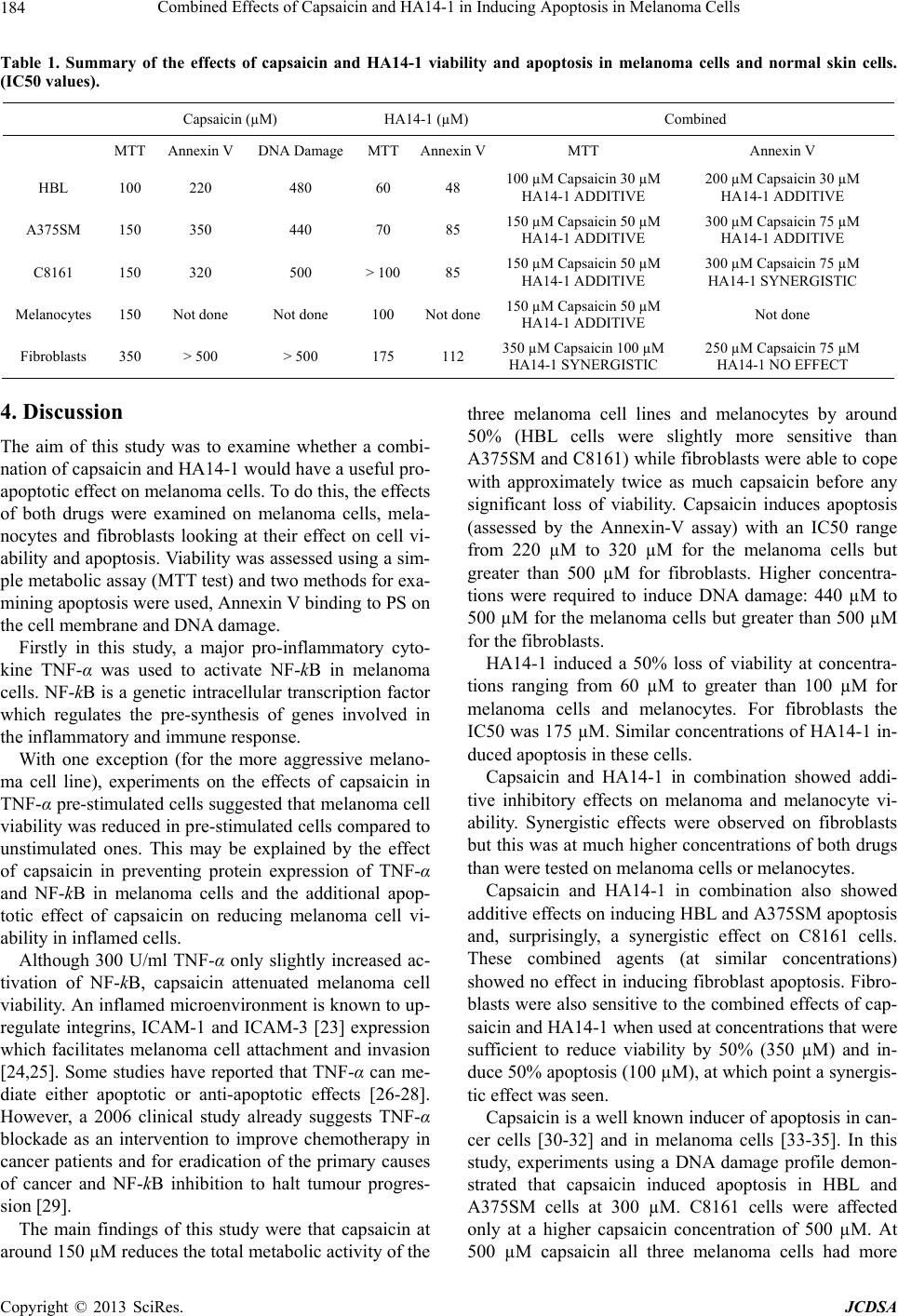 Combined Effects of Capsaicin and HA14-1 in Inducing Apoptosis in Melanoma Cells 184 Table 1. Summary of the effects of capsaicin and HA14-1 viability and apoptosis in melanoma cells and normal skin cells. (IC50 values). Capsaicin (µM) HA14-1 (µM) Combined MTT Annexin VDNA Damage MTTAnnexin VMTT Annexin V HBL 100 220 480 60 48 100 µM Capsaicin 30 µM HA14-1 ADDITIVE 200 µM Capsaicin 30 µM HA14-1 ADDITIVE A375SM 150 350 440 70 85 150 µM Capsaicin 50 µM HA14-1 ADDITIVE 300 µM Capsaicin 75 µM HA14-1 ADDITIVE C8161 150 320 500 > 10085 150 µM Capsaicin 50 µM HA14-1 ADDITIVE 300 µM Capsaicin 75 µM HA14-1 SYNERGISTIC Melanocytes 150 Not done Not done 100 Not done 150 µM Capsaicin 50 µ M HA14-1 ADDITIVE Not done Fibroblasts 350 > 500 > 500 175 112 350 µM Capsaicin 100 µM HA14-1 SYNERGISTIC250 µM Capsaicin 75 µM HA14-1 NO EFFECT 4. Discussion The aim of this study was to examine whether a combi- nation of capsaicin and HA14-1 would have a useful pro- apoptotic effect on melanoma cells. To do this, the effects of both drugs were examined on melanoma cells, mela- nocytes and fibroblasts looking at their effect on cell vi- ability and apopto sis. Viab ility was assessed using a sim- ple metabolic assay (MTT test) and two methods for exa- mining apoptosis were used, Annexin V binding to PS on the cell membrane and DN A damage. Firstly in this study, a major pro-inflammatory cyto- kine TNF-α was used to activate NF-kB in melanoma cells. NF-kB is a genetic intracellular transcription factor which regulates the pre-synthesis of genes involved in the inflammatory and immune response. With one exception (for the more aggressive melano- ma cell line), experiments on the effects of capsaicin in TNF-α pre-stimulated cells suggested that melanoma cell viability was reduced in pre-stimulated cells compared to unstimulated ones. This may be explained by the effect of capsaicin in preventing protein expression of TNF-α and NF-kB in melanoma cells and the additional apop- totic effect of capsaicin on reducing melanoma cell vi- ability in inflamed cells. Although 300 U/ml TNF-α only slightly increased ac- tivation of NF-kB, capsaicin attenuated melanoma cell viability. An inflamed microenv ironment is known to up- regulate integrins, ICAM-1 and ICAM-3 [23] expression which facilitates melanoma cell attachment and invasion [24,25]. Some studies have reported that TNF-α can me- diate either apoptotic or anti-apoptotic effects [26-28]. However, a 2006 clinical study already suggests TNF-α blockade as an intervention to improve chemotherapy in cancer patients and for eradication of the primary causes of cancer and NF-kB inhibition to halt tumour progres- sion [29]. The main findings of this study were that capsaicin at around 150 µM reduces the total metabolic activ ity of the three melanoma cell lines and melanocytes by around 50% (HBL cells were slightly more sensitive than A375SM and C8161) while fibrob lasts were able to cope with approximately twice as much capsaicin before any significant loss of viability. Capsaicin induces apoptosis (assessed by the Annexin-V assay) with an IC50 range from 220 µM to 320 µM for the melanoma cells but greater than 500 µM for fibroblasts. Higher concentra- tions were required to induce DNA damage: 440 µM to 500 µM for the melanoma cells but greater than 500 µM for the fibroblasts. HA14-1 induced a 50% loss of viability at concentra- tions ranging from 60 µM to greater than 100 µM for melanoma cells and melanocytes. For fibroblasts the IC50 was 175 µM. Similar con centrations of HA14-1 in- duced apoptosis in these cells. Capsaicin and HA14-1 in combination showed addi- tive inhibitory effects on melanoma and melanocyte vi- ability. Synergistic effects were observed on fibroblasts but this was at much higher concentrations of both drugs than were tested on melanoma cells or melanocytes. Capsaicin and HA14-1 in combination also showed additive effects on inducing HBL and A375SM apoptosis and, surprisingly, a synergistic effect on C8161 cells. These combined agents (at similar concentrations) showed no effect in inducing fibroblast apoptosis. Fibro- blasts were also sensitive to the combined effects of cap- saicin and HA14-1 when used at concentrations that were sufficient to reduce viability by 50% (350 µM) and in- duce 50% apop tosis (100 µM), at which po int a synergis- tic effect was seen. Capsaicin is a well known inducer of apoptosis in can- cer cells [30-32] and in melanoma cells [33-35]. In this study, experiments using a DNA damage profile demon- strated that capsaicin induced apoptosis in HBL and A375SM cells at 300 µM. C8161 cells were affected only at a higher capsaicin concentration of 500 µM. At 500 µM capsaicin all three melanoma cells had more Copyright © 2013 SciRes. JCDSA  Combined Effects of Capsaicin and HA14-1 in Inducing Apoptosis in Melanoma Cells 185 than a 50% reduction in cell viability. Fibroblasts were not affected by capsaicin at up to 500 µM. These results suggest that capsaicin may cause DNA breakage more readily in melanoma cells than in normal skin cells such as fibroblasts. The selective apoptosis inducing effect of capsaicin has been previously reported [36]. There are many reports suggesting mechanisms of ac- tion of capsaicin inducing apoptosis. For example, extra production of ROS by mitochondrial NADH oxidase [31,37], ras activation inducing apoptosis in transformed cells [35], Bcl-2 down-regulation and caspase 3 activa- tion [38,39] and inhibition of interleukin-6-induced STAT3 activation [40]. In this study, the effects of cap- saicin on inducing DNA damage are very clear suggest- ing that this is one mechanism for explaining apoptosis. What are the molecular mechanisms by which cap- saicin selectively induces apoptosis in transformed cells without affecting normal skin cells? One report from Bodó [41] demonstrated that functional VR1 (Vanilloid Receptor-1) which capsaicin interacts with, was present on human epidermal cells, specifically keratinocytes and Langerhans cells, but not on melanocytes; it was also present in dermal cells, mast cells, sweat gland epithet- lium, sebocytes, endothelial cells and smooth muscle cells but not, interestingly, in connective tissue fibro- blasts. The lack of response of fibroblasts to capsaicin may be based on their lack of receptors but on the other hand, Kim et al. [42] reported the existence of VR1 re- ceptors on fibroblasts. Clearly this is an area that requires further investigation. As summarised in Table 1 capsaicin and HA14-1 each reduced cell viability and induced apoptosis in the mela- noma cells, melanocytes and fibroblasts but at different concentrations. With respect to metabolic activity of the cells (which is what the MTT assay measures) this was reduced by capsaicin with the melanoma cells and me- lanocytes showing a similar sensitivity. Fibroblast viabil- ity was also reduced by capsaicin (arguing for the pres- ence of VR1 receptors on these cells) but it required ap- proximately twice the concentration of capsaicin com- pared to that which reduced viability in melanocytes and melanoma cells. For all cells higher concentrations of capsaicin (approximately twice as h igh) were required to show induction of apoptosis as evidenced by using the Annexin-V assay and still higher concentrations as mea- sured by the assay for DNA damage. The differences found using these different assays are much as expected. The MTT assay detects a reduction in cell viability and as such is quite sensitive. Not all cells with a reduced viability will necessarily go on to become apoptotic or die. Many cells may recover. With the apoptotic assessment (Annexin-V and DNA damage assays), it was found that the cells had the same sensitivity pattern, in that HBL were more sensitive than A375SM which were in turn more sensitive than C8161 cells and fibroblasts but higher capsaicin concentrations were required to demonstrate apoptosis. Annexin-V binds specifically to phospholipids at an early stage of the apoptotic process (a disruption of membrane phospholipids asymmetry exposes PS on the outer cytoplasmatic membrane). In contrast the DNA damage assay is based on breaks in doubled-stranded DNA resulting in the phosphorylation of the histone variant H2AX at serine 139. This measures late apoptosis (by which time physical breaks in the DNA have oc- curred) so it is entirely as expected that the IC50 values for capsaicin assessed using this assay were higher than when using Annexin-V. With respect to HA14-1, its mechanism of action was identified by Wang [43]. These authors verified that HA14-1 interacts with Bcl-2 in vitro. This protein in- duces apoptosis in a variety of tumour cell lines and co- operates wi t h o t he r drugs [44, 45]. Melanoma cells and melanocytes had a roughly similar sensitivity to HA14-1 as assessed by a loss of cell viabi- lity with IC50 values ranging from 60 µM to over 100 µM. For fibroblasts the IC50 was 175 µM, again sug- gesting that these cells are more resistant than melano- cytes and melanoma cells. With respect to induction of apoptosis slightly lower concentrations of HA14-1 were required to produce apo- ptosis (compared to reducing cell viability) as assessed by Annexi n- V (DNA damage was not undertaken i n the se experiments). The IC50 values were 48 µM - 85 µM for the three melanoma cells and 112 µM for fibroblasts. This again is much as expected as this drug is known to interact directly with Bcl-2 to induce apop tosis directly. The two agents were then tested together to see if agents thought to act by different mechanisms would show any useful additivity or synergy in their actions on melanoma cells. Combination therapies have been repor- ted by others [ 46,47]. For both HBL and A375SM cells the effects of com- bining both were additive rather than synergistic, but interestingly for C8161 cells there was eviden ce of some synergy when Annexin-V was measured as an early in- dicator of apoptosis. This was encouraging as C8161 melanoma cells are particularly aggressive with respect to metastases. Concerning loss of viability the effects of combining the two were additive rather than synergistic. For melanocytes combining the two gave a loss of vi- ability that was additive (effects on apoptosis were not studied in these cells). For fibroblasts the results appear contradictory at first glance (Table 1) in that there is ap- parent synergy with respect to loss of viability but no effect when these two agents were combined on apop- tosis. However this is explained by the concentrations used. Copyright © 2013 SciRes. JCDSA  Combined Effects of Capsaicin and HA14-1 in Inducing Apoptosis in Melanoma Cells 186 The concentration s of capsaicin and HA14-1 that were used in measuring early stage apoptosis in fibroblasts were similar to those used for the melanoma cells. How- ever, in studying viability, as the fibroblasts had proven relatively resistant to capsaicin and HA14-1, higher con- centrations of both were studied and then a combination of the two was found to be synergistic. This argues strongly for fibroblasts having a lower concentration of the VR1 receptor rather than being entirely lacking in it. Capsaicin and HA14-1 showed a synergistic effect on C8161 cells. This study suggests that these agents may have different mechanisms of action in inducing apop- tosis in melanoma cells. As previously reported capsaicin induces oxidative stress [10,11,31], regulates activation of NF-kB and IL-8 [40] and hypoxia inducing factor- 1-alpha in human melanoma [34], but also inhibits Bcl-2 anti-apoptotic activity [38,39], whilst HA14-1 blocks the anti-apoptotic Bcl-2 protein [17,18] inducing melanoma cell apoptosis. Thus there is some overlap in their activity in that both are reported to inhibit Bcl-2 anti-apoptotic protein. Capsaicin has been used as an anti-inflammatory agent [47-49]. Topical capsaicin has been used for peripheral neuropathies [50] and patients with soft tissue pain and chronic back pain [51] and for treating burning mouth syndrome [52]. Capsaicin instillation has been used for postoperative pain following knee arthroplasty [53]. It has been also reported that capsaicin can be topically used as an add-on therapy in systematic pain medication [54]. Finally the topic of this study, systemic administra- tion of capsaicin has been demonstrated to induce tumour cell apoptosis [55 ]. It has been suggested that HA14-1 can be used in the- rapeutic combination with other anticancer ag ents [17] as it has no effect in normal cells. It has been reported to inhibit the expression of anti-apoptotic Bcl-2 proteins which are associated with chemotherapy resistance in various human cancers. Preclinical studies have shown that agents targeting antiapop totic Bcl-2 family members can be used as a single agent and in combination with other anticancer agents. Its mechanism of action is re- lated to the intrinsic apoptotic pathway which can be ini- tiated by many signals such as cellular damage or cyto- kine deprivation [57]. Another molecular antagonist of the Bcl-2 family members kno wn as deoxyglucose-A BT- 263/737 has been demonstrated to be a potent apoptotic inducer by releasing cytochrome c from the mitochon- dria activating caspases which are required to complete the apoptosis process and has also been suggested as a combined therapy for cancer treatment [58]. There are now some experimental and pre-clinical trials in which capsaicin has been used as a treatment for pain in ad- vanced cancer [59]. 5. Conclusion In conclusion this comparative study has shown that melanoma cells and melanocytes have a similar sensitiv- ity to capsaicin while fibroblasts are more resistant to this agent. HA14-1 induces apoptosis at relatively low con- centrations and a co mbination o f the two agents pro duces a useful additive affect for 2 out of 3 of the melanoma cancer lines and studies and encouragingly for the most metastatically aggressive cancer cell line (C8161), a com- bination of the two showed some evidence of synergy. The results suggest that a natural agent (which is per- haps common in one’s diet), such as capsaicin, can be used in combination with another organic compound, HA14-1, as a pro-apoptotic agent. The advantages of a combined therapy include using the two drugs at lower concentrations which reduces toxicity and side effects for the patient while promoting improved cell apoptosis. This may be a promising alternative therapy for those patients with malignent melanoma, as there are few ef- fective chemotherapy approaches at present. The newer more promising agents of ipilimumab and vemurafenib could also be studied in combination with capsaicin in future s t udies. 6. Acknowledgements The authors would like to thank CAPES Foundation and State University of Santa Catarina (UDESC) for sup- porting CMG Marques PhD Studentship. REFERENCES [1] F. Balkwill and A. Mantovani, “Inflammation and Cancer: Back to Virchow?” Lancet, Vol. 357, No. 9255, 2001, pp 539-545. doi:10.1016/S0140-6736(00)04046-0 [2] L. M. Coussens and Z. Werb, “Inflammation and Cancer,” Nature, Vol. 240, No. 6917, 2002, pp. 860-867. doi:10.1038/nature01322 [3] M. Karin, “Cancer Research in Flames,” The Scientist, Vol. 19, No. 23, 2005, pp. 24-25. [4] J. Marx, “Inflammation and Cancer: The Link Grows Stronger,” Science, Vol. 306, No. 5698, 2004, pp. 966-968. doi:10.1126/science.306.5698.966 [5] A. Mantovani, “Inflammation by Remote Control,” Na- ture, Vol. 435, No. 704, 2005, pp. 752-753. doi:10.1038/435752a [6] F. Balkwill, “Cancer and the Chemokine Network,” Na- ture, Vol. 4, No. 7, 2004, pp. 540-550. [7] E. Shacter and S. A. Weitzman, “Chronic Inflammation and Cancer,” Oncology, Vol. 16, No. 2, 2002, pp. 217- 232. [8] R. E. Harris, J. Beebe-Donk, H. Doss and D. B. Doss, “Aspirin, Ibuprofen, and Other Non-Steroidal Anti-Infla- mmatory Drugs in Cancer Prevention: A Critical Review of Non-Selective COX-2 Blockade,” Oncology Reports, Copyright © 2013 SciRes. JCDSA  Combined Effects of Capsaicin and HA14-1 in Inducing Apoptosis in Melanoma Cells 187 Vol. 13, No. 4, 2005, pp. 559-583. [9] P. Dasgupta, V. Chandiramani, M. C. Parkinson, A. Beckett and C. J. Fowler, “Treating the Human Bladder with Capsaicin: Is It Safe?” European Urology, Vol. 33, No. 1, 1998, pp. 28-31. doi:10.1159/000019531 [10] D. J. Morré, E. Sun, C. Geilen, L. Y. Wu, R. de Cabo, K. Krasagakis, C. E. Orfanos and D. M. Morre, “Capsaicin Inhibits Plasma Membrane NADH Oxidase and Growth of Human and Mouse Melanoma Lines,” European Journal of Cancer, Vol. 32, No. 11, 1996, pp. 1995-2003. doi:10.1016/0959-8049(96)00234-1 [11] S. S. Brar, T. P. Kennedy, A. R. Whorton, A. B. Sturrock, T. P. Huecksteadt, A. J. Ghio and J. R. Hoidal, “Reactive Oxygen Species from NAD(P)H:Quinone Oxidoreductase Constitutively Activate NF-kappaB in Malignant Mela- noma Cells,” Cell Physiology: American Journal of Phy- siology, Vol. 280, No. 3, 2001, pp. 659-676. [12] V. A. Trinh, “Current Management of Metastatic Mela- noma,” American Journal of Health-System Pharmacy, Vol. 65, No. 24, 2008, pp. S3-S8. doi:10.2146/ajhp080460 [13] J. Poust, “Targeting Metastatic Melanoma,” American Journal of Health-System Pharmacy, Vol. 65, No. 24, 2008, pp. S9-S15. doi:10.2146/ajhp080461 [14] S. S. Agarwala, U. Keilholz, E. Gilles, A. Y. Bedikian, J. Wu, R. Kay, C. A. Stein, L. M. Itri, S. Suciu and A. M. Eggermont, “LDH Correlations with Survival in Advan- ced Melanoma from Two Large, Randomised Trials (Ob- limersen GM301 and EORTC 18951),” European Journal of Cancer, Vol. 45, No. 10, 2009, pp. 1807-1814. doi:10.1016/j.ejca.2009.04.016 [15] F. S. Hodi, S. J. O’Day, D. F. McDermott, R. W. Weber, J. A. Sosman, J. B. Haanen, R. Gonzalez, C. Robert, D. Schadendorf, J. C. Hassel, W. Akerley, A. J. van den Eertwegh, J. Lutzky, P. Lorigan, J. M. Vaubel, G. P. Linette, D. Hogg, C. H. Ottensmeier, C. Lebbé, C. Pe- schel, I. Quirt, J. I. Clark, J. D. Wolchok, J. S. Weber, J. Tian, M. J. Yellin, G. M. Nichol, A. Hoos and W. J. Urba, “Improved Survival with Ipilimumab in Patients with Metastatic Melanoma,” The New England Journal of Medicine, Vol. 363, No. 8, 2010, pp. 711-723. doi:10.1056/NEJMoa1003466 [16] J. A. Sosman, K. B. Kim, L. Schuchter, R. Gonzalez, A. C. Pavlick, J. S. Weber, G. A. McArthur, T. E. Hutson, S. J. Moschos, K. T. Flaherty, P. Hersey, R. Kefford, D. Law- rence, I. Puzanov, K. D. Lewis, R. K. Amaravadi, B. Chmielowski, H. J Lawrence, Y. Shyr, F. Ye, J. Li, K. B. Nolop, R. J. Lee, A. K. Joe and A. Ribas, “Survival in BRAF V-600-Mutant Advanced Melanoma Treated with Vemurafenib,” The New England Journal of Medicine, Vol. 366, No. 8, 2012, pp. 707-714. doi:10.1056/NEJMoa1112302 [17] D. Tian, S. G. Das, J. M Doshi, J. Peng, J. Lin and C. Xing, “sHA14-1, a Stable and ROS-Free Antagonist against Anti-Apoptotic Bcl-2 Proteins, Bypasses Drug Resistances and Synergizes Cancer Therapies in Human Leukaemia Cell,” Cancer Letters, Vol. 259, No. 2, 2007, pp. 198-208. doi:10.1016/j.canlet.2007.10.012 [18] J. L. Wang, D. Liu, Z. L. Zhang, S. Shan, X. Han, S. M. Srinicasula, C. M. Croce, E. S. Alnemri and Z. Huang, “Structure-Based Discovery of an Organic Compound that Binds Bcl-2 Protein and Induces Apoptosis of Tumor Cells,” Proceeding of National Acadamiy Sciences of the United State of America, Vol. 97, No. 13, 2000, pp. 712- 719. doi:10.1073/pnas.97.13.7124 [19] U. Zangemeister-Wittke and A. Ziegler, “Bcl-2 Antisense Therapy for Cancer: The Art of Persuading Tumour Cells to Commit Suicide,” Apoptosis, Vol. 3, No. 2, 1998, pp. 67-74. doi:10.1023/A:1009636722713 [20] J. M. Kozlowski, I. R. Hart, I. J. Fidler and N. Hanna, “A Human Melanoma Line Heterogeneous with Respect to Metastatic Capacity in Athymic Nude Mice,” Journal of the National Cancer Institute, Vol. 72, No. 4, 1984, pp 913-917. [21] G. E. Ghanem, G. Comunale, A. Libert, A. Vercammen- Grandjean and F. J. Lejeune, “Evidence for Alpha-Mela- nocytes-Stimulating Hormone (Alpha-MSH) Receptors on Human Malignant Melanoma Cells,” International Journal of Cancer, Vol. 41, No. 2, 1988, pp. 248-255. doi:10.1002/ijc.2910410216 [22] P. Eves, C. Layton, S. Hedley, R. Dawson, M. Wagner, R. Morandini, G. Ghanem and S. MacNeil, “Characterisation of an In Vitro Model of Human Melanoma Invasion Ba- sed on Reconstructed Human Skin,” British Journal of Dermatology, Vol. 142, No. 2, 2000, pp. 210-222. doi:10.1046/j.1365-2133.2000.03287.x [23] Y. G. Kim, M. J. Kim, J.-S. Lim, M.-S. Le e, J. S. Kim and Y. D. Yoo, “ICAM-3-Induced Cancer Cell Proliferation through the PI3K/Akt Pathway,” Cancer Letters, Vol. 239, No. 1, 2006, pp. 103-110. doi:10.1016/j.canlet.2005.07.023 [24] E. Katerinaki, J. W. Haycock, R. Lalla, K. E. Carlson, Y. Yang, R. P. Hill, P. C. Lorigan and S. MacNeil, “Sodium Salicylate Inhibists TNF-Alpha-Induced NF-kappaB Acti- vation, Cell Migration, Invasion and ICAM-1 Expression in Human Melanoma Ce lls,” Melanoma Research, Vol. 16, No. 1, 2006, pp. 11-22. doi:10.1097/01.cmr.0000195698.58013.53 [25] B. B. Aggarwal, “Signalling Pathways of the TNF Super- family: A Double Edged Sword,” Nature Reviews Immu- nology, Vol. 3, No. 9, 2003, pp. 745-756. doi:10.1038/nri1184 [26] S. Aggarwal, Y. Takada, A. M. Mhashilkar, K. Sieger, S. Chada and B. B. Aggarwal, “Melanoma Differentiation- Associated Gene7/IL-24 Gene Enhances NF-kB Activa- tion and Suppresses Apoptosis Induced by TNF,” Journal of Immunology, Vol. 173, No. 7, 2004, pp. 4368-4376. [27] E. Pikarsky and Y. Ben-Neriah, “NF-kB Inhibition: A Double-Edged Sword in Cancer?” European Journal of Cancer, Vol. 42, No. 6, 2006, pp. 779-784. doi:10.1016/j.ejca.2006.01.011 [28] W.-W. Lin and M. Karin, “A Cytokine-Mediated Link between Innate Immunity, Inflammation, and Cancer,” Journal of Clinical Investigation, Vol. 117, No. 5, 2007, pp. 1175-1183. doi:10.1172/JCI31537 [29] J. P. Monk, G. Phillips, R. Waite, J. Kuhn, L. J. Schaaf, G. A. Otterson, D. Guttridge, C. Rhoades, M. Shah, T. Criswell, M. A. Caligiuri and M. A. Villalona-Calero, “Assessment of Tumor Necrosis Factor Alpha Blockade Copyright © 2013 SciRes. JCDSA  Combined Effects of Capsaicin and HA14-1 in Inducing Apoptosis in Melanoma Cells 188 as an Intervention to Improve Tolerability of Dose-Inten- sity Chemotherapy in Cancer Patients,” Journal of Clini- cal Oncology, Vol. 24, No. 12, 2006, pp. 1852-1859. doi:10.1200/JCO.2005.04.2838 [30] C. H. Lin, W. C. Lu, C. W. Wang, Y. C. Chan and M. K. Chen, “Capsaicin Induces Cell Cycle Arrest and Apop- tosis in Human KB Cancer Cells,” BMC Complementary and Alternative Medicine, Vol. 13, No. 1, 2013, pp. 46. doi:10.1186/1472-6882-13-46 [31] D. J. Morré, S. Caldwell, A. Mayorga, L. Y. Wu and D. M. Morre, “NADH Oxidase Activity from Sera Altered by Capsaicin is Widely Distributed among Cancer Patients,” Archives of Biochemistry and Biophysics, Vo l. 342, No. 2, 1997, pp. 224-230. doi:10.1006/abbi.1997.0110 [32] A. Mori, S. Lehmann, J. O’Kelly, T. Kumagai, J. C. Desmond, M. Pervan, W. H. McBride, M. Kizaki and H. P. Koeffler, “Capsaicin, a Component of Red Peppers, In- hibits the Growth of Androgen-Independent, p53 Mutant Prostate Cancer Cells,” Cancer Research, Vol. 66, No. 6, 1996, pp. 3222-3229. doi:10.1158/0008-5472.CAN-05-0087 [33] P. S. Patel, M. L. Varney, B. J. Dave and R. K. Singh, “Regulation of Constitutive and Induced NF-kappaB Ac- tivation in Malignant Melanoma Cells by Capsaicin Mo- dulates Interleukin-8 Production and Cell Proliferation,” Journal of Interferon & Cytokine Research, Vol. 22, No. 4, 2002, pp. 427-435. doi:10.1089/10799900252952217 [34] P. S. Patel, S. Yang, A. Li, M. L. Varney and R. K. Singh, “Capsaicin Regulates Endothelial Cell Growth Factor Ex- pression by Modulation of Hypoxia Inducing Factor- 1-Alpha in Human Malignant Melanoma Cells,” Journal of Cancer Research and Clinical Oncology, Vol. 129, No. 9, 2002, pp. 461-468. doi:10.1007/s00432-002-0368-8 [35] X. F. Gong, M. W. Wang and T Ikejima, “Mechanisms of Capsaicin-Induced Apoptosis of Human Melanoma A375- S2 Cells,” Zhonghua Zhong Liu Za Zhi, Vol. 27, No. 7, 2005, pp. 401-403. [36] H. J. Kang, Y. Soh, M. S. Kim, E. J. Lee, Y. J. Surh, H. R. Kim, S. H. Kim and A. Moon, “Roles of JNK-1 and p38 in Selective Induction of Apoptosis by Capsaicin in Ras-Transformed Human Breast Epithelial Cells,” Inter- national Journal of Cancer, Vol. 103, No. 4, 2003, pp. 475-482. doi:10.1002/ijc.10855 [37] Y. J. Surh, “More Than Spice: Capsaicin in Hot Chilli Peppers Makes Tumor Cells Commit Suicide,” Journal of the National Cancer Institute, Vol. 94, No. 17, 2002, pp. 1263-1265. doi:10.1093/jnci/94.17.1263 [38] H. S. Jun, T. Park, C. K. Lee, M. K. Kang, M. S. Park, H. I. Kang, Y. L. Surh and O. H. Kim, “Capsaicin Induced Apoptosis of B16-F10 Melanoma Cells through Down- Regulation of Bcl-2,” Food Chem Toxicol , Vol. 45, No. 5, 2007, pp. 708-715. doi:10.1016/j.fct.2006.10.011 [39] M. Y. Jung, H. J. Kang and A. Moon, “Capsaicin-Induced Apoptosis in SK-Hep1 Hepatocarcinoma Cell Involves Bcl-2 Down-Regulation and Caspase-3 Activation,” Can- cer Letters, Vol. 165, No. 2, 2001, pp. 139-145. doi:10.1016/S0304-3835(01)00426-8 [40] M. Bhutani, A. K. Pathak, A. S. Nair, A. B. Kunnumak- kara, S. Guha, G. Sethi and B. B. Aggarwal, “Capsaicin Is a Novel Blocker of Constitutive and Interleukin-6-In- ducible STAT3 Activation,” Clinical Cancer Research, Vol. 13, No. 10, 2007, pp. 3024-3032. doi:10.1158/1078-0432.CCR-06-2575 [41] E. Bodó, I. Kovács, A. Telek, A. Varga, R. Paus, L. Kovácsa and T. Bíró, “Vanilloid Receptor-1 (VR1) Is Wi- dely Expressed on Various Epithelial and Mesenchymal Cell Types of Human Skin,” Journal of Investigative Der- matology, Vol. 123, No. 2, 2004, pp. 410-413. doi:10.1111/j.0022-202X.2004.23209.x [42] C. S. Kim, W. H. Park, J. Y. Park, J. H. Kang, M. O. Kim, T. Kawada, H. Yoo, I. S. Han and R. Yu, “Capsaicin, a Spicy Component of Hot Pepper, Induces Apoptosis by Activation of the Peroxisome Proliferators-Activated Re- ceptor Gamma in HT-29 Human Colon Cancer Cells,” Journal of Medicinal Food, Vol. 7, No. 3, 2004, pp. 267- 273. [43] J. L. Wang, D. Liu, Z. J. Zhang, S. Shan, X. Han, S. M. Sinivasula, C. M. Croce, E. S. Alnemri and Z. Huang, “Structure-Based Discovery of an Organic Compound that Binds Bcl-2 Protein and Induces Apoptosis of Tumor Cells,” Proceeding of National Acadamiy Sciences of the United State of America, Vol. 97, No. 13, 2000, pp. 7124- 7129. doi:10.1073/pnas.97.13.7124 [44] C. Campàs, A. M. Cosialls, M. Barragán, D. Iglesias- Serret, A. F. Santidrián, L. Coll-Mulet, M de Frias, A. Domingo, G. Pons and J. Gil, “Bcl-2 Inhibitors Induce Apoptosis in Chronic Lymphocytic Leukemia Cells”, Experimental Hematology, Vol. 34, No. 12, 2006, pp. 1663-1669. doi:10.1016/j.exphem.2006.07.008 [45] S. Mena, M. Benlloch, A. Ortega, J. Carretero, E. Obrador, M. Asensi, I. Petchen, B. D. Brown and J. M. Estrella, “Bcl-2 and Glutathione Depletion Sensitizes B16 Mela- noma to Combination Therapy and Eliminates Metastatic Disease,” Clinical Cancer Research, Vol. 13, No. 9, 2007, pp. 2658-2666. doi:10.1158/1078-0432.CCR-06-2642 [46] L. Dal Lago, V. D’Hondt and A. Awada, “Selected Com- bination Therapy with Sorafenib: A Review of Clinical dAta and Perspectives in Advanced Solid Tumors,” Onco- logist, Vol. 13, No. 8, 2008, pp. 845-858. doi:10.1634/theoncologist.2007-0233 [47] K. Y. Woo, L. K. Abbot and L. Linrach, “Evidence-Based Approach to Manage Persistent Wound-Related Pain,” Current Opinion in Supportive and Palliative Care, Vol. 7, No. 1, 2013, pp. 86-94. doi:10.1097/SPC.0b013e32835d7ed2 [48] A. Leiherer, A. Mündlein and H. Drexel, “Phytochemicals and Their Impact on Adipose Tissue Inflammation and Diabetes,” Vascular Pharmacology, Vol. 58, No. 1-2, 2012, pp. 3-20. doi:10.1016/j.vph.2012.09.002 [49] M. P. Flores, A. P. Castro and J. dos S. Nascimento, “Topical Analgesics,” Revista Brasileira de Anestesiolo- gia, Vol. 62, No. 2, 2012, pp. 244-252. doi:10.1590/S0034-70942012000200010 [50] P. Anand and K. Bley, “Topical Capsaicin for Pain Man- agement: Therapeutic Potential and Mechanisms of Ac- tion of the New High-Concentration Capsaicin 8% Pat- ch,” British Journal of Anaesthesia, Vol. 107, No. 4, 2011, pp. 490-502. doi:10.1093/bja/aer260 Copyright © 2013 SciRes. JCDSA  Combined Effects of Capsaicin and HA14-1 in Inducing Apoptosis in Melanoma Cells Copyright © 2013 SciRes. JCDSA 189 [51] S. Chrubasik, T. Weiser and B. Beime, “Effectiveness and Safety of Topical Capsaicin Cream in the Treatment of Chronic Soft Tissue Pain,” Phytotherapy Research, Vol. 24, No. 12, 2012, pp. 1877-1885. doi:10.1002/ptr.3335 [52] F. J. Silvestre, J. Silvestre-Rangil, J. C. Tanarit-Santafé and D. Bautista, “Application of a Capsaicin Rinse in the Treatment of Burning Mouth Syndrome,” Medicina Oral Patologia Oral y Cirugia Bucal, Vol. 17, No. 1, 2012, pp. 1-4. doi:10.4317/medoral.17219 [53] C. T. Hartrick, C. Pestano, N. Carlson and S. Hartrick, “Capsaicin Instillation for Postoperative Pain Following Total Knee Arthroplasty: A Preliminary Report of a Ran- domized, Double-Blind, Parallel-Group, Placebo-Con- trolled, Multicentre Trial,” Clinical Drug Investigation, Vol. 31, No. 12, 2011, pp. 877-882. doi:10.1007/BF03256925 [54] R. Baron and F. Mahn, “Types of Topical Treatment for Peripheral Neuropathic Pain: Mechanism of Action and Indications,” Schmerz, Vol. 24, No. 4, 2012, pp. 317-325. doi:10.1007/s00482-010-0939-6 [55] A. K. Ghosh and S. Basu, “Tumor Macrophages as a Tar- get for Capsaicin Mediated Immunotherapy,” Cancer Let- ters, Vol. 324, No. 1, 2012, pp. 91-97. doi:10.1016/j.canlet.2012.05.002 [56] C. Hall, S. M. Troutman, D. K. Price, W. D. Figg and M. H. Kang, “Bcl-2 Family of Proteins as Therappeutic Targets in Genitourinary Neoplasms,” Clinical Genitouri- nary Cancer, Vol. 11, No. 1, 2013, pp. 9-10. doi:10.1016/j.clgc.2012.09.002 [57] M. H. Kang and C. P. Reynolds, “Bcl-2 Inhibitors: Tar- geting Mitochondrial Apoptotic Pathways in Cancer The- rapy,” Clinical Cancer Research, Vol. 15, No. 4, 2009, pp. 1126-1132. doi:10.1158/1078-0432.CCR-08-0144 [58] R. Yamaguchi, E. Janssen, G. Perkins, M. Ellisman, S. Kitada and J. C. Reed, “Efficient Elimination of Cancer Cells by Deoxyglucose-ABT-263/737 Combination The- rapy,” PLoS One, Vol. 6, No. 9, 2011, pp. 1-14. doi:10.1371/journal.pone.0024102 [59] M. J. Iadarola and A. J. Mannes, “The Vanilloid Resinif- eratoxin for Interventional-Based Pain Control,” Current Topics in Medicinal Chemistry, Vol. 11, No. 17, 2011, pp. 2171-2179. doi:10.2174/156802611796904942
|