 Psychology 2013. Vol.4, No.8, 669-675 Published Online August 2013 in SciRes (http://www.scirp.org/journal/psych) http://dx.doi.org/10.4236/psych.2013.48095 Copyright © 2013 SciRes. 669 Autonomic Mechanisms of Emotional Reactivity and Regulation Catherine C. Uy1, Iain A. Jeffrey2, Matthew Wilson3, Viswanath Aluru1, Anita Madan4, Ying Lu5, Preeti Raghavan1,5* 1Department of Rehabilitation Medicine, New York University School of Medicine, New York, USA 2Touro College of Osteopathic Medicine, New York, USA 3Hunter College, New York, USA 4Department of Psychiatry, New York University School of Medicine, New York, USA 5Education and Human Development, Steinhardt School of Culture, New York University, New York, USA Email: *Preeti.Raghavan@nyumc.org Received May 15th, 2013; revised June 29th, 2013; accepted July 23rd, 2013 Copyright © 2013 Catherine C. Uy et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The ability to perceive and regulate our emotions appropriately is essential for social behavior. Our sub- jective emotional states to changing external cues are accompanied by physiological changes in heart rate variability (HRV), which is regulated by the sympathetic and parasympathetic branches of the autonomic nervous systems (ANS). In this pilot study, we sought to elucidate the autonomic basis of emotional reac- tivity and regulation in response to ecologically-valid emotional stimuli—presented in the form of film- clips—in healthy subjects. Subjects watched a series of videos, validated to elicit feelings of amusement, sexual amusement, sadness, fear, and disgust. Subjects were also asked to regulate the outward expression of their response to disgust by suppressing or amplifying it when instructed. Electrodes placed on the torso measured cardiac and respiratory signals, which were processed to compute HRV, which when ana- lyzed with the concurrent respiratory signal calculates measures of parasympathetic activity (RFA, Res- piratory Frequency Area, from higher frequencies) and sympathetic activity (LFA, Low Frequency Area, from lower frequencies). Fluctuations in LFA and RFA were computed by the coefficient of variation, and the intensity of the emotional response to the film-clips was captured via questionnaires. Our results suggest that in healthy individuals, higher intensities of subjective emotional experience, both positive (e.g., amusement) and negative (e.g., amplified disgust) elicit higher LFA (sympathetic) responses, whereas emotional regulation is mediated primarily by fluctuations in RFA (parasympathetic) activity. Furthermore, correlations between emotional intensity and components of HRV suggest that higher posi- tive or lower negative emotional states may increase the capacity for emotional regulation via modulation of the parasympathetic component. Our results suggest that a sense of humor might facilitate emotional control. Keywords: Heart Rate Variability; Sympathetic; Parasympathetic; Emotions Introduction Emotional perception and regulation inform the way we react to different situations, both in terms of how we feel inside and how we present ourselves to others. To form relationships and behave according to social norms we need to regulate our feel- ings based on ever-changing external cues. Based on these cues, we must decide quickly if our feelings are appropriate to the specific situation and adapt our behavior accordingly (Izard, Fine et al., 2001). In many conditions, such as traumatic brain injury (Thurman, Alverson et al., 1999; Bornhofen & McDonald, 2008; McDonald, Bornhofen et al., 2009; de Sousa, McDonald et al., 2012; Hammond, Davis et al., 2012), post-traumatic stress disorder (Aupperle, Allard et al. 2012), and autism (Bal, Har- den et al., 2010), this ability is impaired leading to overreaction or inappropriate behavior. A basic understanding of how healthy individuals perceive, react to, and regulate their response to various emotions is first necessary before we can begin to de- termine how these processes may differ in those with emotional dysfunction and dysregulation. When we feel an emotion, our subjective emotional experi- ences are accompanied by measurable physiological changes in heart rate, skin conductance and respiratory rate (Zuckerman, Klorman et al., 1981; Gross & Levenson, 1993; Gross & Le- venson, 1997). These parameters measure arousal, reflecting activity of the autonomic nervous system (ANS), but do not differentiate between activity of the sympathetic and parasym- pathetic branches of the ANS. According to Porges’ polyvagal theory, parasympathetic influence on the heart allows one to adjust metabolic output to optimize social interactions; in con- trast, sympathetic activity is necessary for mobilization during threatening situations (Porges, 1995; Porges, 2007). While both branches influence heart rate, they do so by different mecha- nisms which are activated by specific environmental circum- stances. For example, an increase in heart rate in response to a fearful stimulus may, in some cases, be a result of sympathetic activation, while in others may arise from parasympathetic *Corresponding author.  C. C. UY ET AL. withdrawal (Stifter, Dollar et al., 2011). Therefore, to better understand the autonomic mechanisms of emotional reactivity and regulation, it would be prudent to study activity patterns of both the sympathetic and parasympathetic branches of the ANS and examine how they interact in response to distinct affective stimuli. Sympathetic and parasympathetic output is thought to be regulated by the brain’s central autonomic network (CAN) (Benarroch, 1993), which includes regions of the cortex, brain- stem and limbic systems, and is involved in a wide range of processes from homeostasis to goal-directed behavior (Appel- hans & Luecken, 2008). By integrating information about the external environment with that of the body’s internal physio- logical state, the CAN modulates activity of the sympathetic and parasympathetic branches of the nervous system to influ- ence heart rate. Sympathetic signals influence the sinoatrial node via the neurotransmitter norepinephrine to gradually in- crease heart rate, achieving peak effect after 4 seconds and returning to baseline after 20 seconds. In contrast, parasympa- thetic signaling via acetylcholine slows heart rate with a short response latency, achieving peak effect after 0.5 seconds and returning to baseline within 1 second (Bazhenova, Plonskaia et al., 2001; Pumprla, Howorka et al., 2002). The frequency spec- trum of heart rate variability (HRV) thus reflects sympathetic and parasympathetic influences on heart rate (Appelhans & Luecken, 2006). HRV, when analyzed concurrently with the respiratory signal calculates measures of parasympathetic activ- ity (RFA, Respiratory Frequency Area, from higher frequencies) and sympathetic activity (LFA, Low Frequency Area, from lower frequencies) (Akselrod, Gordon et al., 1981; Aysin & Aysin, 2006). The goal of this study is to elucidate the autonomic mecha- nisms of emotional reactivity and regulation in response to ecologically-valid affective stimuli. We used film clips vali- dated to elicit specific emotional responses (Rottenberg, Ray et al., 2007; Schaefer, Nils et al., 2010) and measured the fre- quency spectra of HRV and respiratory activity when partici- pants watched the videos. Methods Subjects Seven healthy subjects, ranging in age from 23 to 40 years (mean ± SD = 29.8 ± 6.2 years), with no history of psychiatric disease or complicating medical problems, such as uncontrolled hypertension, diabetes, neurological illness such as stroke, epi- lepsy, or demyelinating disease participated in the study. Four of the subjects were female [57%]. Procedure After obtaining informed consent, subjects were seated at a table, in front of a computer monitor, in a well-lit, 11' × 23' room. Three electrodes, one placed below each clavicle and one on the left lower ribcage, measured electrocardiographic and respiratory signals (ANSAR Medical Technologies, 2005). First, a baseline electrocardiographic recording was obtained with no video stimulus. Subjects were instructed to look for- ward, relax, and breathe normally for 2 minutes. Subjects were then told that they were going to watch a se- ries of eight video clips (2 - 5 minutes long) (Table 1). Each subject was randomly assigned to watch one of two sets of Table 1. Summary of the emotion-eliciting film stimuli. Target emotion Movie title & description Time (min) Neutral Sticks—Different colored sticks gradually accumulate on a black background (non-commercial screen saver, soundless). 4.6 Amusement Something About Mary—A man fights with a small dog. 4.33 Sadness City of Angels—The aftermath of a woman on a bike hit by a truck and dying in a man’s arms. 4.35 Sexual amusement When Harry M et Sally—A woman loudly simulates an orgasm in a crowded diner. 3.75 Fear The Shining— A boy plays alone in an empty hallway. 2.5 Trainspotting—A man defecates then dives into a filthy toilet. 2.38 Amputation—noncommercial recording of an arm amputation. 2.3 Video set 1 Disgust Bad Taste—A group of young men eat vomit. 2.96 Neutral Sticks—Different colored sticks accumulate gradually on a black background (screen saver, soundless). 4.6 Amusement Benny and Joon—A man fools around in a diner. 3.33 Sadness The Champ—A young boy grieves over his dying father. 3.83 Sexual amusement A Fish Calle d Wanda—A woman is sexually aroused while her male companion is found naked by the owners of the house they are in. 4.08 Fear Silence of the Lambs—A woman chases a dangerous man at gunpoint and comes across a corpse in a bathtub. 4.6 Vampire’s Kiss—A man eats a cockroach. 1.65 Black Swan—A woman peels back a hangnail.1.87 Video set 2 Disgust Pink Flamingoes—A woman eats dog feces. 2.5 videos, validated to elicit the same target emotions. Each clip began with 10 seconds of written instructions, stating “Please relax and watch the +”, followed by 60 seconds of a white + sign on a black background. This was provided to bring the subjects back to baseline prior to each film-clip, which would then automatically start playing. Subjects were not informed of the sequence of the film clips. After watching each video, sub- jects completed a post-film questionnaire to assess the intensity of their emotional response to the film clip. Module 1: Positive and Negative Affect. Subjects watched five short video clips targeting the following emotions: neutral, amusement, sadness, sexual amusement, and fear. The order of the clips was the same across all subjects and was chosen to counter-balance positively- and negatively-valenced affective stimuli, to prevent subjects from being overwhelmed in either direction. Before each video, subjects were told, “We will now be showing you a short film clip. It is important to us that you watch the film clip carefully.” Module 2: Disgust Regulation. Subjects watched three dif- ferent video clips validated to elicit a disgust response. For the first video, subjects just watched the film clip while their reac- tivity was measured as in module 1 (unregulated response). For the second video, subjects were instructed to suppress their reactions. They were told, “Watch the film clip carefully. If you have any feelings as you watch the film, please try your best not to let those feelings show.” For the third video, subjects were instructed to amplify their reactions. They were told, Copyright © 2013 SciRes. 670  C. C. UY ET AL. “Watch the film clip carefully. If you have any feelings as you watch the film clip, try your best to let those feeli ngs show.” Apparatus Physiological Measurements. Electrocardiographic and res- piratory signals were sampled at 250 Hz and 50 Hz, respec- tively and collected using ANSAR ANX 3.0 software (ANSAR Medical Technologies, Inc., Philadelphia, PA). Heart rate vari- ability (HRV) was computed every 0.25 seconds and time- frequency spectral analysis was performed to quantify ANS activity. The respiratory frequency area (RFA) measured para- sympathetic activity from higher frequency areas of the HRV spectrum as determined from time-frequency analyses of respi- ratory activity (Aysin & Aysin, 2006). RFA represents the fre- quency ranges associated with Respiratory Sinus Arrhythmia, known to be a cardio-vagal response, reflecting parasympa- thetic activity (Akselrod, Gordon et al., 1981; Appelhans & Luecken, 2008). Low frequency area (LFA) is defined as the area under the heart rate spectral curve over the frequency range from 0.04 - 0.10 Hz, or the lower limit of RFA range (ANSAR Medical Technologies, 2005; Colombo, Shoemaker et al., 2008). By localizing and omitting the parasympathetic in- fluence (e.g., from Respiratory Sinus Arrhythmia) from the low frequency range of HRV, LFA primarily corresponds to activity from the sympathetic nervous system (Aysin & Aysin, 2006; Colombo, Shoemaker et al., 2008). Self-Reported Emotional Responses. After each film clip, subjects completed a short post-film questionnaire to rate the intensity of their feelings of amusement, anger, anxiety, confu- sion, contempt, disgust, embarrassment, fear, guilt, happiness, interest, joy, love, pride, sadness, shame, surprise and unhappy- ness on a scale of 0 (none) to 8 (extreme). They were also asked to indicate if they had seen the film clip prior to the study (Rottenberg, Ray et al., 2007). Data Reduction and Statistical Analyses For the purposes of this study, we focused on the interval of interest (IOI)—the 30 seconds of the film clip that most strongly elicited the target emotion. Therefore, all data analysis pertains to ANSAR recordings during the IOI of each film clip. LFA and RFA values for time points at which RFA 0.1 bpm2 were dropped, as they are un-interpretable for healthy subjects. For each target emotion, we measured 1) the mean LFA and RFA activity, 2) the geometric mean of the ratio between LFA and RFA (LFA/RFA) that quantifies their interrelationship, and 3) the coefficient of variability of both LFA and RFA that quantifies the fluctuation in LFA and RFA independent of the mean level. A linear mixed effect model was used to examine how the activity levels, interrelationship and variability in LFA and RFA change across different target emotions. A random effect at subject level was used to control for individual heterogeneity. Statistical software package R 2.15.2 was used and package lmer was used to fit the model. To respect the normality as- sumption, a logarithm transformation was done to the geomet- ric mean of the ratio of LFA over RFA. The results based on this particular model were then converted into multiplicative effects at the original scale. Relationships between self-reported emotional responses and autonomic parameters were examined using Spearman Rank correlations. Three levels of statistical significance (1%, 5% and 10%) are reported. Results Positive Affective Stimuli Increase LFA and Fluctuation in RFA Physiology. Figure 1(a) shows mean LFA and RFA values during the IOI across all subjects. Compared to neutral, mean LFA increased significantly during both amusement (mean difference (md) = 12.42, p < 0.01) and sexual amusement (md = 6.08, p < 0.05). There were no statistical differences in mean RFA across any of the stimuli compared to neutral. The mean LFA was significantly higher than the mean RFA only during amusement (md = 10.25, p < 0.01). At the subject level, the ratio between LFA and RFA (Figure 1(b)) for the neutral stimulus was close to 1 (LFA/RFA = 0.91), suggesting that LFA and RFA were well balanced. For both positively-valenced stimuli, the LFA/RFA ratios were signifi- cantly higher than for the neutral or the negatively-valenced stimuli (LFA/RFA amusement = 4.84, p < 0.01; sexual amuse- ment = 2.53, p < 0.05), suggesting that positively-valenced stimuli elicited greater autonomic activity in the lower fre- quency bandwidth of HRV. The coefficient of variation of LFA (cvLFA) was relatively constant across all affective stimuli (Figure 1(c)). Only the cvRFA for amusement was significantly higher than that for neutral (md = 0.26, p < 0.01). In addition, the difference be- tween the cvRFA and cvLFA was significant during amuse- ment (md = 0.47, p < 0.01) and fear (md = 0.314, p < 0.05). Note however that 4 out of 7 subjects had seen the fear-pro- voking film clip previously. Subjective experience. To confirm that the film clips were eliciting the intended target emotion, we examined the intensity of the various emotions elicited by the film-clips on the post- film questionnaire (Figure 1(d)). Note that the emotions that were felt most intensely for each film clip corresponded with the target emotion for that clip. Sexual amusement provoked some embarrassment while non-sexual amusement did not. There was also a strong correlation between reported embar- rassment and the cvRFA (r = 0.971, p < 0.01), suggesting that those who experienced more embarrassment showed greater fluctuation in the higher frequency component of HRV. Inter- estingly, there was a negative correlation between self-reported fear and both mean LFA (r = −0.564, p = 0.18) and mean RFA (r = −0.873, p < 0.01). Regulation of Disgust Is A ssociated wit h Fluctu at ions in RFA Physiology. Figure 2(a) shows the mean LFA and RFA dur- ing unregulated and regulated disgust film-clips compared to neutral. The mean LFA during disgust amplification was sig- nificantly higher compared to neutral (md = 13.125, p < 0.01), unregulated disgust (md = 10.366, p < 0.01) and disgust sup- pression (md = 10.043, p < 0.01). The difference between mean LFA and RFA during disgust amplification was also significant (md = 18.21, p < 0.05). No significant differences were found in mean RFA across the stimuli. The ratio of LFA/RFA was higher during unregulated disgust (LFA/RFA = 2.39, p < 0.10) and disgust amplification (LFA/RFA = 2.66, p < 0.05) com- pared to neutral and disgust suppression (Figure 2(b)). Inter- estingly, LFA/RFA ratios during disgust suppression ap- Copyright © 2013 SciRes. 671  C. C. UY ET AL. Copyright © 2013 SciRes. 672 Figure 1. Emotional reactivity in response to positively-valenced and negatively-valenced film stimuli. The symbols indicate statistical signifi- cance compared to the neutral stimulus: ‡p < 0.01, *p < 0.05. (a) Mean LFA and RFA levels; (b) Mean ratio of LFA to RFA (LFA/RFA); (c) Coefficient of variation of LFA and RFA; (d) Self-reported emotional intensity scores for various emotions elicited by the film-clip on the post-film questionnaire (PFQ). In sexual amusement, EMB = embarrassment. In sadness, INTER = interest. Discussion proached that of the neutral stimulus, suggesting that healthy subjects were indeed able to regulate their autonomic activity when prompted to do so. The cvRFA was significantly higher than the cvLFA during disgust suppression (md = 0.31, p < 0.05) and amplification (md = 0.31, p < 0.05) (Figure 2(c)), suggesting that emotional regulation is reflected in increased higher frequency HRV fluctuations. In this study, we measured autonomic activity as reflected in the frequency spectrum of HRV, modulated by respiratory ac- tivity, when healthy subjects watched a series of film clips, which were validated to elicit specific target emotions. Our data suggest that in healthy individuals, emotional reactivity or arousal is reflected in the mean level of activity in the Low Frequency Area (LFA), a measure of sympathetic activity, whereas emotional regulation is reflected in fluctuations of the Respiratory Frequency Area (RFA), a measure of parasympa- thetic activity. Subjective experience. Although disgust, surprise and anxiety were experienced with all three disgust-inducing film clips, the intensity of these emotions varied during suppression and am- plification (Figure 2(d)). Disgust suppression was associated with less intense feelings of disgust compared with disgust am- plification (md = 1.85 units, p < 0.1), but greater anxiety (md = 3 units, p < 0.05). Interestingly, the intensity of anxiety during disgust suppression was negatively correlated with the cvRFA (r = −0.927, p < 0.01), suggesting that those who experienced less anxiety showed greater fluctuation in the higher frequency component of HRV. In contrast, during amplification, anxiety was positively correlated with cvRFA (r = 0.778, p < 0.05), suggesting that those with more anxiety showed greater cvRFA, which is consistent with the direction of regulation. In addition, a strong positive correlation was noted between the intensity of amusement and cvRFA during disgust suppression (r = 0.082, p < 0.05), suggesting that individuals who experienced higher intensity of amusement also showed greater fluctuation in the higher frequency component of HRV. Both processes may occur simultaneously depending on the emotional cues, suggesting a complex interplay between sym- pathetic and parasympathetic mechanisms in response to chang- ing environmental cues. Furthermore, correlations between self- reported emotional intensity and components of HRV suggest that higher positive or lower negative emotional states may increase the capacity for emotional regulation. Autonomic Basis of Emotional Reactivity In our study, subjects responded to positively-valenced amus- ing stimuli with relatively high mean LFA, and high LFA/RFA ratios compared to neutral; these emotions also evoked intense subjective feelings. Increased autonomic activity when one is amused may be due to expressive behavior such as laughing, 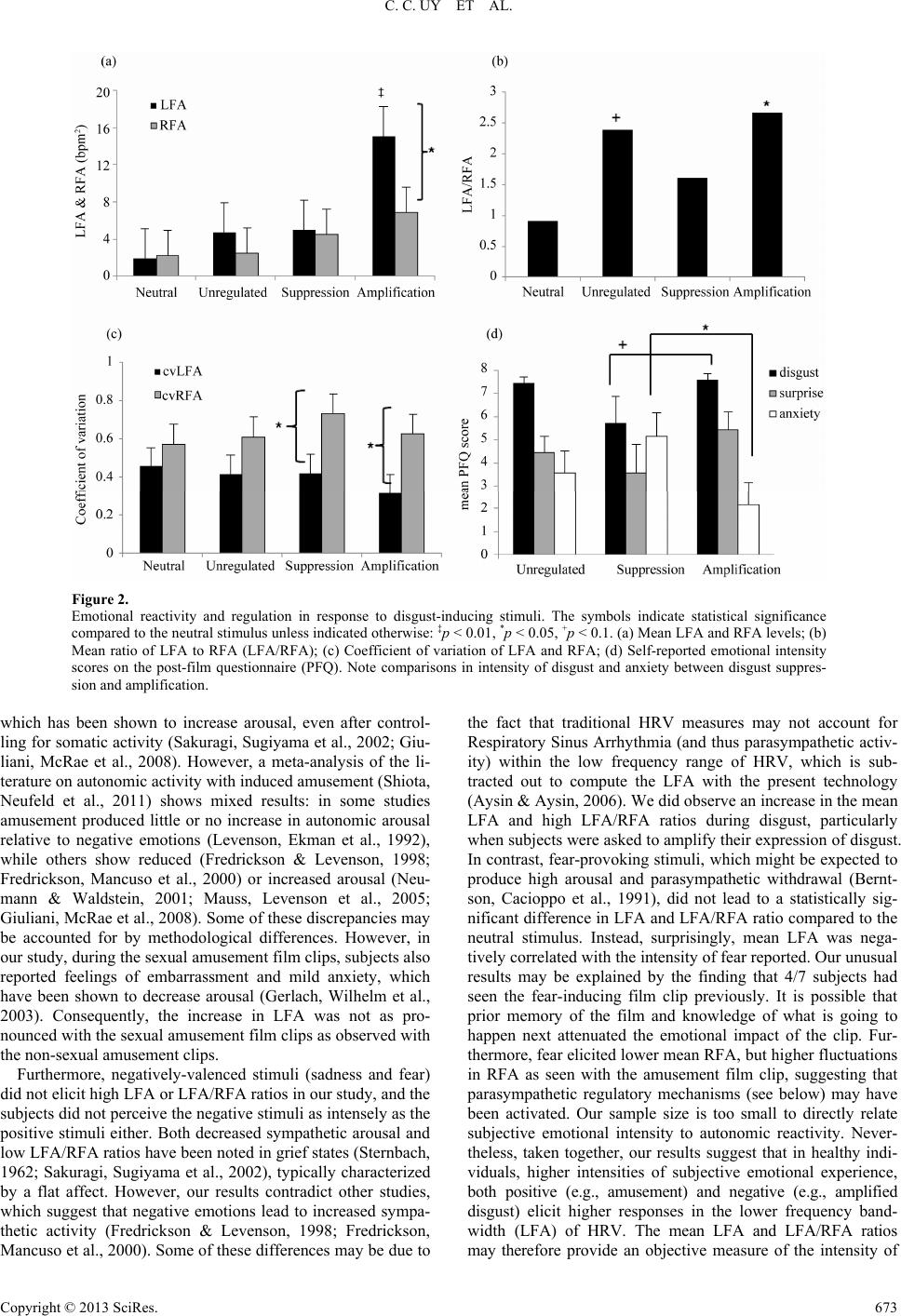 C. C. UY ET AL. Figure 2. Emotional reactivity and regulation in response to disgust-inducing stimuli. The symbols indicate statistical significance compared to the neutral stimulus unless indicated otherwise: ‡p < 0.01, *p < 0.05, +p < 0.1. (a) Mean LFA and RFA levels; (b) Mean ratio of LFA to RFA (LFA/RFA); (c) Coefficient of variation of LFA and RFA; (d) Self-reported emotional intensity scores on the post-film questionnaire (PFQ). Note comparisons in intensity of disgust and anxiety between disgust suppres- sion and amplification. which has been shown to increase arousal, even after control- ling for somatic activity (Sakuragi, Sugiyama et al., 2002; Giu- liani, McRae et al., 2008). However, a meta-analysis of the li- terature on autonomic activity with induced amusement (Shiota, Neufeld et al., 2011) shows mixed results: in some studies amusement produced little or no increase in autonomic arousal relative to negative emotions (Levenson, Ekman et al., 1992), while others show reduced (Fredrickson & Levenson, 1998; Fredrickson, Mancuso et al., 2000) or increased arousal (Neu- mann & Waldstein, 2001; Mauss, Levenson et al., 2005; Giuliani, McRae et al., 2008). Some of these discrepancies may be accounted for by methodological differences. However, in our study, during the sexual amusement film clips, subjects also reported feelings of embarrassment and mild anxiety, which have been shown to decrease arousal (Gerlach, Wilhelm et al., 2003). Consequently, the increase in LFA was not as pro- nounced with the sexual amusement film clips as observed with the non-sexual amusement clips. Furthermore, negatively-valenced stimuli (sadness and fear) did not elicit high LFA or LFA/RFA ratios in our study, and the subjects did not perceive the negative stimuli as intensely as the positive stimuli either. Both decreased sympathetic arousal and low LFA/RFA ratios have been noted in grief states (Sternbach, 1962; Sakuragi, Sugiyama et al., 2002), typically characterized by a flat affect. However, our results contradict other studies, which suggest that negative emotions lead to increased sympa- thetic activity (Fredrickson & Levenson, 1998; Fredrickson, Mancuso et al., 2000). Some of these differences may be due to the fact that traditional HRV measures may not account for Respiratory Sinus Arrhythmia (and thus parasympathetic activ- ity) within the low frequency range of HRV, which is sub- tracted out to compute the LFA with the present technology (Aysin & Aysin, 2006). We did observe an increase in the mean LFA and high LFA/RFA ratios during disgust, particularly when subjects were asked to amplify their expression of disgust. In contrast, fear-provoking stimuli, which might be expected to produce high arousal and parasympathetic withdrawal (Bernt- son, Cacioppo et al., 1991), did not lead to a statistically sig- nificant difference in LFA and LFA/RFA ratio compared to the neutral stimulus. Instead, surprisingly, mean LFA was nega- tively correlated with the intensity of fear reported. Our unusual results may be explained by the finding that 4/7 subjects had seen the fear-inducing film clip previously. It is possible that prior memory of the film and knowledge of what is going to happen next attenuated the emotional impact of the clip. Fur- thermore, fear elicited lower mean RFA, but higher fluctuations in RFA as seen with the amusement film clip, suggesting that parasympathetic regulatory mechanisms (see below) may have been activated. Our sample size is too small to directly relate subjective emotional intensity to autonomic reactivity. Never- theless, taken together, our results suggest that in healthy indi- viduals, higher intensities of subjective emotional experience, both positive (e.g., amusement) and negative (e.g., amplified disgust) elicit higher responses in the lower frequency band- width (LFA) of HRV. The mean LFA and LFA/RFA ratios may therefore provide an objective measure of the intensity of Copyright © 2013 SciRes. 673  C. C. UY ET AL. feelings. Autonomic Basis of Emotional Regulation In order to evaluate emotional regulation, we focused on the response to disgust-inducing stimuli. Disgust is a primal, uni- versal emotion that may be less prone to external influences or personal preferences compared with other emotions such as amusement or sadness. In our study, subjects always started with the unregulated disgust condition, which elicited subjects’ natural response to disgust. We then asked subjects to suppress their disgust; and finally, we asked them to amplify their re- sponse to disgust. We believe that this order of stimulus pres- entation would override any desensitization that may occur from repeated exposure to disgust-inducing stimuli. Note that three different disgust film-clips were used for the three condi- tions. Unregulated disgust elicited higher mean LFA and LFA/RFA ratios compared with the neutral film clip; however, the differ- ence only showed a trend toward significance, perhaps due to our small sample size. The self-report questionnaire indicated that disgust was strongly elicited. Previous studies have noted increased arousal in response to disgust-eliciting film stimuli (Gross & Levenson, 1993). In contrast, when asked to suppress their disgust, subjects balanced their LFA and RFA and the LFA/RFA ratio approached that of the neutral stimulus. In ad- dition, the intensity of disgust was reduced on the post-film questionnaire, suggesting that subjects were indeed able to suppress their feelings of disgust. The reaction to disgust was also clearly exaggerated with instructions to amplify. The mean LFA, difference between mean LFA and RFA, and LFA/RFA ratio were also higher with disgust amplification. Our data are consistent with previous studies, which found that exaggerating expressive behavior results in higher sympathetic arousal, even on non-somatic measures (Zuckerman, Klorman et al., 1981). Thus healthy subjects can clearly regulate both autonomic arousal, reflected in the mean level of the LFA, and subjective emotional responses when requested to do so. Interestingly, both disgust suppression and amplification led to increased fluctuation in the RFA, indicating higher levels of parasympathetic activation during emotional regulation. This pattern is similar to that observed during amusement and fear film-clips, which also appeared to activate regulatory mecha- nisms (see above). Parasympathetic activation corresponds with a sense of calm (Fredrickson & Levenson, 1998) and higher at- tention (Healy, 2010) which may be needed during regulation. We also noted a strong positive correlation between fluctuation in RFA (cvRFA) and intensity of amusement, and a strong negative correlation with anxiety during disgust suppression. These results suggest that higher positive or lower negative emotional states may increase the capacity for emotional regu- lation, and lend support to the theory of vagal tone as a physi- ologic index of stress (Porges, 1995). Interestingly, anxiety lev- els were greater during disgust suppression than during disgust amplification. Decreased anxiety during amplification may cor- respond with subjects’ ability to give an unrestrained, exagger- ated reaction, while increased anxiety with suppression may re- sult from the stress of not being able to express one’s feelings. Taken together, our results suggest that emotional regulation is mediated by parasympathetic activation resulting in fluctuations in the RFA, and that higher positive or lower negative emo- tional states may enhance the capacity for emotional regulation. Conclusion and Future D i r ections Despite the small sample size, this study leads to a number of important findings: 1) Emotional reactivity or arousal is medi- ated by increase in the mean level of the lower frequency com- ponent of HRV, or LFA, which measures sympathetic activity; 2) the mean LFA and LFA/RFA ratio provide an objective measure of the subjective intensity of one’s feelings whether positive or negative; 3) Moment to moment fluctuations in respiratory activity modulated HRV frequency, or RFA, reflects parasympathetic-discharge-mediated emotional regulation; and 4) Positive subjective feelings appear to enhance the capacity for emotional regulation. These findings provide insight into mechanisms by which we react to and regulate our emotions to changing environmental cues, and form the basis for future research in conditions characterized by emotional dysfunction such as traumatic brain injury, post-traumatic stress disorder, and autism. Acknowledgements This project was funded by pilot grant from the Rusk Insti- tute of Rehabilitation Medicine, New York University School of Medicine. We are grateful for support to IJ through the Re- habilitation Research Experience for Medical Students (RREMS) program sponsored by the Association of Academic Physiatrists and the Foundation for Physical Medicine & Rehabilitation. We are also thankful to Shaun Nanavati for his insights in using ANSAR software, and to Dr. Joseph Colombo for the equip- ment loan from ANSAR. REFERENCES Akselrod, S., Gordon, D. et al. (1981). Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardio- vascular control. Science, 213, 220-222. doi:10.1126/science.6166045 ANSAR Medical Technologies (2005). The ANX version 3.0 autonomic nervous system monitor user’s operations manual. ANSAR Medical Technologies, Inc., 1-20. Appelhans, B., & Luecken L. J. (2006). Heart rate variability as an index of regulated emotional responding. Review of General Psy- chology, 10, 229-240. doi:10.1037/1089-2680.10.3.229 Appelhans, B. M., & Luecken, L. J. (2008). Heart rate variability and pain: Associations of two interrelated homeostatic processes. Bio- logical Psychology, 77, 174-182. doi:10.1016/j.biopsycho.2007.10.004 Aupperle, R. L., Allard, C. B. et al. (2012). Dorsolateral prefrontal cor- tex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Archives of General Psychiatry, 69, 360-371. doi:10.1001/archgenpsychiatry.2011.1539 Aysin, B., & Aysin, E. (2006). Effect of respiration in heart rate vari- ability (HRV) analysis. Conference Proceedings—IEEE Engineering in Medicine and Biology Society, 1 , 1776-1779. doi:10.1109/IEMBS.2006.260773 Bal, E., Harden, E. et al. (2010). Emotion recognition in children with autism spectrum disorders: Relations to eye gaze and autonomic state. Journal of Autism and Developmental Disorders , 40, 358-370. doi:10.1007/s10803-009-0884-3 Bazhenova, O. V., Plonskaia, O. et al. (2001). Vagal reactivity and af- fective adjustment in infants during interaction challenges. Child De- velopment, 72, 1314-1326. doi:10.1111/1467-8624.00350 Benarroch, E. E. (1993). The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clinic Proceedings, 68, 988-1001. doi:10.1016/S0025-6196(12)62272-1 Berntson, G. G., Cacioppo, J. T. et al. (1991). Autonomic determinism: The modes of autonomic control, the doctrine of autonomic space, Copyright © 2013 SciRes. 674  C. C. UY ET AL. Copyright © 2013 SciRes. 675 and the laws of autonomic constraint. Psychological Review, 98, 459-487. doi:10.1037/0033-295X.98.4.459 Bornhofen, C., & McDonald, S. (2008). Emotion perception deficits following traumatic brain injury: A review of the evidence and ra- tionale for intervention. Journal of the International Neuropsycholo- gical Society, 14, 511-525. doi:10.1017/S1355617708080703 Colombo, J., Shoemaker, W. C. et al. (2008). Noninvasive monitoring of the autonomic nervous system and hemodynamics of patients with blunt and penetrating trauma. Journal of Trauma, 65, 1364-1373. doi:10.1097/TA.0b013e31818cc307 de Sousa, A., McDonald, S. et al. (2012). Changes in emotional empa- thy, affective responsivity, and behavior following severe traumatic brain injury. Journal of Clinical and Experimental Neuropsychology, 34, 606-623. doi:10.1080/13803395.2012.667067 Fredrickson, B. L., & Levenson, R. W. (1998). Positive emotions speed recovery from the cardiovascular sequelae of negative emotions. Cognition & Emotion, 12, 191-220. doi:10.1080/026999398379718 Fredrickson, B. L., Mancuso, R. A. et al. (2000). The undoing effect of positive emotions. Motivation and Emotion, 24, 237-258. doi:10.1023/A:1010796329158 Gerlach, A. L., Wilhelm, F. H. et al. (2003). Embarrassment and social phobia: The role of parasympathetic activation. Journal of Anxiety Disorders, 17, 197-210. doi:10.1016/S0887-6185(02)00197-4 Giuliani, N. R., McRae, K. et al. (2008). The up- and down-regulation of amusement: Experiential, behavioral, and autonomic consequences. Emotion, 8, 714-719. doi:10.1037/a0013236 Gross, J. J., & Levenson, R. W. (1993). Emotional suppression: Physi- ology, self-report, and expressive behavior. Journal of Personality and Social Psychology, 64, 970-986. doi:10.1037/0022-3514.64.6.970 Gross, J. J., & Levenson, R. W. (1997). Hiding feelings: The acute effects of inhibiting negative and positive emotion. Journal of Ab- normal Psychology, 106, 95-103. doi:10.1037/0021-843X.106.1.95 Hammond, F. M., Davis, C. S. et al. (2012). Relational dimension of irritability following traumatic brain injury: A qualitative analysis. Brain Injury, 26, 1287-1296. doi:10.3109/02699052.2012.706352 Healy, B. (2010). The effect of attentional control and heart-period variability on negative affect and trait anxiety. Journal of General Psychology, 137, 140-150. doi:10.1080/00221301003645079 Izard, C., Fine, S. et al. (2001). Emotion knowledge as a predictor of social behavior and academic competence in children at risk. Psy- chological Science, 12, 18-23. doi:10.1111/1467-9280.00304 Levenson, R. W., Ekman, P. et al. (1992). Emotion and autonomic ner- vous system activity in the Minangkabau of west Sumatra. Journal of Personality and Social P s y chology, 62, 972-988. doi:10.1037/0022-3514.62.6.972 Mauss, I. B., Levenson, R. W. et al. (2005). The tie that binds? Coher- ence among emotion experience, behavior, and physiology. Emotion, 5, 175-190. doi:10.1037/1528-3542.5.2.175 McDonald, S., Bornhofen, C. et al. (2009). Addressing deficits in emo- tion recognition after severe traumatic brain injury: The role of fo- cused attention and mimicry. Neuropsychological Rehabilitation, 19, 321-339. doi:10.1080/09602010802193989 Neumann, S. A., & Waldstein, S. R. (2001). Similar patterns of cardio- vascular response during emotional activation as a function of affec- tive valence and arousal and gender. Journal of Psychosomatic Re- search, 50, 245-253. doi:10.1016/S0022-3999(01)00198-2 Porges, S. W. (1995). Cardiac vagal tone: A physiological index of stress. Neuroscie n c e & Biobehavioral Re v iews, 19, 225-233. doi:10.1016/0149-7634(94)00066-A Porges, S. W. (1995). Orienting in a defensive world: Mammalian mo- difications of our evolutionary heritage. A polyvagal theory. Psy- chophysiology, 32, 301-318. doi:10.1111/j.1469-8986.1995.tb01213.x Porges, S. W. (2007). The polyvagal perspective. Biological Psychol- ogy, 74, 116-143. doi:10.1016/j.biopsycho.2006.06.009 Pumprla, J., Howorka, K. et al. (2002). Functional assessment of heart rate variability: Physiological basis and practical applications. Inter- national Journal of Cardiology, 84, 1-14. doi:10.1016/S0167-5273(02)00057-8 Rottenberg, J., Ray, R. D. et al. (2007). Emotion elicitation using film. The handbook of motion elicitation and assessment. London: Oxford University Press. Sakuragi, S., Sugiyama, Y. et al. (2002). Effects of laughing and weep- ing on mood and heart rate variability. Journal of Physiological An- thropology and Applied Human Science, 21, 159-165. doi:10.2114/jpa.21.159 Schaefer, A., Nils, F. et al. (2010). Assessing the effectiveness of a large database of emotion-eliciting films: A new tool for emotion re- searchers. Cognition & Emotion, 24, 1153-1172. doi:10.1080/02699930903274322 Shiota, M. N., Neufeld, S. L. et al. (2011). Feeling good: Autonomic nervous system responding in five positive emotions. Emotion, 11, 1368-1378. doi:10.1037/a0024278 Sternbach, R. A. (1962). Assessing differential autonomic patterns in emotions. Journal of Psychosomatic Resea rc h, 6, 87-91. doi:10.1016/0022-3999(62)90059-4 Stifter, C. A., Dollar, J. M. et al. (2011). Temperament and emotion regulation: The role of autonomic nervous system reactivity. Devel- opmental Psychobiology, 53, 266-279. doi:10.1002/dev.20519 Thurman, D. J., Alverson, C. et al. (1999). Traumatic brain injury in the United States: A public health perspective. Journal of Head Trauma Rehabilitation, 14, 602-615. doi:10.1097/00001199-199912000-00009 Zuckerman, M., Klorman, R. et al. (1981). Facial, autonomic, and sub- jective components of emotion: The facial feedback hypothesis ver- sus externalizer-internalizer distinction. Journal of Personality and Social Psychology, 41, 929-944. doi:10.1037/0022-3514.41.5.929
|