R. Chibbar et al. / Case Reports in Clinical Medicine 2 (2013) 302-305
Copyright © 2013 SciRes. OPEN ACCESS
304
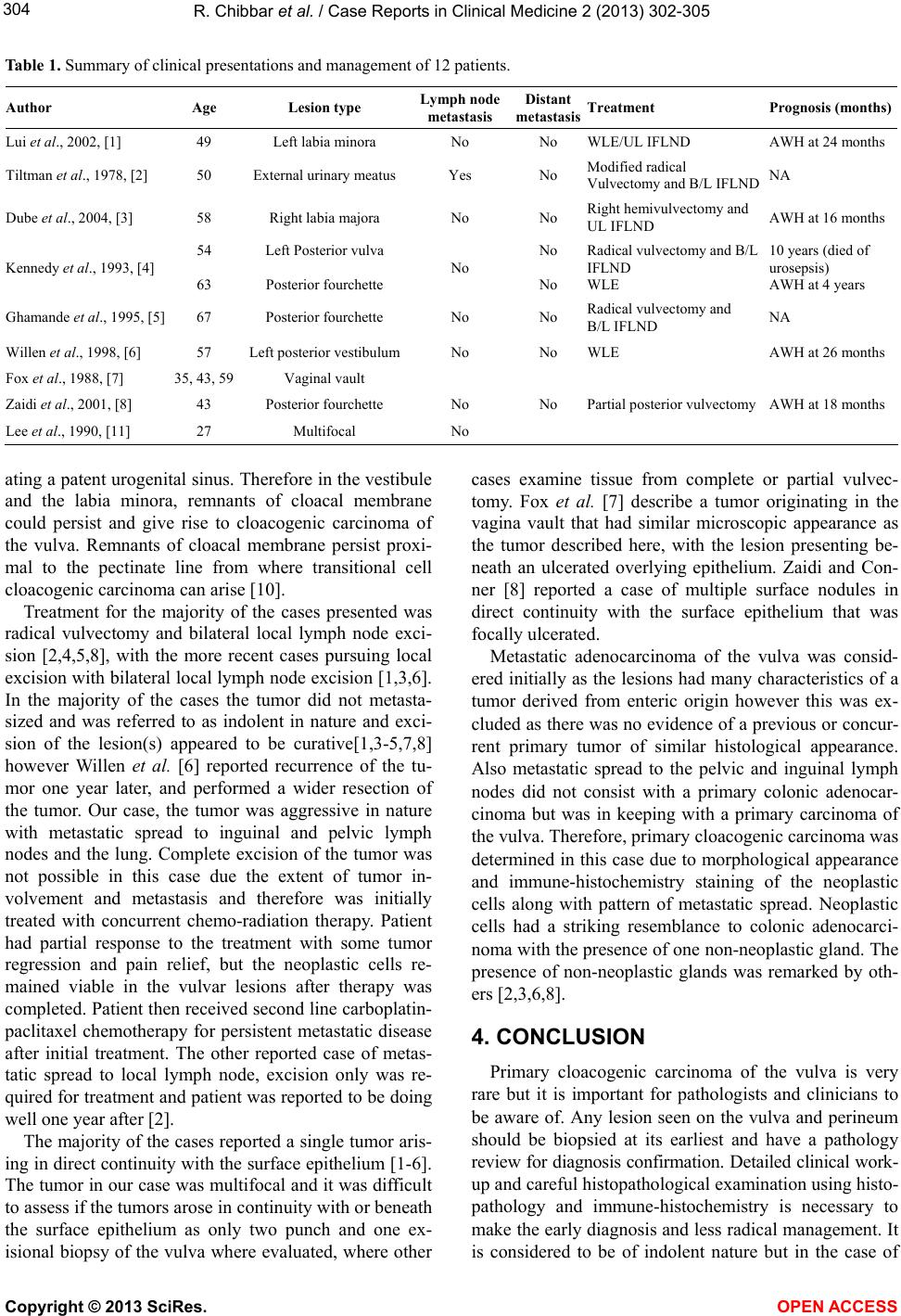
Table 1. Summary of clinical presentations and management of 12 patients.
Author Age Lesion type
Lymph node
metastasis Distant
metastasis Treatment Prognosis (months)
Lui et al., 2002, [1] 49 Left labia minora No No WLE/UL IFLND AWH at 24 months
Tiltman et al., 1978, [2] 50 External urinary meatus Yes No Modified radical
Vulvectomy and B/L IFLND NA
Dube et al., 2004, [3] 58 Right labia majora No No Right hemivulvectomy and
UL IFLND AWH at 16 months
Kennedy et al., 1993, [4] 54
63
Left Posterior vulva
Posterior fourchette No No
No
Radical vulvectomy and B/L
IFLND
WLE
10 years (died of
urosepsis)
AWH at 4 years
Ghamande et al ., 1995, [5] 67 Posterior fourchette No No Radical vulvectomy and
B/L IFLND NA
Willen et al., 1998, [6] 57 Left posterior vestibulumNo No WLE AWH at 26 months
Fox et al., 1988, [7] 35, 43, 59Vaginal vault
Zaidi et al., 2001, [8] 43 Posterior fourchette No No Partial posterior vulvectomy AWH at 18 months
Lee et al., 1990, [11] 27 Multifocal No
ating a patent urogen ital sinus. Therefore in the vestibule
and the labia minora, remnants of cloacal membrane
could persist and give rise to cloacogenic carcinoma of
the vulva. Remnants of cloacal membrane persist proxi-
mal to the pectinate line from where transitional cell
cloacogenic carcinoma can arise [10].
Treatment for the majority of the cases presented was
radical vulvectomy and bilateral local lymph node exci-
sion [2,4,5,8], with the more recent cases pursuing local
excision with bilateral local lymph node excision [1,3,6].
In the majority of the cases the tumor did not metasta-
sized and was referred to as indolent in nature and exci-
sion of the lesion(s) appeared to be curative[1,3-5,7,8]
however Willen et al. [6] reported recurrence of the tu-
mor one year later, and performed a wider resection of
the tumor. Our case, the tumor was aggressive in nature
with metastatic spread to inguinal and pelvic lymph
nodes and the lung. Complete excision of the tumor was
not possible in this case due the extent of tumor in-
volvement and metastasis and therefore was initially
treated with concurrent chemo-radiation therapy. Patient
had partial response to the treatment with some tumor
regression and pain relief, but the neoplastic cells re-
mained viable in the vulvar lesions after therapy was
completed. Patient then received second line carboplatin-
paclitaxel chemotherapy for persistent metastatic disease
after initial treatment. The other reported case of metas-
tatic spread to local lymph node, excision only was re-
quired for treatment and patient was reported to be doing
well one year after [2].
The majority of the cases reported a single tumor aris-
ing in direct continuity with the surface epithelium [1-6].
The tumor in our case was multifocal and it was difficult
to assess if the tumors arose in continuity with or beneath
the surface epithelium as only two punch and one ex-
isional biopsy of the vulva where evaluated, where other
cases examine tissue from complete or partial vulvec-
tomy. Fox et al. [7] describe a tumor originating in the
vagina vault that had similar microscopic appearance as
the tumor described here, with the lesion presenting be-
neath an ulcerated overlying epithelium. Zaidi and Con-
ner [8] reported a case of multiple surface nodules in
direct continuity with the surface epithelium that was
focally ulcerated.
Metastatic adenocarcinoma of the vulva was consid-
ered initially as the lesions had many characteristics of a
tumor derived from enteric origin however this was ex-
cluded as there was no evidence of a previous or concur-
rent primary tumor of similar histological appearance.
Also metastatic spread to the pelvic and inguinal lymph
nodes did not consist with a primary colonic adenocar-
cinoma but was in keeping with a primary carcinoma of
the vulva. Therefore, primary cloacogenic carcinoma was
determined in this case due to morphological appearance
and immune-histochemistry staining of the neoplastic
cells along with pattern of metastatic spread. Neoplastic
cells had a striking resemblance to colonic adenocarci-
noma with the presence of one non-neoplastic gland. The
presence of non-neoplastic glands was remarked by oth-
ers [2,3,6,8].
4. CONCLUSION
Primary cloacogenic carcinoma of the vulva is very
rare but it is important for pathologists and clinicians to
be aware of. Any lesion seen on the vulva and perineum
should be biopsied at its earliest and have a pathology
review for diagnosis confirmation. Detailed clinical work-
up and careful histopathological examination using histo-
pathology and immune-histochemistry is necessary to
make the early diagnosis and less radical management. It
is considered to be of indolent nature but in the case of