Paper Menu >>
Journal Menu >>
 Journal of Cancer Therapy, 2010, 1, 229-231 doi:10.4236/jct.2010.14035 Published Online December 2010 (http://www.scirp.org/journal/jct) Copyright © 2010 SciRes. JCT 229 Long Term Progression Free Survival Achieved by Maintenance “Second–Line” Polychemotherapy with Gemcitabine/Paclitaxel in Metastatic Urothelial Cancer of the Renal Pelvis—A Case Report Andreas Bannowsky1, Hermann Van Ahlen1, Klaus-Peter Jünemann2 1Department of Urology, Klinikum Osnabrück, Germany; 2Department of Urology, University Hospital Schleswig-Holstein, Campus Kiel, Germany. Email:andreas.bannowsky@klinikum-os.de Received June 21st, 2010; revised July 5th, 2010; accepted July 22nd, 2010. ABSTRACT Moderate activity of systemic chemotherapy for advanced urothelial cancer has been reported for more than 30 years. Only with the advent of potent combination therapy clinically significant response rates as well as prolonged survival were documented. The therapeutic effect of a “second-line” polychemotherapy in metastatic upper tract urothelial cancer is largely unknown caused by the small number of cases and poor prognosis. We report an interesting case of a 59-year-old man suffering from urothelial cancer of the renal pelvis with pulmonary, lymphogenic and bone metastases who showed an unexpected response to a “second-line”chemotherapy after only 2 treatment cycles of Gemcit- abine/Paclitaxel (partial remission) after 24 treatment cycles Gemcitabine / Cisplatin in “stable disease” and progres- sion between the treatment intervals. We performed maintenance “second-line” therapy for 24 cycles and the patient showed a remarkable persisting response 54 months after operat i on. Keywords: Chemotherapy, Renal Pelvis, Urothelial Cancer, Paclitaxel 1. Introduction The gold standard treatment for advanced urothelial can- cer of the renal pelvis is radical nephroureterectomy with the complete removal of a bladder cuff around the ipsi- lateral ureteral orifice. In case of metastatic disease a cisplatin based polychemotherapy follows as an adjuvant or palliative treatment. These, often nephrotoxic chemo- therapy can lead to long term remission or permanent cure in only a small number of patients. We report the remarkable progression of a patient with a metastasized urothelial cancer of the renal pelvis in the time before vinflunine had become available for second-line treat- ment. 2. Case Report A tumor nephrectomy on the right side was performed on a 59 year old patient after suspicion of a pulmonary, lymphogenic and bone metastasized kidney cancer (sug- gested RCC). The pathology indicated an advanced urothelial cancer of the renal pelvis (tumor stage pT3a). The primary staging computertomography (CT) showed extensive metastatic disease with involvement of the retroperitoneal lymph nodes, pulmonary metastases and bone metastatic disease in the right Os ileum. After pal- liative analgetic and stabilizising radiation (30 Gy) of the right Os ileum, six cycles of polychemotherapy with Gemcitabine (1200 mg/m2) and Cisplatin (70 mg/m2) were performed. At the first re-staging a “stable disease” (SD) was shown by computertomography. During trea- ment, we again performed six cycles of polychemother- apy with Gemcitabine/Cisplatin, due to progressive dis- ease. After this treatment, SD for five months occurred, followed by another progression. In total the patient re- ceived 24 cycles of polychemotherapy with Gemcit- abine/Cisplatin because of recurrent progressive disease 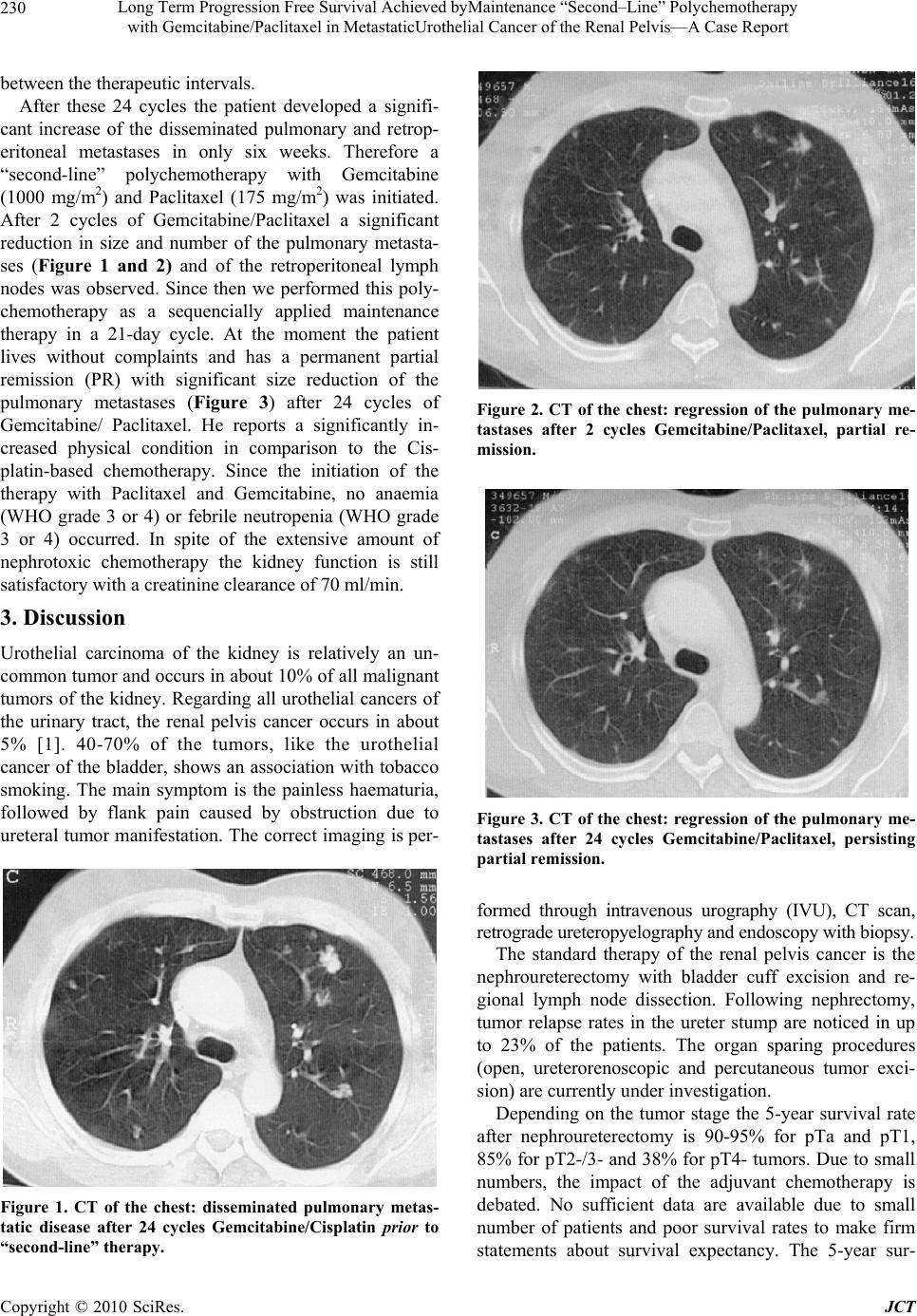 Long Term Progression Free Survival Achieved byMaintenance “Second–Line” Polychemotherapy with Gemcitabine/Paclitaxel in MetastaticUrothelial Cancer of the Renal Pelvis—A Case Report Copyright © 2010 SciRes. JCT 230 between the therapeutic intervals. After these 24 cycles the patient developed a signifi- cant increase of the disseminated pulmonary and retrop- eritoneal metastases in only six weeks. Therefore a “second-line” polychemotherapy with Gemcitabine (1000 mg/m2) and Paclitaxel (175 mg/m2) was initiated. After 2 cycles of Gemcitabine/Paclitaxel a significant reduction in size and number of the pulmonary metasta- ses (Figure 1 and 2) and of the retroperitoneal lymph nodes was observed. Since then we performed this poly- chemotherapy as a sequencially applied maintenance therapy in a 21-day cycle. At the moment the patient lives without complaints and has a permanent partial remission (PR) with significant size reduction of the pulmonary metastases (Figure 3) after 24 cycles of Gemcitabine/ Paclitaxel. He reports a significantly in- creased physical condition in comparison to the Cis- platin-based chemotherapy. Since the initiation of the therapy with Paclitaxel and Gemcitabine, no anaemia (WHO grade 3 or 4) or febrile neutropenia (WHO grade 3 or 4) occurred. In spite of the extensive amount of nephrotoxic chemotherapy the kidney function is still satisfactory with a creatinine clearance of 70 ml/min. 3. Discussion Urothelial carcinoma of the kidney is relatively an un- common tumor and occurs in about 10% of all malignant tumors of the kidney. Regarding all urothelial cancers of the urinary tract, the renal pelvis cancer occurs in about 5% [1]. 40-70% of the tumors, like the urothelial cancer of the bladder, shows an association with tobacco smoking. The main symptom is the painless haematuria, followed by flank pain caused by obstruction due to ureteral tumor manifestation. The correct imaging is per- Figure 1. CT of the chest: disseminated pulmonary metas- tatic disease after 24 cycles Gemcitabine/Cisplatin prior to “second-line” therapy. Figure 2. CT of the chest: regression of the pulmonary me- tastases after 2 cycles Gemcitabine/Paclitaxel, partial re- mission. Figure 3. CT of the chest: regression of the pulmonary me- tastases after 24 cycles Gemcitabine/Paclitaxel, persisting partial remis sion. formed through intravenous urography (IVU), CT scan, retrograde ureteropyelography and endoscopy wit h biopsy. The standard therapy of the renal pelvis cancer is the nephroureterectomy with bladder cuff excision and re- gional lymph node dissection. Following nephrectomy, tumor relapse rates in the ureter stump are noticed in up to 23% of the patients. The organ sparing procedures (open, ureterorenoscopic and percutaneous tumor exci- sion) are currently under investigation. Depending on the tumor stage the 5-year survival rate after nephroureterectomy is 90-95% for pTa and pT1, 85% for pT2-/3- and 38% for pT4- tumors. Due to small numbers, the impact of the adjuvant chemotherapy is debated. No sufficient data are available due to small number of patients and poor survival rates to make firm statements about survival expectancy. The 5-year sur-  Long Term Progression Free Survival Achieved byMaintenance “Second–Line” Polychemotherapy with Gemcitabine/Paclitaxel in MetastaticUrothelial Cancer of the Renal Pelvis—A Case Report Copyright © 2010 SciRes. JCT 231 vival rate of patients with lymphogenic or haematogenic metastasized urothelial cancer is only 15-20% [2]. The median survival with a metastatic upper urinary tract cancer with visceral metastasis (pulmonary/liver/bone) is about 10 months and can be increased to 14-15 months with a polychemotherapy with a combination of Gem- citabine and Cisplatin or the “gold standard” chemother- apy with MVAC (methotrexate, vinblastine, adriamycine and cisplatin) with a total resp onse rate of 49% for Gem- citabine/Cisplatin and 46% with MVAC-treated patients [3,4]. The most common toxicities in both therapy schemes are the haematological toxicities with grade III/IV-anaemia (18-27%), thrombopenia (21-57%) as well as grade III/IV-neutropenia (71-82%). Due to the proven advantages in the toxicity profile with most likely the same effectiveness, Gemcitabine/Cisplatin has be- come the standard treatment [4]. Other combinations with Paclitaxel and Carboplatine show an increase of a 5-year survival rate up to 52% for the lymphogenic me- tastasized urothelial cancer [5]. In case of further disease progression, no data was available at the moment of pro- gression in this patient with regards to an effective “sec- ond-line” therapy of this tumor entity. Vinflunine is a new promising “second-line” strategy after first-line cis- platin-containing regimen for metastatic bladder cancer [6], and is considered as an option for “third-line” treat- ment for this particular patient. In agreement with the therapy protocol of the study: AUO-Nr. AB20/99 (randomized phase III study for “second-line” therapy of the cisplatin pre-treated metas- tasized urothelial cancer with Gemcitabine/Paclitaxel), we successfully performed the therapy with Gemcitabine and Paclitaxel as a sequencially maintenance therapy. Including the patient in this particular stud y was not pos- sible at that time, due to the formerly exclusion criteria of a prior gemcitabine-based therapy. Despite initially extreme unfavourable prognosis and excessive cumulative dosage of chemotherapy, in this case the patient lives 54 months after operation in overall good condition (Karnofsky-performance status 100%) after a total of 24 cycles of Gemcitabine/ Cisplatin and 24 cycles of Gemcitabine/Paclitaxel and is in partial re- mission. This patient had an unexpected and remarkable long-term process with persisting response to a “sec- ond-line” chemotherapy with Gemcitabine/Paclitaxel. Especially in treating younger patients, this interesting case impressively shows the need for using newer poly- chemotherapy schemes as a therapy, despite obvious therapy failure of a well-established chemotherapy and therefore being able to offer the patient an additional therapeutic option. The authors would like to point out, to include suitable patients in controlled multicentre studies to guarantee the collection of data and therefore lay a foundation of further therapy recommendations. REFERENCES [1] S. Hause r and U. E. Stude r, “Therapy of th e Renal Pelvis Carcinoma,” Urologe, Vol. 40, No. 6, 2001, pp. 452-455. [2] J. J. Munoz, L. M. Ellison, “Upper Tract Urothelial Neo- plasms: Incidence and Survival during the Last 2 Dec- ades,” Journal of Urology, Vol. 164, No. 5, 2000, pp. 1523-1525. [3] C. N. Sternberg, “Metastatic Bladder Cancer: Role of Chemotherapy and New Agents,” EAU Update Series, Vol. 1, No. 2, 2003, pp. 108-117. [4] J. Lehmann, M. Retz, M. Hac k, S. Siemer and M. Stöckle, “Systemic Chemotherapy of Urothelial Cancer,” Onkolo- gie, Vol. 26, Sup. 4, 2003, pp. 18-25. [5] A. Bamias, C. Deliveliotis, G. Fountzilas, et al., “Adju- vant Chemotherapy with Paclitaxel and Carboplatin in Patients with Advanced Carcinoma of the Upper Urinary Tract: a study by the Hellenic Cooperative Oncology Group,” Journal of Clinical Oncology, Vol. 22, No. 11, 2004, pp. 2150-2154. [6] S. Culine, C. Theodore, M. De Santis, et al., “A phase II study of Vinflunine in Bladder Cancer Patients Progress- ing after First-line Platinum-containing Regimen,” British Journal of Cancer, Vol. 94, 2006, pp. 1395-1401. |

