Paper Menu >>
Journal Menu >>
 Advances in Bioscience and Biotechnology, 2010, 1, 439-443 ABB doi:10.4236/abb.2010.15057 Published Online December 2010 (http://www.SciRP.org/journal/abb/). Published Online December 2010 in SciRes. http://www.scirp.org/journal/ABB Influence of magnetic iron oxide nanoparticles on red blood cells and Caco-2 cells Daniel Moersdorf1, Pierre Hugounenq1, Lai Truonc Phuoc2, Hind Mamlouk-Chaouachi2, Delphine Felder-Flesch2, Sylvie Begin-Colin2, Geneviève Pourroy2, Ingolf Bernhardt1 1Laboratory of Biophysics, Saarland University, Campus, Building A2.4, 66123 Saarbruecken, Germany; 2Institut de Physique et Chimie des Matériaux de Strasbourg, UMR 7504 CNRS-Université de Strasbourg-ECPM, 23 rue du Loess, 67034 Strasbourg Cedex 2, France. Email: i.bernhardt@mx.uni-saarland.de Received 14 September 2010; revised 22 October 2010; accepted 26 October 2010. ABSTRACT The interactions of two types o f cells (red blood cells, Caco-2 cells) with magnetic iron oxide nanoparticles (non-grafted, citrate-grafted, dendrimer-grafted) of 11 nm in size have been investigated. We focused on two important physiological parameters of the cells, the intracellular pH and the intracellular Ca2+ con- tent. The results show that the nanoparticles do not have a significant influence on the pH and Ca2+ con- tent of Caco-2 cells. The Ca2+ content of red blood cells is also not affected but the intracellular pH is slightly reduced. Keywords: Red Blood Cells; Caco-2 Cells; Ca2+ Content; Intracellular PH; Mag netic Iron Oxide Nanoparticles 1. INTRODUCTION One of the main topics in biotechnology relies on the development of various systems, ranging in the nano- and submicronic scales for diagnostics, imaging, drug delivery, or cancer therapy [1,2]. These systems com- prise association between organic self-assembled vehi- cles (vesicles, emulsions, microbubbles, dendrimers) and inorganic nanoparticles. Designing and synthesizing hybrid materials combining at least two of these com- ponents are of the utmost importance since such materi- als can ensure a strong versatility and adaptability. This is the aim of project in which this study has been achieved [Nanomagdye FP7 EU Project grant agreement nr NMP3-SL-2008-214032]. However, before combin- ing each component, the toxicity towards cells has to be studied. In our studies, magnetic iron oxide nanoparticles non- coated or coated with citrate or dendrons have been tested on cells. We first investigate the effect on physio- logical parameters of RBCs. As fundamental physio- logical parameters, the intracellular Ca2+ content as well as the intracellular pH (pHi) have been chosen. These parameters can be measured using fluorescent dyes. For comparison and as an example of cancer cells, which are targets for nanoparticles in medical treatment, Caco-2 cells have been chosen. It should be mentioned that RBCs and Caco-2 cells differ in one main fundamental aspect – no end ocytosis occurs in RBCs whereas Caco-2 cells have such a mechanism [3]. Many publications report experiments on the interac- tion of targeted tissues or cells and nanoparticles. How- ever, it is sometimes unclear whether these particles show unspecific reactions with other tissues or cells they come in contact with [4]. For example, nanoparticles injected into the blood stream and therefore, coming in contact with red blood cells (RBCs), could adhere to the surface of the cells (and in this case not move to the tar- get cells) or even enter the cells. Such an uptake of nanoparticles by RBCs has been reported by Geiser et al. [5] and Rothen-Rutishauser et al. [6]. In addi tion, in bot h cases nanoparticles could affect physiological parame- ters of the cells. For instance, it has been reported that iron oxide nanoparticles can increase the membrane permeability of human microvascular endothelial cells [7]. 2. MATERIAL AND METHODS The influence of bare iron oxide nanoparticles (11 nm diameter) and decorated with an organic shell (citrate or dendrimer) on the intracellular pH (pHi) and the intra- cellular Ca2+ content of RBCs and Caco-2 cells has been investigated. 2.1. Nanoparticles The bare, citrated or dendronized nanoparticles used were  D. Moersdorf et al. / Advances in Bioscience and Biotechnology 1 (2010) 439-443 Copyright © 2010 SciRes. ABB 440 synthesized at IPCMS, Strasbourg. The non grafted iron oxide nanoparticles were prepared according to the method of Daou et al. [8] by coprecipitation at 70℃ from ferrous Fe2+ and ferric Fe3+ ions and a (N(CH3)4OH) solution. The nanoparticles were characterized by X-ray diffraction (XRD), magnetic measurements and micros- copy techniques. They exhibit the magnetite spinel structure and a lattice parameter of 0.8379 ± 0.0004 nm corresponding to an intermediate phase between maghe- mite and magnetite written Fe3-xO4. They are 11 ± 2 nm in size deduced from TEM measurements and XRD characterizations. A generation zero dendritic molecule (C41H77O22P, 953.02 g/mol) was grafted at their surface by mean of a phosphonate coupling agent following the method described by Daou et al. [9] and Basly et al. [10]. Citrate coated nanoparticles were obtained by intro- ducing sodium citrate in a water suspension of nanopar- ticles and adjusting the pH at 4.0 with a buffer solution. Then the suspension was mechanically stirred for 24 h, washed with water and centrifuged several times. Water is finally added in order to obtain a stable suspension of citrated nanoparticles. 2.2. Preparation of RBCs The blood used for experiments was given by healthy human donors, stored at 4℃ not longer than 24 h, and washed three times by centrifug ation at 2000 g for 5 min at room temperature in a physiological solution contain- ing 145 mM NaCl, 7.5 mM KCl, 10 mM glucose, 10 mM HEPES, pH 7.4. 2.3. Preparation of CaCo-2 Cells The Caco-2 cells were cultured in Dulbeccos modified Eagle medium. For detaching and separation the cells were trypsinated and washed three times by centrifuga- tion at 2000 g for 5 min at room temperature in physio- logical solution. 2.4. Loading with Fluorescent Dyes For intracellular Ca2+ and the intracellular pH measure- ments the fluorescent dye fluo-4 AM and BCECF AM were used, respectively. Stock solutions of the fluores- cent dyes (1 mM) in Pluronic F-127 20% in DMSO (Molecular Probes, USA)) were prepared. The dyes were added to the washed and re-suspended cells (0.2% v/v) at a concentration of 2 nM. After incubation for 30 min at 37℃ the cells were washed three times (2000 g, 5 min) to remove free dye in the medium surrounding the cells. 2.5. Treatment of Cells with Nanoparticles The cells loaded with the dye (fluo-4 AM or BCECF AM) were incubated in a ph ysiological solution con taining 5 - 50 µg/ml of nanoparticles for 30 min at 37℃. The ex- posure time of 30 min has been selected to be able to measure changes of fast as well as slow processes of cellular reactions. 2.6. Fluorescence Measurements After the 30 min incubatio n the cells were transferred on a cover slip and after 5 min (necessary time to let the cells settle down) the fluorescence intensity was meas- ured with a fluorescence microscope (Eclipse TE2000-E, Nikon, Japan) for single cell investigations. Flow cy- tometry (FACScalibur, Becton Dickinson Bioscience, USA) measurements were carried out to investigate an higher amount of cells. In this case the cell suspension was immediately measured after the 30 min incubation. 2.7. Calibration for PHi Determination A calibration curve of the fluorescence intensity de- pending on pH was created, using the K+/H+ ionophore nigericin. For this reason 5 µM nigericin was added to RBCs suspended in the calibration buffer solutions con- taining 135 mM KCl, 10 mM NaCl, 10 mM glucose, 10 mM HEPES/NaOH [11,12]. The calibration curve was linear in the pH range 6.5-8.0. 2.8. Reagents and Dyes The chemicals used were purchased from VWR (Ger- many) and Sigma Aldrich (USA). Fluorescent dyes from Molecular Probes (USA) were used for analysing the physiological parameters. 2.9. Statistical Treatment of Results The data are presented as mean values ± SD of inde- pendent experiments. The significance of differences was tested by paired or unpaired Student’s t-test. Differ- ences were considered significant if p < 0.05. Each ex- periment was repeated at least 3 times on blood from different donors. For Ca2+ and pHi determinations using the fluorescence microscope at least 3 measurements (with blood from 3 different donors and at least 10 single cells from each blood, i.e. 30 single cells) were carried out for each experimental condition. For the FACS ex- periments 3 measurements (with blood from 3 different donors and 30.000 cells from each blood) were carried out and analysed und er each experimental condition. 3. RESULTS AND DISCUSSION The effect of non grafted, citrate grafted or dendronized nanoparticles on the intracellular Ca2+ content as well as on the pHi of RBCs and Caco-2 cells is shown in Figure 1. These two cellular parameters are largely independent from each other and play an important role for many relevant physiological processes. For example, they in- 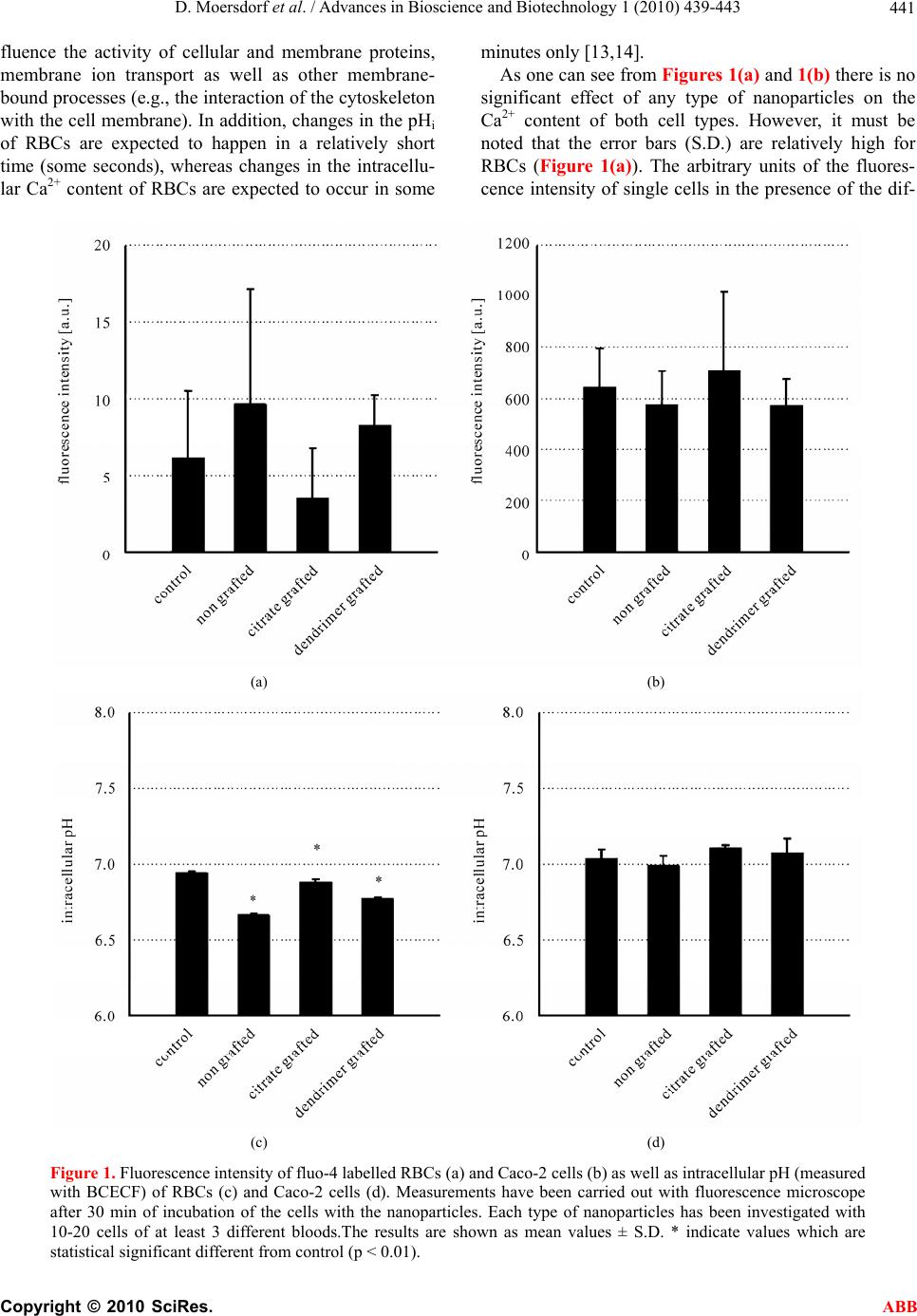 D. Moersdorf et al. / Advances in Bioscience and Biotechnology 1 (2010) 439-443 Copyright © 2010 SciRes. ABB 441 fluence the activity of cellular and membrane proteins, membrane ion transport as well as other membrane- bound processes ( e.g., the interaction of the cytoskeleton with the cell membrane). In addition , changes in the pHi of RBCs are expected to happen in a relatively short time (some seconds), whereas changes in the intracellu- lar Ca2+ content of RBCs are expected to occur in some minutes only [13,14]. As one can see from Figures 1(a) and 1(b) there is no significant effect of any type of nanoparticles on the Ca2+ content of both cell types. However, it must be noted that the error bars (S.D.) are relatively high for RBCs (Figure 1(a)). The arbitrary units of the fluores- cence intensity of single cells in the presence of the dif- (a) (b) (c) (d) Figure 1. Fluorescence intensity of fluo-4 labelled RBCs (a) and Caco-2 cells (b) as well as intracellular pH (measured with BCECF) of RBCs (c) and Caco-2 cells (d). Measurements have been carried out with fluorescence microscope after 30 min of incubation of the cells with the nanoparticles. Each type of nanoparticles has been investigated with 10-20 cells of at least 3 different bloods.The results are shown as mean values ± S.D. * indicate values which are statistical significant different from control (p < 0.01).  D. Moersdorf et al. / Advances in Bioscience and Biotechnology 1 (2010) 439-443 Copyright © 2010 SciRes. ABB 442 ferent nanoparticles vary only between 0 and 20. The reason is that the intracellular Ca 2+ content of these cells is very low and close to the detection minimum using fluo-4 as the Ca2+-sensitive fluorescent dye. It is well known that the intracellular Ca2+ content of RBCs is very low [15,16] and it has been previously reported by our group that the ratiometric Ca2+-sensitive fluorescent dye fura-2 is not applicable for RBCs and that only fluo-4 is useful to monitor reasonable qualitative changes of the intracellular Ca2+ content (see [15] for more de- tails). To clarify the big variation of the fluorescence inten- sities of fluo-4, i.e. the Ca2+ content of the RBCs shown in Fig. 1a, comparable flow cytometry (FACS) investi- gations have been carried out. This method allows con- sidering 30.000 cells in one single experiment, i.e. for one blood sample. Figure 2 shows a characteristic ex- ample of such flow cytometry measurement (one of three) for 2 types of nanoparticles in comparison to con- trol. It can be clearly seen that the fluorescence peaks are identical, supporting the finding that there is no signifi- cant increase of the intracellular Ca2+ content when the RBCs are exposed to the nanoparticles. It should be mentioned that one big advantage of fluorescence mi- croscopy is that single cells can be monitored over a certain time period. This allows seeing differences in cellular reactions and morphological changes and possi- ble destructions of individual cells. In addition, some physiological parameters of RBCs are affected by shear forces [17,18], which might be also the case during the process of cell flow through the capillary in a flow cy- tometer. Figure 1(c) shows that the pHi of RBCs is slightly but significantly lowered after the cells are in contact with the different nanoparticles (compared to the control value). However, such effect in the range of 0.1 to 0.3 pH units seems not of fundamental physiological rele- vance. It has to be stated that the pHi value for the con- trol is already about 0.1 to 0.2 units below the expected (literature) value [13]. The small effect of the nanoparti- cles on the pHi of RBCs could be due to the fact that RBCs can regulate their pH very efficiently and rapidly. Each RBC contains 106 copies of a protein (band 3) re- sponsible for the gas (HCO3-/Cl-) exchange and some other proteins for pH regulation like the Na+/H+ ex- changer [14,19]. The band 3 protein occupying a sub- stantial area of the outer membrane surface [20] could be easily affected by the nanoparticles. Although HCO3- was not explicitly added to the so lutions, there is enough bicarbonate present (from the air-containing CO2 dis- solving in the solutions) that the HCO3-/Cl- exchanger is fully functioning. Furthermore, this exchanger also op- erates as a Cl-/Cl- exchanger and therefore normally no Figure 2. Fluorescence intensity of fluo-4 labelled RBCs. Measurements have been carried out with flow cytometry after 30 min of incubation of the cells without (control) and with nanoparicles (citrate grafted, dendromer grafted). For each measurement 30.000 cells were analysed. The figure shows one single experiment, representative for 3 different blood samples. HCO3- has to be added when investigating RBC mem- brane transport [14]. Figure 1(d) shows that there is no significant effect of the various nanoparticles on pHi of Caco-2 cells. In the described experiments investigating the effect of nanoparticles on RBCs (Figs. 1a and 1c) no haemoly- sis was observed. On the contrary, a haemolytic effect of magnetic nanoparticles (magnetite colloidal nanoparti- cles stabilised with citric acid) on animal RBCs was re- ported by Creanga et al. [21]. However, the authors mentioned that further investigations must be done to explain how nanoparticles can induce haemolysis of RBCs. 4. CONCLUSION Nanoparticles can influence physiological parameters of cells. In our investigations we found a small but signifi- cant effect of nanoparticles on the intracellular pH (pHi) of RBCs. The pHi of Caco-2 cells was not affected by the nanoparticles. The intracellular Ca2+ content of RBCs as well as Caco-2 cells did not significantly change when the cells were exposed to nanop articles. Therefore, nanoparticles have to be tested not only with respect to their effect on target cells but also in respect of interac- tions with cells they can co me in contact with, especially RBCs. 5. ACKNOWLEDGEMENTS The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7 2007- 2013) under grant agreement nr NMP3-SL-2008-214032. REFERENCES [1] Pankhurst, Q.A., Connolly, J., Jones, S.K. and Dobson, J. (2003) Applications of magnetic nanoparticles in bio- medicine. J Phys D: Appl Phys, 36, R167-R181.  D. Moersdorf et al. / Advances in Bioscience and Biotechnology 1 (2010) 439-443 Copyright © 2010 SciRes. ABB 443 [2] Park, K., Lee, S., Kang, E., Kim, K., Choi, K. and Kwon, I.C. (2009) New generation of multifunctional nanoparti- cles for cancer imaging and therapy. Adv Funct Mater, 19, 1553-1566. [3] Win, K.Y. and Feng, S. (2005) Effects of particle size and surface coating on cellular uptake of polymeric nanopar- ticles for oral delivery of anticancer drugs. Biomaterials, 26, 2713-2722. [4] Hillyer, J.F. and Albrecht, R.M. (2001) Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J Pharm Sci, 90, 1927-1936. [5] Geiser, M., Rothen-Rutishauser, B., Kapp, N., Schürch, S., Kreyling, W., Schulz, H., Semmler, M., Im Hof, V., Heyder, J. and Gehr, P. (2005) Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect, 11 3, 1555-1560. [6] Rothen-Rutishauser, B., Schürch, S., Haenni, B., Kapp, N. and Gehr, P. (2006) Interaction of fine particles and nanoparticles with red blood cells visualized with ad- vanced microscopic techniques. Environ Sci Technol, 40, 4353-4359. [7] Apopa, P.L., Qian, Y., Shao, R., Guo, N.L., Schwe- gler-Berry, D., Pacurari, M., Porter, D., Xianglin, S., Vallyathan, V., Castranova, V. and Flynn, D.C. (2009) Iron oxide nanoparticles induce human microvascular endothelial cell permeability through reactive oxygen species production and microtubule remodelling. Part Fibre Toxicol, 6:1. [8] Daou, T.J., Pourroy, G., Begin-Colin, S., Greneche, C. Ulhaq-Bouillet, J.M., Legare, P., Bernhardt, P., Leuvrey, C. and Rogez, G. (2006) Hydrothermal synthesis of monodisperse magnetite nanoparticles. Chem Mater, 18, 4399-4404. [9] Daou, T.J., Pourroy, G., Greneche, J.M., Bertin, A., Felder-Flesch, D. and Begin-Colin, S. (2009) Water soluble dendronized iron oxide nanoparticles. Dalton Transactions, 23, 4442-4449. [10] Basly, B., Felder-Flesch, D., Perriat, P., Billotey, C., Taleb, J., Pourroy, G. and Begin-Colin, S. (2010) Den- dronized iron oxide nanoparticles as contrast agents for MRI. Chem Comm, 46, 985-987. [11] Grinstein, S., Cohen, S. and Rothstein, A. (1984) Cyto- plasmic pH regulation in thymic lymphocytes by an amiloride-sensitive Na+/H+ antiport. J Gen Physiol, 83, 341-369. [12] Kummerow, D., Hamann, J., Browning, J.A., Wilkins, R., Ellory, J.C. and Bernhardt, I. (2000) Variations of intra- cellular pH in human erythrocytes via K+(Na+)/H+ ex- change under low ionic strength conditions. J Membr Biol, 176, 207-216. [13] Bernhardt, I. and Weiss, E. (2003) Passive membrane permeability for ions and the membrane potential. In: Bernhardt, I. and Ellory, J.C., Eds., Red Cell Membrane Transport in Health and Disease, Springer-Verlag, Berlin, 83-109. [14] Knauf, P.A. and Pal, P. (2003) Band 3 mediated transport. In: Bernhardt, I. and Ellory, J.C., Eds., Red Cell Mem- brane Transport in Health and Disease, Springer-Verlag, Berlin, 253-301. [15] Kaestner, L., Tabellion, W., Weiss, E., Bernhardt, I. and Lipp, P. (2006) Calcium imaging of individual erythro- cates: Problems and approaches. Cell Calcium, 39, 13-19. [16] Bennekou, P. and Christophersen, P. (2003) Ion channels. In: Bernhardt, I. and Ellory, J.C., Eds., Red Cell Mem- brane Transport in Health and Disease, Springer-Verlag, Berlin, 139-152. [17] Leveritt, L.B., Hellums, J.D., Alfrey, C.P. and Lynch, E. C. (1972) Red blood cell damage by shear stress. Biophys J, 12, 257-273. [18] Wan, J., Ristenpart, W.D. and Stone, H.A. (2008) Dy- namics of shear-induced ATP release from red blood cells. PNAS, 105, 16432-16437. [19] Nikinmaa, M. (2003) Gas transport. In: Bernhardt, I. and Ellory, J.C., Eds., Red Cell Membrane Transport in Health and Disease, Springer-Verlag, Berlin, 489-509. [20] Betz, T., Bakowsky, U., Mueller, M.R., Lehr, C.M. and Bernhardt, I. (2007) Conformational change of mem- brane proteins leads to shape changes of red blood cells. Bioelectrochemistry, 70, 122-126. [21] Creanga, D.E., Culea, M., Nadejde, C., Oancea, S., Curecheriu, L. and Racuciu, M. (2009) Magnetic nanoparticle effects on the red blood cells. J Phys: Conf Ser, 170, 012019. |

