Paper Menu >>
Journal Menu >>
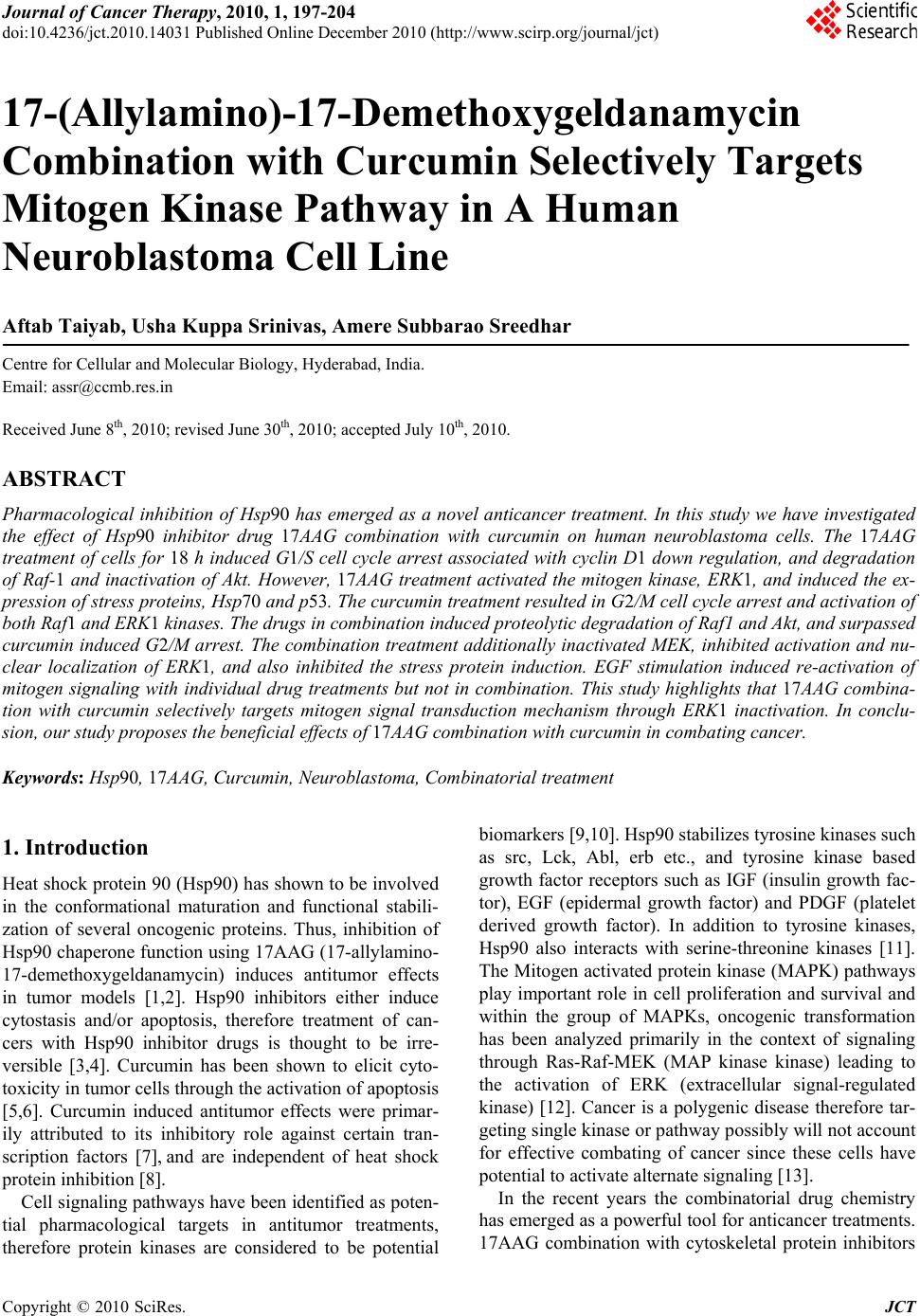 Journal of Cancer Therapy, 2010, 1, 197-204 doi:10.4236/jct.2010.14031 Published Online December 2010 (http://www.scirp.org/journal/jct) Copyright © 2010 SciRes. JCT 197 17-(Allylamino)-17-Demethoxygeldanamycin Combination with Curcumin Selectively Targets Mitogen Kinase Pathway in A Human Neuroblastoma Cell Line Aftab Taiyab, Usha Kuppa Srinivas, Amere Subbarao Sreedhar Centre for Cellular and Molecular Biology, Hyderabad, India. Email: assr@ccmb.res.in Received June 8th, 2010; revised June 30th, 2010; accepted July 10th, 2010. ABSTRACT Pharmacological inhibition of Hsp90 has emerged as a novel anticancer treatment. In this study we have investigated the effect of Hsp90 inhibitor drug 17AAG combination with curcumin on human neuroblastoma cells. The 17AAG treatment of cells for 18 h induced G1/S cell cycle arrest associated with cyclin D1 down regulation, and degradation of Raf-1 and inactivation of Akt. However, 17AAG treatment activated the mitogen kinase, ERK1, and induced the ex- pression of stress proteins, Hsp70 and p53. The curcumin treatmen t resulted in G2/M cell cycle arrest and activation of both Raf1 and ERK1 kinases. The drugs in combination induced proteolytic degradation of Raf1 and Akt, and surpassed curcumin induced G2/M arrest. The combination treatment additionally inactivated MEK, inhibited activation and nu- clear localization of ERK1, and also inhibited the stress protein induction. EGF stimulation induced re-activation of mitogen signaling with individual drug treatments but not in combination. This study highlights that 17AAG combina- tion with curcumin selectively targets mitogen signal transduction mechanism through ERK1 inactivation. In conclu- sion, our study proposes the beneficial effects of 17AAG combination with curcumin in combating cancer. Keywords: Hs p90, 17AAG, Curcumin, Neuroblastoma, Combinatorial treatment 1. Introduction Heat shock protein 9 0 (Hsp90) has shown to be involved in the conformational maturation and functional stabili- zation of several oncogenic proteins. Thus, inhibition of Hsp90 chap erone function usin g 17AAG (17-allylamin o- 17-demethoxygeldanamycin) induces antitumor effects in tumor models [1,2]. Hsp90 inhibitors either induce cytostasis and/or apoptosis, therefore treatment of can- cers with Hsp90 inhibitor drugs is thought to be irre- versible [3,4]. Curcumin has been shown to elicit cyto- toxicity in tumor cells throu gh th e activation of apop tosis [5,6]. Curcumin induced antitumor effects were primar- ily attributed to its inhibitory role against certain tran- scription factors [7], and are independent of heat shock protein inhibition [8]. Cell signaling pathways have been identified as poten- tial pharmacological targets in antitumor treatments, therefore protein kinases are considered to be potential biomarkers [9,10]. Hsp90 stabilizes tyrosine kinases such as src, Lck, Abl, erb etc., and tyrosine kinase based growth factor receptors such as IGF (insulin growth fac- tor), EGF (epidermal growth factor) and PDGF (platelet derived growth factor). In addition to tyrosine kinases, Hsp90 also interacts with serine-threonine kinases [11]. The Mitogen activated protein kinase (MAPK) pathways play important role in cell proliferation and survival and within the group of MAPKs, oncogenic transformation has been analyzed primarily in the context of signaling through Ras-Raf-MEK (MAP kinase kinase) leading to the activation of ERK (extracellular signal-regulated kinase) [12]. Cancer is a polygenic disease therefore tar- geting single kinase or pathway possibly will not account for effective combating of cancer since these cells have potential to activate alternate signa ling [13]. In the recent years the combinatorial drug chemistry has emerged as a powerful tool for anticancer treatments. 17AAG combination with cytoskeletal protein inhibitors  17-(Allylamino)-17-Demethoxygeldanamycin Combination with Curcumin Selectively Targets Mitogen Kinase Pathway in A Human Neuroblastoma Cell Line Copyright © 2010 SciRes. JCT 198 such as taxol was reported to be effective against cancer [14]. Similarly curcumin combination with EGCG (epi- gallocatechin gallate), cisplatin and doxorubicin were shown to target pro-survival pathways via NF-kB (nu- clear factor-kappa B) [15]. There were reports that cur- cumin combination with piplartine augments the cyto- toxic effects via ERK and cdk2 (cyclin dependent kinase-2) inactivation [8], and its combination with vi- tamin D3 induces differentiation [16]. Considering the present clinical interest with Hsp90 inhibitors and cur- cumin in anticancer treatments, we have examined and evaluated the effect of 17AAG in combination with cur- cumin in human neuroblastoma tumor cells, and report that the combination treatment effectively targets mito- gen signaling through ERK1. 2. Materials and Methods 2.1. Cell Culture and Chemicals The human neuoroblatoma tumor cells (IMR32) were maintained in Dulbecco’s Modified Eagles Medium (DMEM) containing 10% fetal calf serum (FCS), penicil- lin (100 U/ml), and streptomycin (50 µg/ml) in a humidi- fied incubator chamber (37C) supplied with 5% CO2. All the chemicals are procured from Sigma Chemical Com- pany, USA unless otherwise indicated. 2.2. Drug Treatments Exponentially growing tumor cells (1x106/ml) in complete medium were treated with different concentrations of 17AAG (Invitrogen; 0.5 to 10 µM), and curcumin (Sigma Aldrich; 1 to 25 µM) either alone or in combination. The EGF at a concentration 50 ng/ml (S igma Aldrich) was used to confron t drug tre atments. All the treat ments were con tin- ued with respective time intervals at 37 C bef ore ha rve stin g for further experiments. 2.3. The Morphology, Viability, and FACS Analysis Cells after respective drug treatments were subjected to morphological examination using a regular phase con- trast microscope (Nikon, TMS) attached to a 35 mm camera. Cell viability was determined by trypan blue exclusion assay before processing cells for further ex- periments. Control and drugs treated cells were washed with PBS (phosphate buffered saline), stained with 50 µg/ml propidium iodide (containing 0.1% triton X-100 and 22 µg/ml RNase), and analyzed in a fluorescence activated cell sorter (FACS Calibur, BD). 2.4.MTT (3-(4,5-Dimethylthiazol-2-yl) -2, 5-diphenyltetrazolium bromide) Assay Neuroblastoma cells (3x105 cells/ml) were grown on 96 well plates, and the final volume of the medium in each well was maintained to 100 µl. Cells were treated with 17AAG, curcumin and their combination for 24 h fol- lowed by treatment with 10 µl MTT (100 mg MTT/ 20 ml DMEM), for 6 h at room temperature with gentle shaking. The absorbance recorded at 590 nm using ELISA reader, and the formazan values obtained were interpr eted such tha t an increase in ab sorbance is ch arac- teristic to increased cell death. 2.5. Immunofluorescence and Laser Scanning Confocal Microscopy Neuroblastoma cells grown on cover slips (Fisher Scien- tifics, 22x22 mm) after respective drug treatments were rinsed with PBS, fixed in 3.7% paraformaldehyde for 10 min, and permeabilized with 0.2% Triton X-100 for 10 min. Cells were then washed with PBS, blocked with PBS-Tween 20 (0.5%; PBS-T) containing 2% BSA for 30 min, and incubated with primary antibody. After be- ing washed with PBS-T, cells were incubated with cor- responding FITC-conjugated secondary antibody (Ban- galore Genie). Cover slips were mounted on slides with Slow-Fade mounting medium (Molecular Probes) and analyzed using a laser scanning confocal microscope (Olympus FV500 microscope). 2.6. SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Western Blot Analysis Cells after respective drug treatments were lysed using the lysis buffer (20 mM HEPES lysis buffer, pH 7.6 , 10 mM NaCl, 1.5 mM MgCl2, 0.1% Triton X-100, 1 mM dithiothretol) for 60 min at 4C. Protein concentration was estimated by Bradford method [17] using bovine serum albumin (BSA) as standard. Twenty micrograms of total cell lysate was mixed with Laemmli buffer [18] containing 100 µl dithiothreitol, boiled for five minutes, and run on 10% SDS-PAGE. Proteins were transferred from the gel to nitro-cellulose membrane using a semidry protein gel transfer apparatus (Amersham Biosciences). Transfer of proteins was confirmed by Ponceau-S stain- ing before processing gels for Western blot analysis. Membrane blocked with 3% BSA followed by incuba- tion with appropriate primary antibody for 1 h and sub- sequent incubation with horseradish peroxidase-conjugated secondary antibody (dilution, 1:3000) for 1 h. The anti- gen- antibody interaction was visualized using luminol from BM-Chemiluminescence kit (Roche-Switzerland), and the protein bands exposed to X--ray film (Kodak). Antibodies used in the present study, ERK1 (1:1000), MEK1/2 (1:100 0), Raf 1 (1 :10 00), A k t (1 :800), cyclin D 1 (1:800) from Santa Cruz (USA). The antibodies actin (1:500), -tubulin (1:500), Hsp70 (1:1000), Hsp27 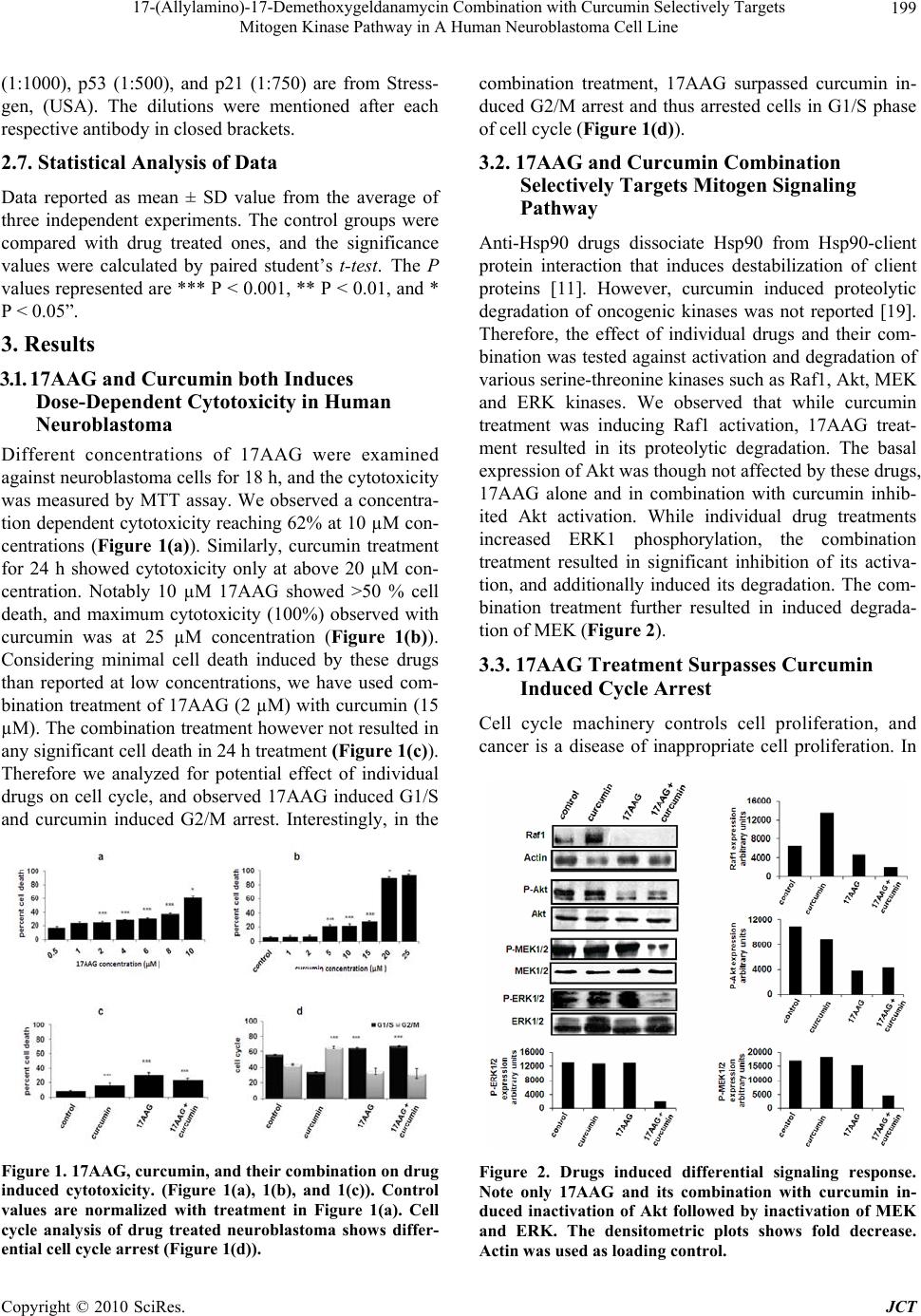 17-(Allylamino)-17-Demethoxygeldanamycin Combination with Curcumin Selectively Targets Mitogen Kinase Pathway in A Human Neuroblastoma Cell Line Copyright © 2010 SciRes. JCT 199 (1:1000), p53 (1:500), and p21 (1:750) are from Stress- gen, (USA). The dilutions were mentioned after each respective antibody in closed brackets. 2.7. Statistical Analysis of Data Data reported as mean ± SD value from the average of three independent experiments. The control groups were compared with drug treated ones, and the significance values were calculated by paired student’s t-test. The P values represented are *** P < 0.001, ** P < 0.01, and * P < 0.05”. 3. Results 3.1. 17AAG and Curcumin both Induces Dose-Dependent Cytotoxicity in Human Neuroblastoma Different concentrations of 17AAG were examined against neuroblastoma cells for 18 h, and the cytotoxicity was measured by MTT assay. We observed a concentra- tion dependent cytotoxicity reaching 62% at 10 µM con- centrations (Figure 1(a)). Similarly, curcumin treatment for 24 h showed cytotoxicity only at above 20 µM con- centration. Notably 10 µM 17AAG showed >50 % cell death, and maximum cytotoxicity (100%) observed with curcumin was at 25 µM concentration (Figure 1(b)). Considering minimal cell death induced by these drugs than reported at low concentrations, we have used com- bination treatment of 17AAG (2 µM) with curcumin (15 µM). The combination treatment howev er not resulted in any significant cell death in 24 h treatment (Figure 1(c)). Therefore we analyzed for potential effect of individual drugs on cell cycle, and observed 17AAG induced G1/S and curcumin induced G2/M arrest. Interestingly, in the Figure 1. 17AAG, curcumin, and their combination on drug induced cytotoxicity. (Figure 1(a), 1(b), and 1(c)). Control values are normalized with treatment in Figure 1(a). Cell cycle analysis of drug treated neuroblastoma shows differ- ential cell cycle arrest (Figu r e 1 ( d )). combination treatment, 17AAG surpassed curcumin in- duced G2/M arrest and thus arrested cells in G1/S phase of cell cycle (Figure 1(d)). 3.2. 17AAG and Curcumin Combination Selectively Targets Mitogen Signaling Pathway Anti-Hsp90 drugs dissociate Hsp90 from Hsp90-client protein interaction that induces destabilization of client proteins [11]. However, curcumin induced proteolytic degradation of oncogenic kinases was not reported [19]. Therefore, the effect of individual drugs and their com- bination was tested against activation and degradation of various serine-threonine kinases such as Raf1, Akt, MEK and ERK kinases. We observed that while curcumin treatment was inducing Raf1 activation, 17AAG treat- ment resulted in its proteolytic degradation. The basal expression of Akt w a s though not affected by these drugs, 17AAG alone and in combination with curcumin inhib- ited Akt activation. While individual drug treatments increased ERK1 phosphorylation, the combination treatment resulted in significant inhibition of its activa- tion, and additionally induced its degradation. The com- bination treatment further resulted in induced degrada- tion of MEK (Figure 2). 3.3. 17AAG Treatment Surpasses Curcumin Induced Cycle Arrest Cell cycle machinery controls cell proliferation, and cancer is a disease of inappropriate cell proliferation. In Figure 2. Drugs induced differential signaling response. Note only 17AAG and its combination with curcumin in- duced inactivation of Akt followed by inactivation of MEK and ERK. The densitometric plots shows fold decrease. Actin was used as loading control. 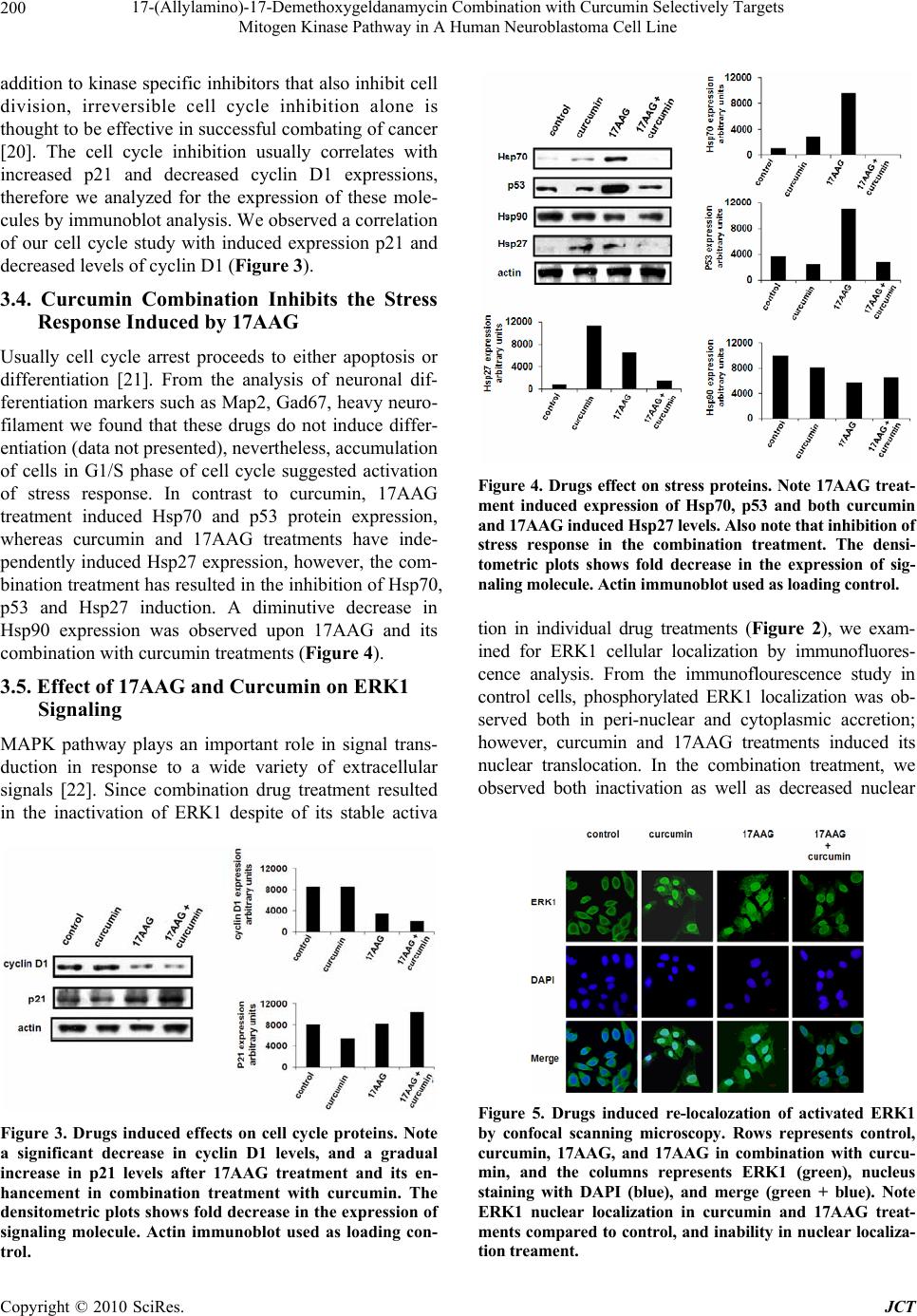 17-(Allylamino)-17-Demethoxygeldanamycin Combination with Curcumin Selectively Targets Mitogen Kinase Pathway in A Human Neuroblastoma Cell Line Copyright © 2010 SciRes. JCT 200 addition to kinase specific inhibitors that also inhibit cell division, irreversible cell cycle inhibition alone is thought to be effective in successful combating of cancer [20]. The cell cycle inhibition usually correlates with increased p21 and decreased cyclin D1 expressions, therefore we analyzed for the expression of these mole- cules by immunoblot analysis. We observed a correlation of our cell cycle study with induced expression p21 and decreased levels of cyclin D1 (Figure 3). 3.4. Curcumin Combination Inhibits the Stress Response Induced by 17AAG Usually cell cycle arrest proceeds to either apoptosis or differentiation [21]. From the analysis of neuronal dif- ferentiation markers such as Map2, Gad67, heavy neuro- filament we found that these drugs do not induce differ- entiation (data not presented), nevertheless, accumulation of cells in G1/S phase of cell cycle suggested activation of stress response. In contrast to curcumin, 17AAG treatment induced Hsp70 and p53 protein expression, whereas curcumin and 17AAG treatments have inde- pendently induced Hsp27 expression, however, the com- bination treatment has resulted in the inhibition of Hsp70, p53 and Hsp27 induction. A diminutive decrease in Hsp90 expression was observed upon 17AAG and its combination with curcumin treatments (Figure 4). 3.5. Effect of 17AAG and Curcumin on ERK1 Signaling MAPK pathway plays an important role in signal trans- duction in response to a wide variety of extracellular signals [22]. Since combination drug treatment resulted in the inactivation of ERK1 despite of its stable activa Figure 3. Drugs induced effects on cell cycle proteins. Note a significant decrease in cyclin D1 levels, and a gradual increase in p21 levels after 17AAG treatment and its en- hancement in combination treatment with curcumin. The densitometric plots shows fold decrease in the expression of signaling molecule. Actin immunoblot used as loading con- trol. Figure 4. Drugs effect on stress proteins. Note 17AAG treat- ment induced expression of Hsp70, p53 and both curcumin and 17AAG induced Hsp27 levels. Also note that inhibition of stress response in the combination treatment. The densi- tometric plots shows fold decrease in the expression of sig- naling molecule. Actin immunoblot used as loading control. tion in individual drug treatments (Figure 2), we exam- ined for ERK1 cellular localization by immunofluores- cence analysis. From the immunoflourescence study in control cells, phosphorylated ERK1 localization was ob- served both in peri-nuclear and cytoplasmic accretion; however, curcumin and 17AAG treatments induced its nuclear translocation. In the combination treatment, we observed both inactivation as well as decreased nuclear Figure 5. Drugs induced re-localozation of activated ERK1 by confocal scanning microscopy. Rows represents control, curcumin, 17AAG, and 17AAG in combination with curcu- min, and the columns represents ERK1 (green), nucleus staining with DAPI (blue), and merge (green + blue). Note ERK1 nuclear localization in curcumin and 17AAG treat- ments compared to control, and inability in nuclear localiza- tion treament.  17-(Allylamino)-17-Demethoxygeldanamycin Combination with Curcumin Selectively Targets Mitogen Kinase Pathway in A Human Neuroblastoma Cell Line Copyright © 2010 SciRes. JCT 201 localization of ERK1 (Figure 5). 3.6. Effect of Epidermal Growth Factor in the Reversal of Mitogen Signal Inactivation The membrane receptors, including EGF, transduce sig- nals by activating the MAPK family of proteins; there- fore tumor cells take advantage of these kinases for con- stitutive activation of survival signals. In the present study, to examin e whether our combination is selectively targeting the mitogen signaling pathway, we evaluated EGF effect in 17AAG and curcumin combination drug pre-treatment (EGF treatment followed by combination drugs), co-treatment (EGF treatment along with combi- nation of drugs) and post-treatments (EGF treatment af- ter the combination drug treatment). The EGF treatment induced reversal of cytotoxicity in the combination treatments significantly, however, was not found to be effective with 17AAG treatment alone. Among the pre-, co-, and post- EGF treatments, the co- and post-treatments showed decreased cell recovery com- pared to the pre-treatment (Figure 6). The improved growth in EGF pre-treatment could be related to en- hanced proliferative signaling prior to drugs treatment, whereas co- and post-treatments suggests absence or lowered proliferation rate due to combination of drugs. These findings invoked existence of possible signal transduction dependent regulatory mechanism operated by EGF in the reversal of 17AAG effect however on ly in the combination treatment. In support, the immunoblot analysis of EGF treated cells showed restrained Raf1, Akt and MEK activities but resulted in enhanced prote- olytic degradation of ERK1 (Figure 7). 4. Discussion Among several anticancer drugs that are under clinical evaluation, Hsp90 inhibitor drug 17AAG [23-24] and an- tioxidant and anti-inflammatory drug curcumin [6] are attributed for their selectivity and specificity against a large variety of cancer cells. Considering the growing in- terest and beneficial effects of combinatorial drug treat- ments in treating cancer, we have examined for combina- torial effects of 17AAG and curcumin against human neuroblastoma tumor cells. From the present study, we report that the combination drug treatment selectively tar- gets the MAP kinase signal transducti on pathway. Several reports of 17AAG combination with ox- aliplatin, flurouracil [25], carboplatin [26], paclitaxel [27], rapamycin [28], trastuzumab and tanespinycin [29], histone deacetylease [30] etc., suggests that 17AAG combination treatments not only enhance drug specific effects, but exhibit synergistic effects however not though MAP kinases. In a classical mitogen activated Figure 6. Epidermal growth factor (EGF) treatment on cell recovery and mitogen signal activation. Compared to 17AAG treatment its combination with curcumin shows reversal of phenotype. Note that only post-treatment of cells with EGF shows maximum survival compared to co- and pre-treatments. Figure 7. Drugs induced differential signaling response after EGF recovery. Note only 17AAG and its combination with curcumin induced inactivation of Akt, MEK and ERK. The densitometric plots shows fold decrease. Tubulin used as loading control. signaling pathway MEK binds to ERK and this coordi- nated binding is essential for the activation of ERK. De- regulated signal transduction mechanism is one of the hall marks of cancers [31,32]. From our studies it is evi- dent that Raf1 activation by curcumin or inactivation by 17AAG has no or little effect on MEK and ERK signal- ing, therefore, the Akt or Raf1 activation appears to be independent of do wnstream effector s of MAPK pathway. Usually only one form of dual phopshorylated ERK ex- ists in the cell with greater specificity, which translocates to the nucleus and activates certain transcription al factors. In agreement with curcumin induced ERK and JNK  17-(Allylamino)-17-Demethoxygeldanamycin Combination with Curcumin Selectively Targets Mitogen Kinase Pathway in A Human Neuroblastoma Cell Line Copyright © 2010 SciRes. JCT 202 pathways [33], we report ERK1 activation and its nuclear localization with 17AAG and curcumin, however, the combination has significantly inhibited its activation and nuclear translocation . Despite the fact that curcumin alone can activate stress response in experimental models [34,35], in our system curcumin did not induce stress response. Curcumin on the other hand had inhibited stress response induced by 17AAG. Similar results were obtained with different cell types such as rat histiocytoma (BC-8 ), rat hepatoma cells (CRL-1600), human T-lymphocyte cells (Jurkat), mouse embryonal carcinoma cells (PCC4). Induced stress pro- teins are known to hamper cancer treatments, therefore, drugs that induce cytotoxic effects without inducing the stress response are considered to be effective anticancer agents [36]. Only a decrease in BCl2 expression in asso- ciation with increased stress proteins further suggests activation of only the stress response, but not apoptotic response. Treatment of tumor cells with ROS (reactive oxygen species) scavenger, NAC (N-acetyl cysteine), ascorbic acid, vitamin K etc., though decreased ROS levels, they did not show any effect on combination treatment induced mitogen signal targeting (data not shown). Contrary to curcumin induced p21 and degrada- tion of cyclin D1 [5,37], we did not find curcumin tar- geting of these two molecule. However, once again it is only the combination treatment that had resulted in the degradation of cyclin D1, which is otherwise found de- regulated in majority of cancers [38]. The EGF and EGF-receptor signaling stimulates growth of cells in culture without affecting DNA synthe- sis [39]. Neuroblastoma tumors are known to have in- duced expression of EGF family of receptors [40]. Ob- serving a MAPK dependent targeting in our combinato- rial drug treatment with 17AAG and diferuloylmethane, we have examined for EGF effect on drug treated cells and found that EGF stimulation though resulted in the restitution of normal division potential, selective target- ing of ERK signaling was established. We present that 17AAG combination with curcumin potentiated 17AAG effect and add ition ally targ eted MAP kinases. Combination treatment further inhibited 17AAG induced stress response. Our results indicate that 17AAG may be a promising candidate to use in combinatorial treatments with curcumin to combat cancer where mito- gen kinase expression is challenging the anticancer treatment. 5. Acknowledgements Authors thank Ms. Nandini for confocal microscopy. Department of Biotechnology and Department of Sci- ence and Technology, Ministry of Human Resource De- velopment, Government of India supported the work in author’s laboratory. REFERENCES [1] L. Whitesell, E. G. Mimnaugh, B. De Costa, C. E. Myers and L. M. Neckers, “Inhibition of Heat Shock Protein Hsp90-pp60v-src Heteroprotein Complex Formation by Benzoquinone Ansamycins: Essential Role for Stress Proteins in Oncogene Transformation,” Proceedings of National Academy of Sciences USA, Vol. 91, No. 18, 1994, pp. 8324-8328. [2] A. Kamal, L. Thao, J. Sensintaffar, L. Zhang, M. F. Boehm, L. C. Fritz and F. J. Burrows, “High Affinity Conformation of Hsp90 Confers Tumor Selectivity on Hsp90 Inhibitors,” Nature, Vol. 425, No. 6956, 2003, pp. 207-410. [3] T. Vanden Berghe, M. Kalai, G. van Loo, W. Declerq and P. Vandenbeele, “Disruption of HSP90 Function Reverts Tumor Necrosis Factor-induced Necrosis to Apoptosis,” Journal of Biological Chemistry, Vol. 278, No. 9, 2003, pp. 5622-5629. [4] I. Hostein, D. Robertson, F. DiSte fa n co, P. Workman, and P. A. Clarke, “Inhibition of Signal Transduction -by the Hsp90 Inhibitor 17-allylamino-17-demethoxygeldanamycin results in Cytostasis and Apoptosis,” Cancer Research, Vol. 61, No. 10, May 2001, pp. 4003-4009. [5] B. B. Aggarwal, A. Kumar and A. C. Bharti, “Anticancer Potential of Curcumin: Preclinical and Clinical Studies,” Anticancer Research, Vol. 23, No. 1A, 2003, pp. 263-398. [6] A. L. Cheng, C. H. Hsu, J. K. Lin, M. M. Hsu, Y. M. Ho, T. S. Shen, J. Y. Ko, Lin J. T, Lin B. R, Ming-Shiang W, Yu H. S, Jee S. H, Chen G. S, Chen T. M, Chen C. A, Lai M. K, Lai M. K, Pu Y. S, Pan M. H, Wang Y. J, Tsai C. C and Hsieh C. Y, “Phase I Clinical Trial of Curcumin, a Chemopreventive Agent, in Patients with High-risk or Pre-malignant Lesions,” Anticancer Research, Vol. 21, No. 4B,2001, pp. 2895-2900. [7] A. Duvoix, R. Blasius, S. Delhalle, M. Schnekenburger, F. Morceau, E. Henry, M. Dicato and M. Diederich, “Che- mopreventive and Therapeutic Effects of Curcumin,” Cancer Letters, Vol. 223, No. 2, 2005, pp. 181-190. [8] D. Jyothi, P. Vanathi, P. Mangala Gowri, V. Rama Subba Rao, J. Madhusudana Rao and A. S. Sreedhar, “Diferu- loylmethane Augments the Cytotoxic Effects of Piplar- tine Isolated from Piper Chaba,” Toxicology In Vitro, Vol. 23, No. 6, 2009, pp. 1085-1091. [9] A. H. Bild, G. Yao, J. T. Chang, Q. Wang, A. Potti, D. Chasse, M. B. Joshi, D. Harpole, J. M. Lanca ster, A. Be r- chuck, J. A. Olson Jr, J. R. Marks, H. K. Dressman, M. West and J. R. Nevins, “Oncogenic Pathway Signatures in Human Cancers as a Guide to Targeted Therapies,” Nature, Vol. 439, No. 7074, 2006, pp. 353-357. [10] A. H. Bild, A. Potti and J. R. Nevins, “Linking Onco- genic Pathways with Therapeutic Opportunities,” Nature Review Cancer, Vol. 6, No. 9, 2006, pp. 735-741.  17-(Allylamino)-17-Demethoxygeldanamycin Combination with Curcumin Selectively Targets Mitogen Kinase Pathway in A Human Neuroblastoma Cell Line Copyright © 2010 SciRes. JCT 203 [11] W. B. Pratt and D. O. Toft, “Regulation of Signaling Protein Function and Trafficking by the Hsp90/hsp70- based Chaperone Machinery,” Experimental Biology and Medicine, Vol. 228, No. 2, 2003, pp.111-133. [12] P. Blume-Jensen and T. Hunter, “Oncogenic Kinase Sig- naling,” Nature, Vol. 411, No. 6835, 2001, pp. 355-365. [13] A. S. Sreedhar, P. Vanathi and K. R. Paithankar, “Stress Protein in Biology and Medicine: Evolution, Adaptation and Clinical Evaluation,” International Journal of Pharma and Biosciences, Vol. 1, No. 3, 2010, pp. 1-36. [14] D. B. Solit, A. D. Basso, A. B. Olshen, H. I. Scher and N. Rosen, “Inhibition of Heat Shock Protein 90 Function Down-regulates Akt kinase and Sensitizes Tumors to Taxol,” Cancer Research, Vol. 63, No. 9, 2003, pp. 2139-2144. [15] A. K. Ghosh, N. E. Kay, C. R. Secreto and T. D. Shanafelt, “Curcumin Inhibits Prosurvival Pathways in Chronic Lymphocytic Leukemia B Cells and May Over- come Their Stromal Protection in Combination with EGCG,” Clinical Cancer Research, Vol. 15, No. 4, 2009, pp. 1250-1258. [16] J. A. Sokoloski, K. Shyam and A. C. Sartorelli, “Induc- tion of the Differentiation of HL-60 Promyelocytic Leu- kemia Cells by Curcumin in Combination with Low Lev- els of Vitamin D3,” Oncology Research, Vol. 9, No. 1, 1997, pp. 31-39. [17] M. M. Bradford, “A Rapid and Sensitive Method for the Quantitation of Protein Utilizing the Principle of Pro- tein-dye Binding,” Anal Biochemistry, Vol. 7, 1976, pp. 248-254. [18] U. K. Laemmli, “Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4,” Nature, Vol. 227, No. 5259, 1970, pp. 680-685. [19] L. Neckers, “Screening for Inducers of Kinase Degrada- tion,” Chemistry & Biology, Vol. 10, No. 7, 2003, pp. 587-589. [20] M. B. Kastan and J. Bartek, “Cell-cycle Checkpoints and Cancer,” Nature, Vol. 432, No. 7015, 2004, pp. 316-328. [21] D. Alesiani, R. Cicconi, M. Mattei, C. Montesano, R. Bei and A. Canini, “Cell Cycle Arrest and Differentiation In- duction by 5,7-dimethoxycoumarin in Melanoma Cells. International Journal of Oncology, Vol. 32, No. 2, 2008, pp. 425-434. [22] M. D. Brown and D. B. Sacks, “Protein Scaffold in MAP Kinase Signaling,” Cell Signalling, Vol. 21, No.4, 2009, pp. 462-469. [23] S. Pacey, U. Benerji, I. Judson and P. Workman, “Hsp90 Inhibitors in the Clinic,” Handbook of Experimental Pharmacology, Vol. 172, 2006, pp. 331-358. [24] A. S. Sreedhar, C. Soti and P. Csermely, “Inhibition of Hsp90: A New Strategy for Inhibiting Protein Kinases,” Biochimica et Biophysica Acta, Vol. 1697, No. 3, 2004, pp. 233-242. [25] T. V. Rakitina, I. A. Vasilevskaya and P. J. O'Dwyer, “Inhibition of G1/S Transition Potentiates Oxaliplatin- induced Cell Death in Colon Cancer Cell Lines,” Bio- chemical Pharmacology, Vol. 73, No. 11, 2007, pp. 1715-1726. [26] U. Banerji, N. Sain, S. Y. Sharp, M. Valenti, Y. Asad, R. Ruddle, F. Raynaud F, M. Walton, S. A. Eccles, I. Judson, A. L. Jackman and P. Workman, “An in Vitro and in Vivo Study of the Combination of the Heat Shock Protein Inhibitor 17-allylamino-17-demethoxygeldanamycin and Carboplatin in Human Ovarian Cancer Models,” Cancer Chemotherapy and Pharmacology, Vol. 62, No. 5, 2008, pp. 769-778. [27] N. Sain, B. Krishnan, M. G. Ormerod, A. De Rienzo, W. M. Liu, S. B. Kaye, P. Workman and A. L. Jackman, “Potentiation of Paclitaxel Activity by the HSP90 Inhibi- tor 17-allylamino-17-demethoxygeldanamycin in Human Ovarian Carcinoma Cell Lines with High Levels of Acti- vated AKT,” Molecular Cancer Therapy, Vol. 5, No. 5, 2006, pp. 1197-1208. [28] M. M. Roforth and C. Tan, “Combination of Rapamycin and 17-allylamino-17-demethoxygeldanamycin Abro- gates Akt Activation and Potentiates mTOR Blockade in Breast Cancer Cells,” Anticancer Drugs, Vol. 19, No. 7, 2008, pp. 681-688. [29] S. Modi, A. T. Stopeck, M. S. Gordon, D. Mendelson, D. B. Solit, R. Bagatell, W. Ma, J. Wheler, N. Rosen, L. Norton, G. F. Cropp, R. G. Johnson, A. L. Hannah and C. A. Hudis, “Combination of Trastuzumab and Tanespimy- cin (17-AAG, KOS-953) is Safe and Active in Trastuzu- mab-refractory HER-2 Overexpressing Breast Cancer: A Phase I Dose-escalation Study,” Journal of Clinical On- cology, Vol. 25, No. 34, 2007, pp. 5410-5417. [30] G. Ravi, P. George, P. Bali, J. Tao, F. Guo, C. Sigua, Y. Li, L. Moscin ski, P. Atadja and K. N. Bhalla , “A Combi- nation of Histone Deacetylase Inhibitor LBH589 and the Hsp90 Inhibitor 17-AAG is Highly Active Against Hu- man CML-BC and AML Cells with Constitutively Active Mutant FLT-3 Tyrosine Kinase,” Abstract 3023, Experi- mental and Molecular Therapeutics 28: Novel Targets and approaches for angiogenesis and Signaling pathways, 2004. [31] D. Hanahan and R. A Weinberg, “The Hallmarks of Can- cer,” Cell, Vol. 7, No. 1, 2000, pp. 57-70. [32] D. T. Denhardt, “Oncogene-initiated Aberrant Signaling Engenders the Metastatic Phenotype: Synergistic Tran- scription Factor Interactions are Targets for cancer ther- apy,” Critical Reviews in Oncogenesis, Vol. 7, No. 3-4, 1996, pp. 261-291. [33] B. H. Choi, C. G. Kim, Y. S. Bae, Y. Lim, Y. H. Lee and S. Y. Shin, “P21 Waf1/Cip1 Expression by Curcumin in U-87MG Human Glioma Cells: Role of Early Growth Response-1 Expression,” Cancer Research, Vol. 68, pp. 1369-1377, 2008. [34] A. Khar, A. M. Ali, B. V. V. Pardhasaradhi, C. H. Vara- lakshmi, R. Anjum and A. L. Kumari, “Induction of Stress Response Renders Human Tumor Cell Lines Re- sistant to Curcumin-mediated Apoptosis: Role of Reac-  17-(Allylamino)-17-Demethoxygeldanamycin Combination with Curcumin Selectively Targets Mitogen Kinase Pathway in A Human Neuroblastoma Cell Line Copyright © 2010 SciRes. JCT 204 tive Oxygen Intermediates,” Cell Stress and Chaperones, Vol. 6, No. 4, 2001, pp. 368-376. [35] K. Kato, H. Ito and K. Kamei, “Iwamoto I. Stimulation of the stress-induced expression of stress proteins by Cur- cumin in cultured cells and in rat tissues in vivo,” Cell Stress and Chaperones, Vol. 3, No. 3, 1998, pp. 152-160. [36] A. S. Sreedhar and P. Csermely, “Heat Shock Proteins in the Regulation of Apoptosis: New Strategies in Tu- mor Therapy. A Comprehensive Review,” Pharmacol- ogy and Therapeutics, Vol. 101, No. 3, 2004, pp. 227-257. [37] A. B. Kunnumakkara, P. Diagaradjane, P. Anand, K. B. Harikumar, A. Deorukhkar, J. Gelovani, S. Guha, S. Krishnan and B. B. Aggarwal, “Curcumin Sensitizes Human Colorectal Cancer to Capecitabine by Modulation of Cyclin D1, COX-2, MMP-9, VEGF and CXCR4 Ex- pression in an Orthotopic Mouse Model,” International Journal of Cancer, Vol. 125, No. 9, 2009, pp. 2187-2197. [38] K. Pruitt and C. J. Der, “Ras and Rho Regulation of the Cell Cycle and Oncogenesis,” Cancer Letters, Vol. 171, No. 1, 2001, pp. 1-10. [39] T. Putz, Z. Culig, I. E. Edler, C. Nessler-Menardi, G. Bartsch, H. Grunicke, F. Uberall and H. Klocker, “Epi- dermal Growth Factor (EGF) Receptor Blockade Inhibits the Action of EGF, Insulin-like Growth Factor I, and a Protein Kinase A Activator on the Mitogen-activated Pro- tein kinase Pathway in Prostate Cancer Cell Lines,” Can- cer Research, Vol. 59, No. 1, 1999, pp. 227-233. [40] H. Sartelet, L. L. Oligny and G. Vassal, “AKT Pathway in Neuroblastoma and Its Therapeutic Implication,” Ex- pert Review of Anticancer Therapy, Vol. 8, No. 5, 2008, pp. 757-769. |

